Introduction
Parkinson's disease (PD) is the most common movement
disorder, which occurs worldwide and increases in incidence with
age. In China, the prevalence and incidence of PD are 2 per 100,000
population and 797 per 100,000 person-years (1). The clinical symptoms of PD mainly
manifest as extrapyramidal movement disorders, such as tremor,
rigidity, bradykinesia and a mask-like, expressionless face, which
seriously affect the patients' quality of life (2). The main pathological characteristics of
PD are the progressive degeneration of dopaminergic neurons in the
substantia nigra pars compacta (SNpc) and the appearance of
eosinophilic inclusions (Lewy bodies), which leads to a reduction
of dopamine (DA) levels and extrapyramidal movement disorder; the
underlying mechanism may be associated with, for example, oxidative
stress, mitochondrial dysfunction or protein misfolding (3,4). When
the symptoms begin, 50–80% of dopaminergic neurons have already
been destroyed (5). A worldwide
problem is that good drugs and effective methods for the treatment
of PD are lacking. L-Dopa is one of the most commonly used
medicines for PD. However, the use of L-dopa can be problematic
with regard to the selection of the appropriate dosage and timing.
Therefore, it is necessary to identify the optimal timing and
dosage of L-dopa and evaluate the efficacy of disease treatment in
order to reduce unnecessary drug use.
Positron emission tomography (PET), an imaging
technique for use in the in vitro quantitation and dynamic
observation of physiological and biochemical changes in the body,
has been widely applied in aspects of neurological diseases. The
spatial resolution of PET technology specially designed for use in
small animals (micro-PET) is ≤0.8 mm, which enables dynamic
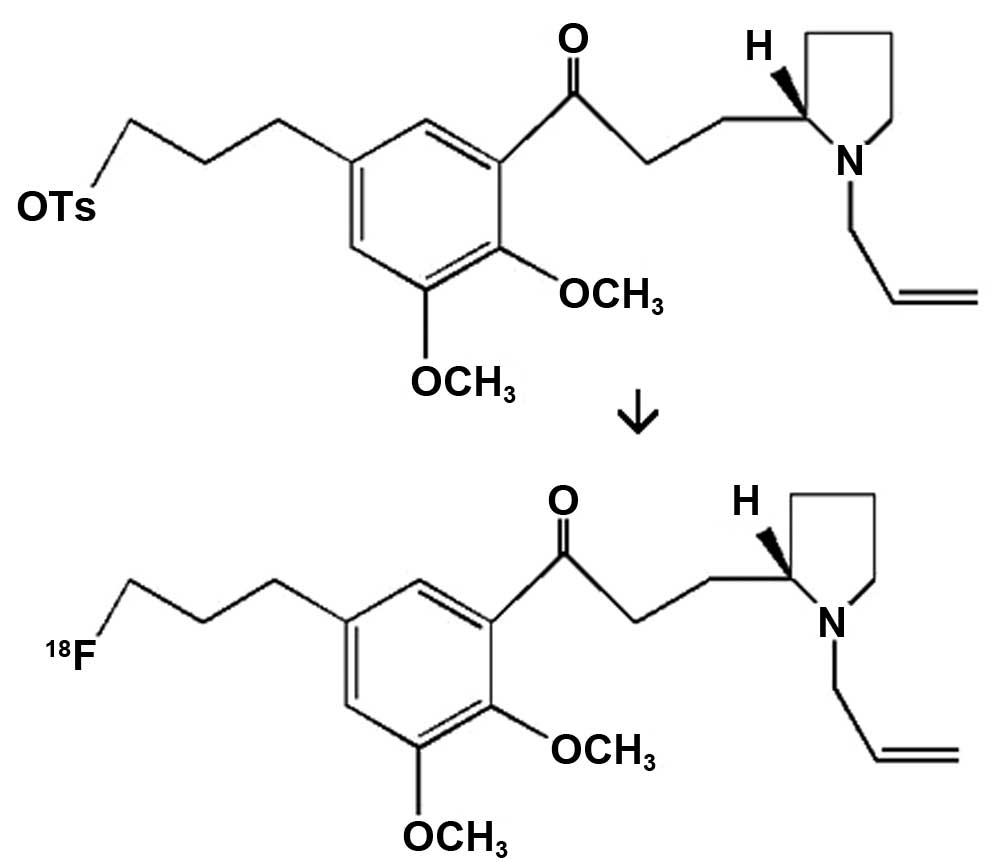
histological analysis to be performed on small volumes (6). 18F-fallypride, the
systematic name of which is
5-(3-18F-fluoropropyl)-2,3-dimethoxy-N-[(2S)-1-prop-2-enylpyrrolidin-2-yl]
methyl] benzamide (structure shown in Fig. 1), is a safe and effective novel
imaging agent for DA receptor D2 (DRD2), due to its strong
lipophilic property and high affinity for DRD2 in the brain, as
well as the short half-life of the carried positron 18F
(~109 min) (7,8). Micro-PET imaging with
18F-fallypride can be used to observe the changes in
brain DRD2 in a live, noninvasive and dynamic manner, and thus
provides a novel means for the study of brain diseases (9).
A decline in the levels of DA and its metabolites is
the major neurobiochemical change of PD, and it is proportional to
the degree of neuronal loss in the substantia nigra (SN).
Therefore, the nigrostriatal midbrain DA content is one of the main
indicators of clinical diagnosis (10). While the main metabolites of DA
include 3,5-dihydroxyphenylacetic acid (DOPAC) and homovanillic
acid (HVA), among others, HVA is the final product; therefore, the
ratio of HVA/DA can directly reflect the metabolism of DA and the
function of residual dopaminergic neurons (11). The immunoreactivity of tyrosine
hydroxylase (TH) can also be used as a marker of dopaminergic
neurons (12). In the present study
a 1-methyl-4-phenyl-1,2,3,6-tetrapyridine (MPTP)-induced mouse
model of PD was established (13),
the role of micro-PET imaging in the evaluation of the treatment of
PD with L-dopa was verified, and the underlying mechanisms were
explored, by methods including observation of general behavior,
swimming test, locomotor activity counts, determination of striatal
contents of glutathione peroxidase (GSH-PX), superoxide dismutase
(SOD), DA, DOPAC and HVA, immunohistochemical analysis of TH and
micro-PET imaging.
Materials and methods
Reagents and animals
MPTP was from Atuka Inc. (Toronto, Canada); L-dopa
tablet was the product of Beijing Shuguang Pharmaceutical Co., Ltd.
(Beijing, China); mouse anti-β-actin antibody was purchased from
Abcam (Cambridge, MA, USA); GSH-PX, SOD and malondialdehyde (MDA)
assay kits were purchased from Nanjing Jiancheng Technology Co.,
Ltd. (Nanjing, China). Other reagents were of analytical grade. The
experimental animals were 63 male 4-week-old ICR mice (20–24 g),
provided by Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai,
China; animal certificate no: 2007000522089).
Synthesis of 18F-fallypride
and determination of the radiochemical purity
The 18F-fallypride was prepared by
reacting the reaction precursor (4 mg; ABX GmbH, Radeberg, Germany)
with resolubilized K[18F]F-K222 (ABX GmbH) in
acetonitrile (1 ml) at 90°C for 20 min. The crude reaction mixture
was mixed with water (8 ml) and passed through a Sep-Pak C-18
column chromatography cartridge (Waters Corporation, Milford, MA,
USA) 3 times. To determine the radiochemical purity,
18F-fallypride was loaded onto one end of an instant
thin layer chromatography (iTLC) paper and separated upward with
dichloromethane:methanol (v/v)=9:1 as the developing agent. The
radiochemical purity was measured with a Mini-Scan TLC scanner
(Bioscan, Inc., Washington, DC, USA).
PD mouse model establishment and
evaluation
Establishment of the PD mouse model
This study was approved by Soochow University review
board/local ethics committee (Suzhou, China), and the experiments
followed the animal management regulations on experiments in China.
A total of 36 ICR mice were intraperitoneally (i.p.) injected with
MPTP (25 mg/kg) for 7 consecutive days.
Assessment of the PD mouse model
i) General behavioral test. The general behavior of
the mice was observed following the injection of MPTP.
ii) Swimming test. According to the method of Donnan
et al (3), the mice were
placed in a Plexiglass tank (20×30×20 cm); the water depth was 10
cm, and the water temperature was 22–25°C. The mice were graded as
follows: Mice continuously swimming for 1 min received 3.0 points;
floating occasionally and swimming most the time scored 2.5 points;
floating for >50% of the time received 2.0 points; swimming
occasionally received 1.5 points; and mice occasionally swimming
with hindlimbs and floating at the side of the water tank were
given 1.0 point. An average score was obtained from 3 independent
tests with a 10-min interval between each test. The mice were
tested once prior to drug administration and again at 1, 4 and 7
days, respectively, after the 40 mg/kg/day treatment, which began
at day 7 after modeling.
iii) Locomotor activity counts. According to the
method of Kawai et al (14),
a homemade 30×30×15 cm Plexiglass box was prepared with 6×6 cm
grids drawn on the bottom. The test was performed in a quiet,
low-light environment. The mice were adapted to the environment for
10 min and then the number of lines crossed and the frequency of
standing posture were determined. An average value was obtained
from 3 independent tests with a 20-min interval between each test.
The mice were tested once prior to the drug administration and
again at 1, 4 and 7 days, respectively, after the treatment.
18F-fallypride imaging and
competitive inhibition experiments
Micro-PET imaging in vivo with
18F-fallypride
Following anesthesia with ketamine, the mice were
placed onto the headboard of micro-PET (Inveon micro-PET; Siemens
AG, Munich, Germany) and injected with 18F-fallypride
(3.7 MBq/each) through the tail vein. The acquisition mode was as
follows: Energy peak, 511 keV; time window, 3.432 nsec; acquisition
time, 120 min. The attenuation-corrected images of
18F-fallypride distribution in vivo in the mice
were obtained using an iterative reconstruction method. Region of
interest (ROI) techniques were used to manually select the striatal
ROI in the coronal section of mice and calculate the maximum uptake
of 18F-fallypride in the ROI.
Competitive inhibition binding experiment with
19F-fallypride
Appropriate amounts of 19F-fallypride
were weighed and used to prepare standard solutions at the
concentrations of 500, 50, 10, 1 and 0.1 µg/ml, respectively.
Eighteen normal ICR mice were randomly divided into 6 groups with 3
animals in each group, which were injected with respective
19F-fallypride standard solutions (100 µl/20 g body
weight) via tail vein. Mice injected with an equal volume of saline
served as the negative control group. Low to high concentrations of
19F-fallypride were sequentially injected into each
group; 10 min later, the 18F-fallypride was injected,
and after another 10 min, the animals were scanned by micro-PET.
ROIs were then used to outline the striatal radioactive counts.
Determination of TH level by
immunohistochemistry
The mice were weighed, anesthetized with ketamine
and perfused with 4% paraformaldehyde. The brains were dissected
immediately after the perfusion, fixed in 4% paraformaldehyde for 2
h and placed sequentially into 20 and 30% sucrose solutions. After
the tissues sank to the bottom, the SNpc and caudate nucleus were
selected and cut into serial coronal frozen sections (thickness, 20
mm). The sections were then washed 3 times in phosphate-buffered
saline (PBS; 0.01 M) by shaking for 15 min each time, incubated
with 0.3% H2O2 for 10 min at room temperature
to deactivate the endogenous peroxidase activity, and blocked with
PBS containing 10% normal goat serum and 0.2% Triton X-100. The
sections were incubated with mouse monoclonal anti-TH antibody
(1:1,000; cat. no. ab49640; Abcam) for 24 h. The immunoreactivity
of TH in the SN was observed under a microscope (CX21; Olympus
Corporation, Tokyo, Japan) at low magnification to calculate the
number of neurons in the mouse brain structure.
Determination of the contents of GSH-PX, SOD and
MDA
The mice were divided into control, model and L-dopa
1 day, 4 day and 7 day treatment groups with 6 animals in each
group. Following anesthesia by the intraperitoneal injection of 10%
chloral hydrate, the mice were decapitated, and the whole brain was
rapidly removed and placed on an ice-cold tray. The striatum was
then separated, weighed and homogenized. The homogenate was
adjusted to a concentration of 10% and stored at −80°C. The
contents of GSH-PX, SOD and MDA in the striatal homogenates were
measured according to the kit instructions.
Transmission electron microscopy (TEM)
The brains of 3 mice per group were fixed by
perfusion with 4% paraformaldehyde and 2% glutaraldehyde; the SN
was then isolated, treated with osmium tetroxide, dehydrated, and
embedded in epoxy resin. Frozen sections (90 nm) were cut and
observed under a Hitachi H600 TEM (70 kV; Hitachi, Tokyo,
Japan).
Determination of the striatal content of DA and
its metabolites by high performance liquid
chromatography-electrochemical detection (HPLC-ECD)
The brains of mice from each group were immediately
dissected following decapitation of the animal to isolate the
striatum, which was preserved in liquid nitrogen prior to testing.
The striatum was homogenized in 200 µl 0.02 M perchloric acid and
centrifuged at 4°C, 12,000 × g for 30 min to remove proteins. The
content of DA and its metabolites DOPAC and HVA in the supernatant
were detected using the HPLC-ECD method under the following
conditions: Mobile phase, 0.1 M NaH2PO4
buffer (pH 3.25) containing 0.85 M sodium 1-octanesulfonate, 11%
methanol and 0.5 mM EDTA-Na2; applied potential, 0–500
mV at an increment of 100 mV; column temperature, 35°C; flow rate,
1.2 ml/min, and injection volume, 50 µl. The DA content was
represented in units of µg/g wet tissue weight.
Statistical analysis
All data were analyzed for single-factor analysis of
variance using SPSS software, version 18.0 (SPSS, Inc., Chicago,
IL, USA). The results were presented as mean ± standard deviation.
Comparison between two groups was conducted by 2-sample t-test.
P<0.05 was considered to indicate a statistically significant
result.
Results
Labeling yield and radiochemical
purity of 18F-fallypride and competitive inhibition
results with 19F-fallypride
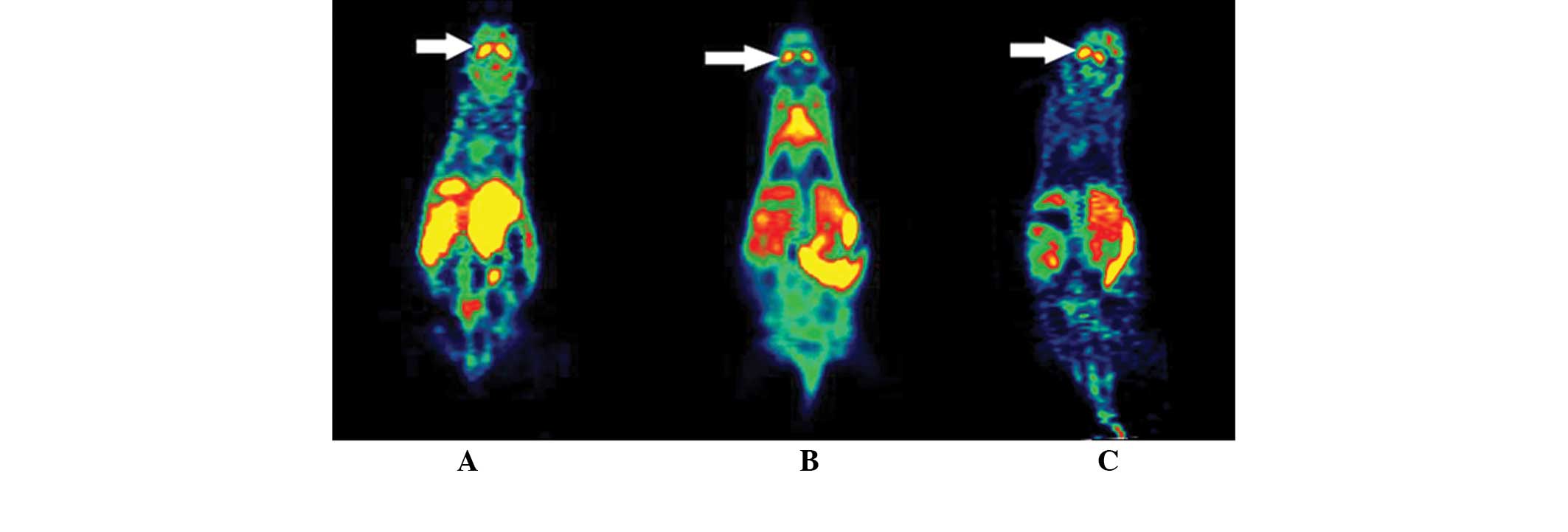
Table I and Fig. 2 show the uptake of
18F-fallypride in the striatal area 10 min after the
injection and the dose of 19F-fallypride. The uptake of
18F-fallypride increased significantly with the lowering
of the 19F-fallypride dosage (Fig. 2).
 | Table I.Striatal uptake of
18F-fallypride under different concentrations of
19F-fallypride (n=3). |
Table I.
Striatal uptake of
18F-fallypride under different concentrations of
19F-fallypride (n=3).
| Concentration
(µg/ml) | Uptake (% ID/g) |
|---|
| Blank | 5.98±0.48 |
| 500 | 0.48±0.04 |
| 50 | 1.03±0.12 |
| 10 | 3.32±0.29 |
| 1 | 4.04±0.17 |
| 0.1 | 4.58±0.31 |
Changes of the evaluation indices in
the mouse model of PD
General behavioral test
The behavioral changes of the mice in the PD model
group were mainly as follows: Slow movement, arched back, hair
erection, tail stiffness, increased salivation, rapid breathing and
trembling head and teeth, which might last for up to 2–3 h. The
behavioral changes in the L-dopa-treated group were mild and with
shorter duration.
Swimming test
The results of the swimming test for mice in the
control, PD model and L-DA groups are shown in Table II. The swimming times of mice in the
model group prior to drug treatment were significantly higher than
those in the control group. After 4 days of L-dopa administration,
while the swimming times in the model group remained shorter than
those in the control group, the swimming times in the treatment
group showed no difference compared with those in the control
group.
 | Table II.Results of the swimming test prior to
treatment and following 1, 4 and 7 days of treatment with L-DA
(n=6). |
Table II.
Results of the swimming test prior to
treatment and following 1, 4 and 7 days of treatment with L-DA
(n=6).
|
| Test score |
|---|
|
|
|
|---|
| Groups | Prior to
treatment | Day 1 | Day 4 | Day 7 |
|---|
| Control | 2.81±0.13 | 2.83±0.18 | 2.81±0.16 | 2.81±0.19 |
| Model |
2.38±0.25a |
2.46±0.08a |
2.50±0.24b |
2.46±0.21b |
| L-DA |
2.44±0.25b |
2.52±0.17b | 2.63±0.21 | 2.75±0.17 |
Mouse locomotor activity
The line-crossing and frequency of standing posture
results of mice in each group are shown in Tables III and IV. Prior to drug administration, the
number of lines crossed and the times of standing of mice in the
model group were significantly less than those in the control
group; however, after 7 days of L-dopa treatment, the number of
lines crossed and the times of standing in the treatment group
showed no significant difference from those in the control
group.
 | Table III.Results of locomotor activity prior
to treatment and following 1, 4 and 7 days of treatment with L-DA
(n=6). |
Table III.
Results of locomotor activity prior
to treatment and following 1, 4 and 7 days of treatment with L-DA
(n=6).
|
| No. of lines
crossed |
|---|
|
|
|
|---|
| Groups | Prior to
treatment | 1 day | 4 days | 7 days |
|---|
| Control | 137.13±22.53 | 142.11±23.75 | 140.33±16.11 | 135.43±21.07 |
| Model |
63.19±18.43a |
67.34±6.53a |
75.25±24.65a |
71.13±33.42b |
| L-DA |
60.43±23.53a |
88.52±30.21b |
111.95±11.07c,d |
129.64±24.53d |
 | Table IV.Frequency of standing posture of mice
in each group (times/5 min) (n=6). |
Table IV.
Frequency of standing posture of mice
in each group (times/5 min) (n=6).
|
| Frequency of
standing posture |
|---|
|
|
|
|---|
| Groups | Prior to
treatment | 1 day | 4 days | 7 days |
|---|
| Control | 38.03±5.39 | 39.15±5.53 | 39.01±5.04 | 38.30±4.53 |
| Model |
24.25±6.34a |
23.53±4.31a |
27.53±6.53b | 31.25±5.35 |
| L-DA |
21.05±7.91c |
32.15±4.53b,d | 35.05±7.35 | 36.13±7.25 |
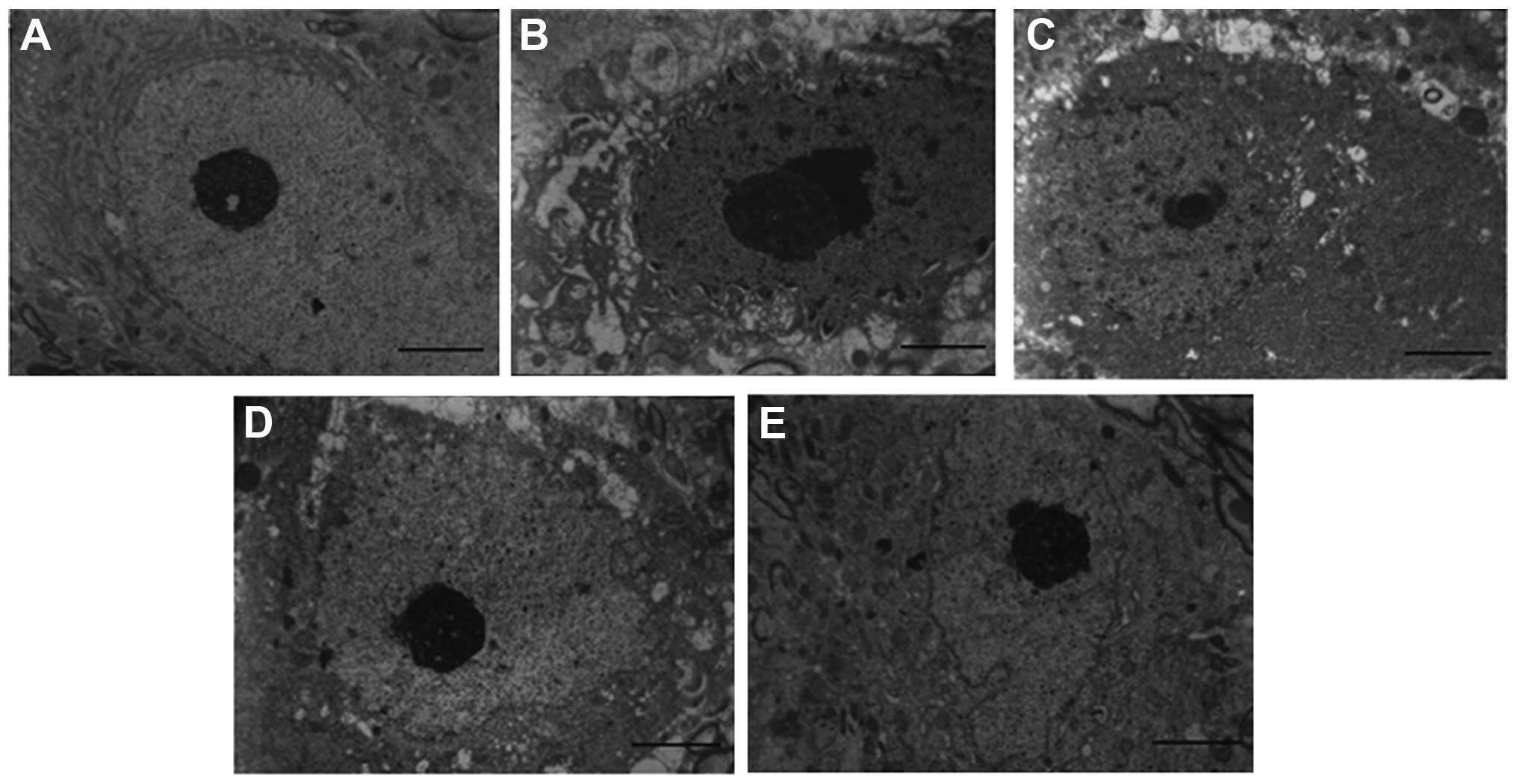
Neuronal changes in the SNpc
Fig. 3 shows the
morphological changes of neurons in the SNpc of mice from each
group. The neurons in the SNpc of the control mice were easily
observed, and their nuclei contained a large circular cluster of
chromatin with clear boundaries; a large amount of complete
mitochondria and endoplasmic reticulum were also observed in the
cytoplasm. However, morphological changes occurred in the cytoplasm
and nuclei of neurons in the SNpc in the PD model group. The
electron density in the nuclei increased and swelling of the
perinuclear region was observed. Cytoplasmic vacuoles and a
multilayer structure with alternating ribosomes and expanded
endoplasmic reticulum were also observed. L-dopa treatment
attenuated the neuronal damage to a certain extent. With prolonged
drug administration, the number of cytoplasmic vacuoles was
reduced, and mitochondria of normal size and shape appeared.
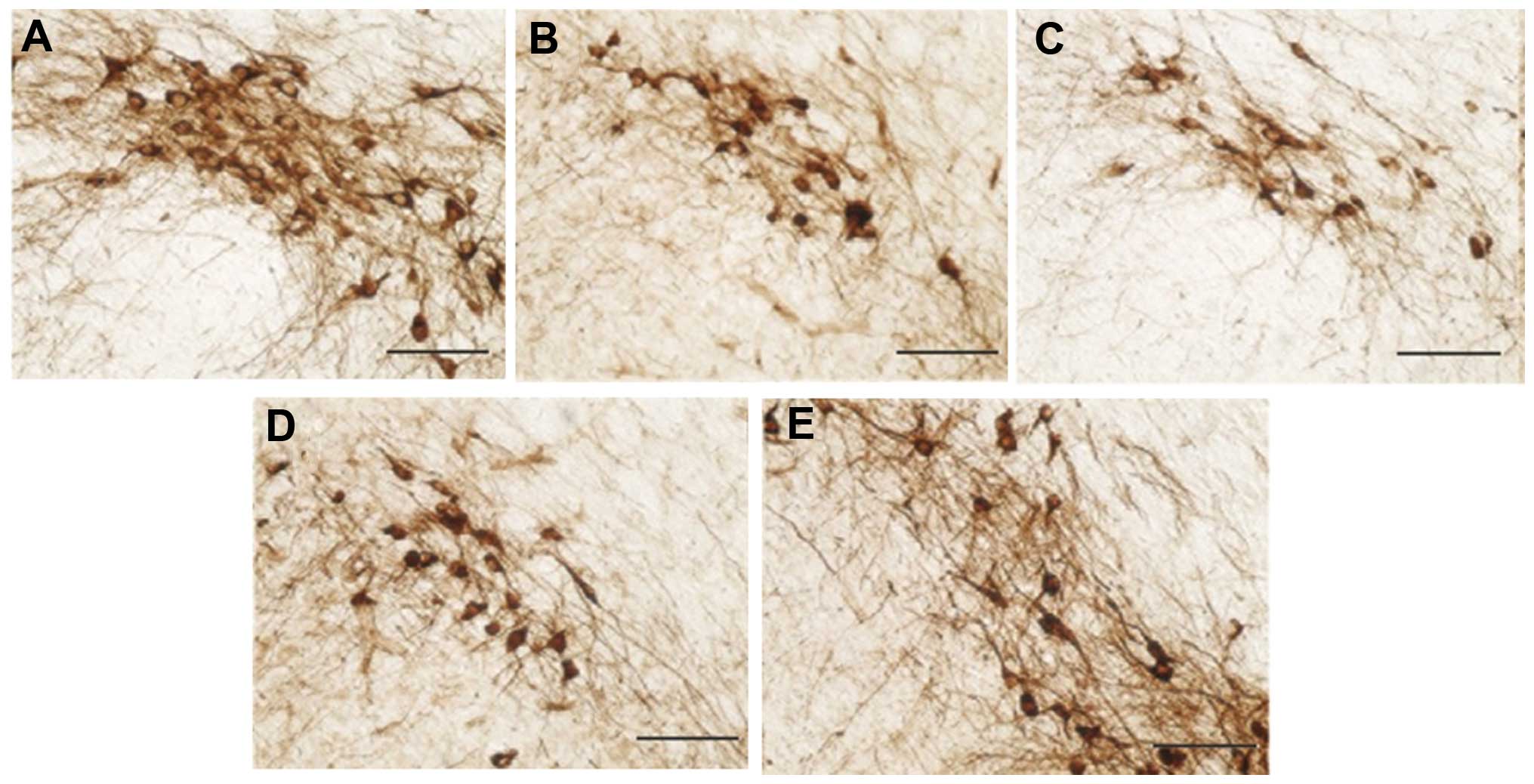
TH immunohistochemistry
Images of TH immunostaining in the mice, captured
under a microscope, are shown in Fig.
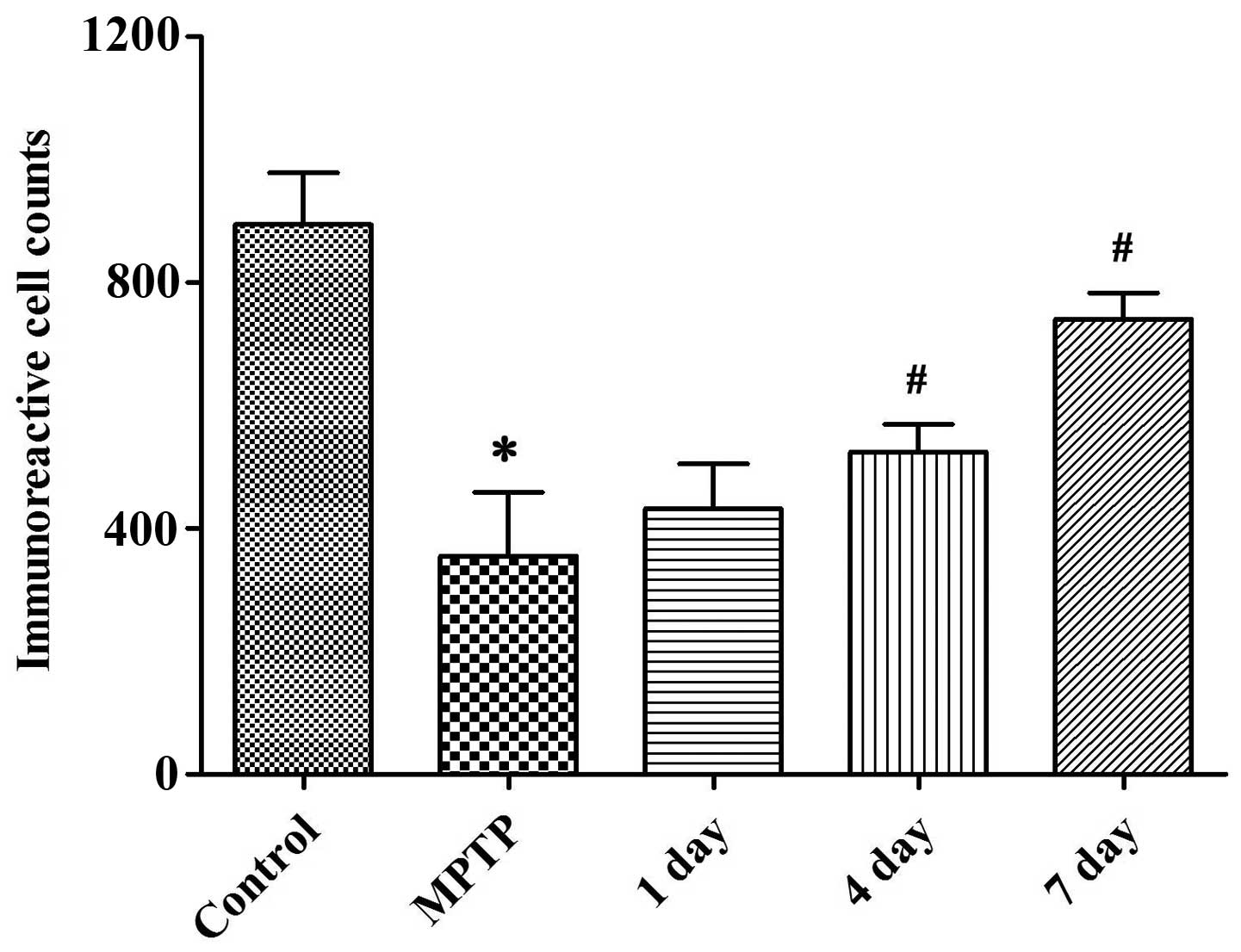
4, while Fig. 5 shows the number
of TH-positive neurons. Compared with the control group, mice in
the PD (MPTP) model group showed a significantly reduced number of
TH-positive neurons, the survival rate of which was only 34.66%.
However, the number of TH-positive neurons in the L-dopa-treated
group was significantly increased (P<0.05).
MDA, SOD and GSH-PX levels in the mouse
striatum
The striatal contents of MDA, SOD and GSH-PX are
shown in Table V. The striatal MDA
content in the PD model group increased significantly compared with
that in the control group, (P<0.05); however, the GSH-PX and SOD
contents were significantly lower in the model group than in the
control group (P<0.05). Compared with the PD model group, the
L-dopa-treated group displayed significantly increased levels of
GSH-PX and SOD, and a reduced content of MDA (P<0.05).
 | Table V.Striatal levels of MDA, SOD and
GSH-PX in mice of each group (n=6). |
Table V.
Striatal levels of MDA, SOD and
GSH-PX in mice of each group (n=6).
| Groups | MDA (nmol/mg
protein) | SOD (U/mg
protein) | GSH-PX (U/mg
protein) |
|---|
| Control |
8.65±0.35 | 4.23±0.16 | 135.13±21.45 |
| Model |
17.12±0.56a |
2.36±0.27a |
49.24±4.63a |
| L-Dopa 1 day | 15.43±0.95 | 2.76±0.54 |
58.42±8.53 |
| L-Dopa 4 day |
10.57±1.25b |
3.15±0.89b |
84.25±8.03b |
| L-Dopa 7 day |
7.53±0.43c |
4.54±0.91b |
114.54±7.34c |
Effect of L-dopa on the striatal contents of DA
and its metabolites in mice
As shown in Fig. 6,
compared with the control group, mice in the PD model group showed
significantly declined striatal contents of DA, DOPAC and HVA
(P<0.05). In comparison with the PD model group, the striatal
contents of DA were significantly increased after 4 days of L-dopa
treatment (P<0.05). In addition, the DOPAC/DA ratio in the PD
model group was significantly higher than that in the control
group, and L-dopa treatment strongly inhibited the effect of
MPTP-induced monoamine oxidase (MAO)-dependent DA metabolism
(P<0.05) on DOPAC/DA and HVA/DA ratios. Similarly, compared with
the control group, the total DA metabolic rate HVA/DA in the PD
model group increased significantly, but L-dopa administration
significantly reduced the DA metabolic rate (P<0.05).
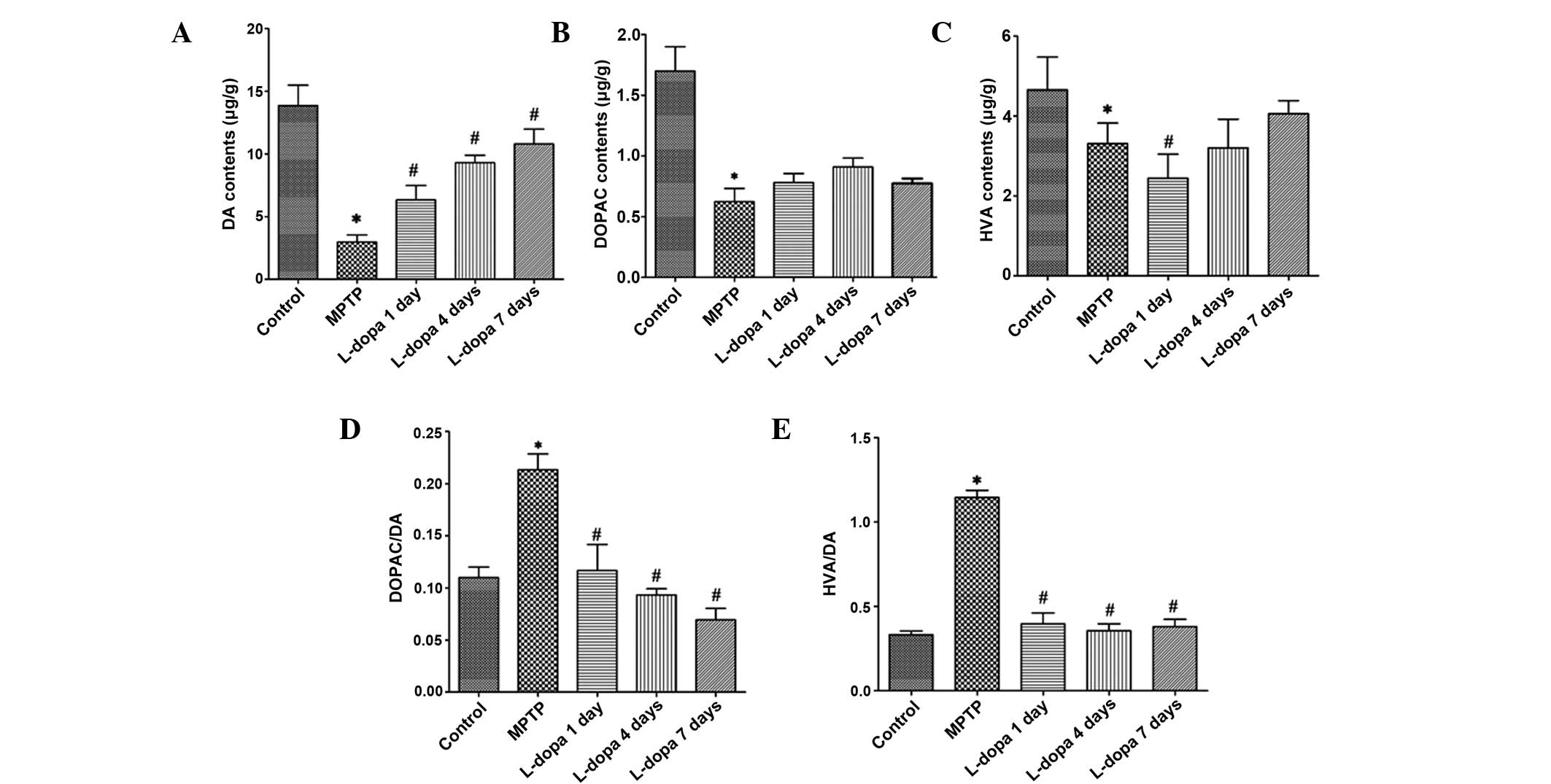 | Figure 6.Effect of L-dopa on the levels of DA
and its metabolites. Levels of (A) DA, (B) DOPAC and (C) HVA, and
the (D) DOPAC/DA and (E) HVA/DA ratios in each group. *P<0.05
vs. the control group; #P<0.05 vs. the PD model
(MPTP) group. DA, dopamine; DOPAC, 3,5-dihydroxyphenylacetic acid;
HVA, homovanillic acid; PD, Parkinson's disease; MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrapyridine. |
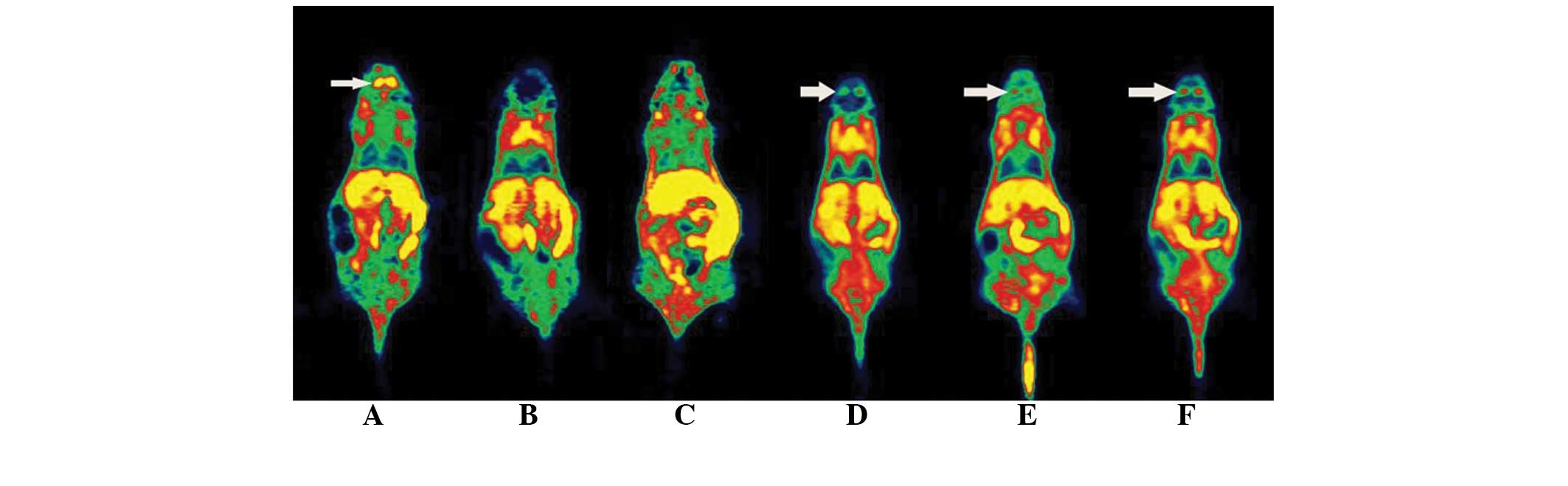
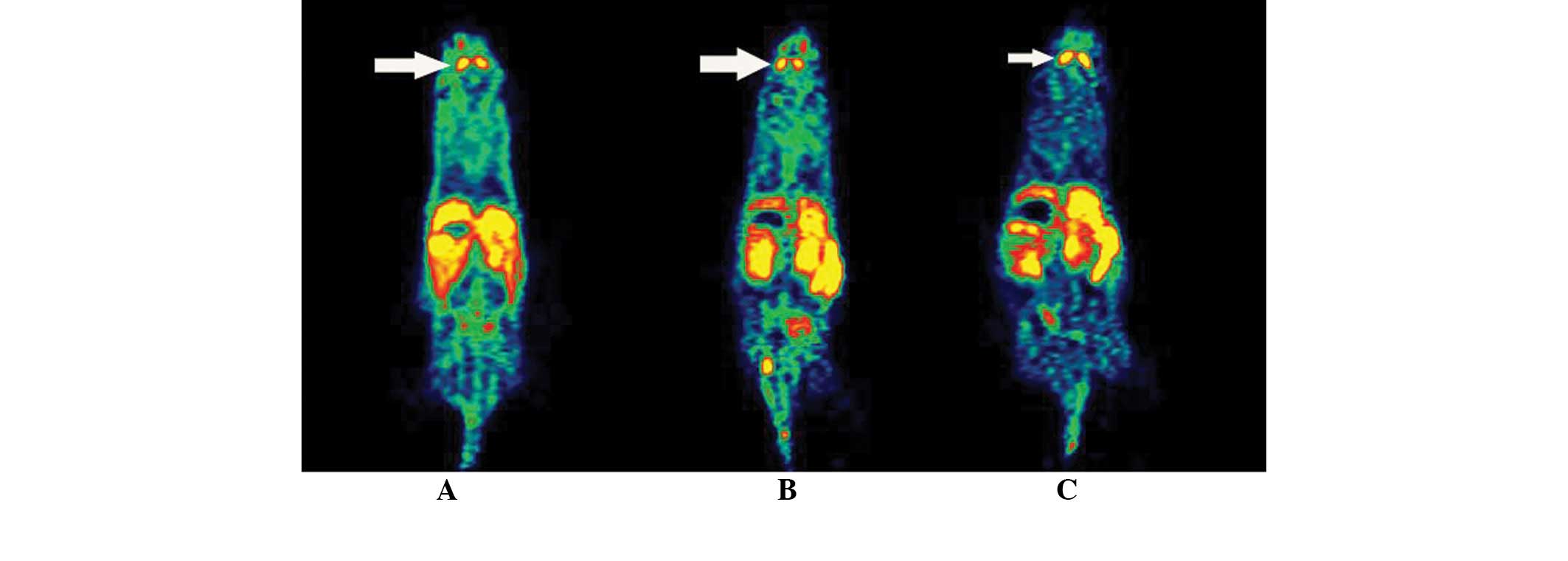
Micro-PET imaging
The results of micro-PET scanning of mice in each
group at different time-points are shown in Table VI and Figs. 7 and 8. The striatal uptake in the model and
treatment groups was significantly lower than that in the control
group prior to the drug administration (P<0.01). After 1 and 7
days of drug treatment, while the striatal uptake in the model
group was lower than that in control group, the L-dopa intervention
group showed an evidently increased striatal uptake compared with
model group, which was not significantly different from that in the
control group.
 | Table VI.Results of the micro-PET imaging of
mice in each group (n=6). |
Table VI.
Results of the micro-PET imaging of
mice in each group (n=6).
|
| SUV (% ID/g) |
|---|
|
|
|
|---|
| Groups | Prior to
treatment | 1 day | 4 days | 7 days |
|---|
| Control | 3.57±0.10 | 3.65±0.38 | 3.62±0.60 | 3.74±0.15 |
| Model |
2.84±0.22a |
2.71±0.13b | 2.97±0.27 |
3.00±0.14a |
| L-DA |
2.77±0.11a |
3.17±0.11c |
3.41±0.08d |
3.42±0.15e |
Discussion
The method for 18F-fallypride synthesis
is simple, with high product stability and synthesis efficiency.
The radiochemical purity of the obtained 18F-fallypride
was >96%. In this study, the 19F-fallypride
competitive inhibition test showed that the uptake of
18F-fallypride increased as the
19F-fallypride dose decreased, which is consistent with
the results of micro-PET imaging, indicating that
18F-fallypride is a specific ligand of the DA receptor
and may be used to reflect the expression of the DA receptor
(15).
An MPTP-induced mouse model of PD was used in the
present study. After MPTP injection, the number of TH-positive
striatal neurons reduced significantly; the behavioral
characteristics manifested as reduced activities and slow movement.
TEM revealed morphological changes in the nuclei and cytoplasm of
neurons in the SNpc, a reduced number of cytoplasmic vacuoles, and
abnormalities in mitochondrial size and morphology in the PD model
group. Micro-PET also showed that compared with the control group,
mice in the PD model group had a significantly declined uptake of
18F-fallypride, which is consistent with the
characteristic pathological changes of PD patients and previous
findings (16,17).
The present study also investigated the effects of
L-dopa treatment on PD model mice, and the results of behavioral
experiments showed that L-dopa alleviated the symptoms of PD to a
certain extent. TEM also demonstrated that L-dopa administration
reduced the MPTP-induced morphological changes in the nuclei and
cytoplasm of neurons in the SNpc; for example, it reduced the
number of cytoplasmic vacuoles and normalized the mitochondrial
size and morphology. However, treatment of the PD mice with L-dopa
significantly increased the number of TH-positive cells, and
compared with the PD model group, L-dopa administration
significantly reduced the levels of DOPAC/DA and HVA/DA, these
ratios were significantly recovered to a level close to that of the
control group, indicating that L-dopa treatment attenuated the
metabolic rate of DA and thereby improved the behavioral disorders
in the PD mice (12).
Previous studies have determined the effect of PD
therapy only through behavioral methods and clinical
characteristics, and there is lack of a scientific research aimed
at identifying a simple and easy noninvasive method of examination.
The present study observed clear images of
18F-fallypride by micro-PET imaging, which showed that
the striatal uptake of 18F-fallypride in the L-dopa
treatment group significantly increased compared with that in the
PD model group on day 1 of treatment (P<0.05), and had no
significant difference from that in control group 7 days after the
treatment (P>0.05). It was also found that the standardized
uptake value (SUV) of 18F-fallypride in the micro-PET
imaging results was clear and reliable, which forms a valuable
reference for monitoring the efficacy of PD therapy in a mouse
model and provides a new means of observation (18).
Studies suggest that the reduction in the number of
TH-positive cells and DA receptor levels in the mice model of PD
reproduces the clinicopathological features of PD patients
(19,20). L-dopa alleviated the symptoms of mice
with PD by upregulating the number of TH-positive cells and the
level of the DA receptor as well as attenuating the metabolic rate
of DA. These changes may be observed noninvasively in vitro
by 18F-fallypride imaging, a new method for monitoring
the early efficacy of PD treatment. However, few current clinical
studies have focused on 18F-fallypride (21). Therefore, whether PET imaging can be
used for the early diagnosis of early-stage PD requires further
verification by clinical imaging studies of patients with PD.
Furthermore, whether 18F-fallypride imaging can be used
as a means to monitor the therapeutic efficacy and evaluate the
prognosis of PD also requires further confirmation through
investigations with drug intervention and PET imaging in patients
with PD.
References
|
1
|
Ma CL, Su L, Xie JJ, Long JX, Wu P and Gu
L: The prevalence and incidence of Parkinson's disease in China: A
systematic review and meta-analysis. J Neural Transm. 121:123–134.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang ZX, Chen H, Chen SD, Shao M, Sun SG,
Qu QM, Zhang BR, Liu YM, Xu Q, Wan X, et al: Chinese culture
permeation in the treatment of Parkinson disease: A cross-sectional
study in four regions of China. BMC Res Notes. 7:652014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Donnan GA, Willis GL, Kaczmarczyk SJ and
Rowe P: Motor function in the
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mouse. J
Neurol Sci. 77:185–191. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tai Y, Chen L, Huang E, Liu C, Yang X, Qiu
P and Wang H: Protective effect of alpha-synuclein knockdown on
methamphetamine-induced neurotoxicity in dopaminergic neurons.
Neural Regen Res. 9:951–958. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marek K, Innis R, van Dyck C, Fussell B,
Early M, Eberly S, Oakes D and Seibyl J: [123I]beta-CIT SPECT
imaging assessment of the rate of Parkinson's disease progression.
Neurology. 57:2089–2094. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu L, Wang Y, Li B, Jia J, Sun Z, Zhang
J, Tian J and Wang X: Evaluation of nigrostriatal damage and its
change over weeks in a rat model of Parkinson's disease: Small
animal position emission tomography studies with
[11C]β-CFT. Nucl Med Biol. 36:941–947. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rominger A, Mille E, Zhang S, Böning G,
Förster S, Nowak S, Gildehaus FJ, Wängler B, Bartenstein P and
Cumming P: Validation of the octamouse for simultaneous
18F-Fallypride small-animal PET recording from 8 mice. J
Nucl Med. 51:1576–1583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tantawy MN, Jones CK, Baldwin RM, Ansari
MS, Conn PJ, Kessler RM and Peterson TE: [18F]fallypride
dopamine D2 receptor studies using delayed microPET scans and a
modified Logan plot. Nucl Med Biol. 36:931–940. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Honer M, Brühlmeier M, Missimer J,
Schubiger AP and Ametamey SM: Dynamic imaging of striatal D2
receptors in mice using Quad-HIDAC PET. J Nucl Med. 45:464–470.
2004.PubMed/NCBI
|
|
10
|
Kura AU, Ain NM, Hussein MZ, Fakurazi S
and Hussein-Al-Ali SH: Toxicity and metabolism of layered double
hydroxide intercalated with levodopa in a Parkinson's disease
model. Int J Mol Sci. 15:5916–5927. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Filipov NM, Stewart MA, Carr RL and
Sistrunk SC: Dopaminergic toxicity of the herbicide atrazine in rat
striatal slices. Toxicology. 232:68–78. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Afonso-Oramas D, Cruz-Muros I,
Castro-Hernández J, Salas-Hernández J, Barroso-Chinea P,
Garcia-Hernández S, Lanciego JL and González-Hernández T: Striatal
vessels receive phosphorylated tyrosine hydroxylase-rich
innervation from midbrain dopaminergic neurons. Front Neuroanat.
8:842014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li S and Pu XP: Neuroprotective effect of
kaempferol against a
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of
Parkinson's disease. Biol Pharm Bull. 34:1291–1296. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawai H, Makino Y, Hirobe M and Ohta S:
Novel endogenous 1,2,3,4-tetrahydroisoquinoline derivatives: Uptake
by dopamine transporter and activity to induce parkinsonism. J
Neurochem. 70:745–751. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ceccarini J, Vrieze E, Koole M, Muylle T,
Bormans G, Claes S and Laere KV: Optimized in vivo detection
of dopamine release using 18F-Fallypride PET. J Nucl
Med. 53:1565–1572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kegeles LS, Slifstein M, Xu X, Urban N,
Thompson JL, Moadel T, Harkavy-Friedman JM, Gil R, Laruelle M and
Abi-Dargham A: Stristal and extrastriatal dopamine D2/D3 receptors
in schizophrenia evaluated with [18F]fallypride PET.
Biol Psychiatry. 68:634–641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Campo ND, Fryer TD, Hong YT, Smith R,
Brichard L, Acosta-Cabronero J, Chamberlain SR, Tait R, Izquierdo
D, Regenthal R, et al: A positron emission tomography study of
nigro-striatal dopaminergic mechanisms underlying attention:
Implications for ADHD and its treatment. Brain. 136:3252–3270.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vučković MG, Li Q, Fisher B, Nacca A,
Leahy RM, Walsh JP, Mukherjee J, Williams C, Jakowec MW and
Petzinger GM: Exercise elevates dopamine D2 receptor in a mouse
model of Parkinson's disease: In vivo imaging with
[18F] Fallypride. Mov Disord. 25:2777–2784. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmidt N and Ferger B: Neurochemical
findings in the MPTP model of Parkinson's disease. J Neural Transm.
108:1263–1282. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bezard E, Dovero S, Bioulac B and Gross C:
Effects of different schedules of MPTP administration on
dopaminergic neurodegeneration in mice. Exp Neurol. 148:288–292.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuepper R, Ceccarini J, Lataster J, van Os
J, van Kroonenburgh M, van Gerven JM, Marcelis M, Van Laere K and
Henquet C: Delta-9-tetrahydrocannabinol-induced dopamine release as
a function of psychosis risk: 18F-Fallypride positron
emission tomography study. PLoS One. 8:e703782013. View Article : Google Scholar : PubMed/NCBI
|