Introduction
Rheumatoid arthritis (RA) is a systemic disease
characterized by progressive synovitis and the degeneration of
joints; however, the underlying pathogenesis of RA remains unclear
(1). For patients with bone and
joint damage caused by RA, the ultimate aim of treatment is to
delay the disability of joint function caused by the disease
(2). During active periods of RA,
the hyperplastic synovial tissue and pannus directly erode
articular cartilage and bone tissue surrounding the joints.
Inhibiting the proliferation of inflammatory synovial tissue and
inducing apoptosis in synovial tissue is therefore the primary aim
of RA treatment (3).
Oxidative stress is closely associated with human
aging, cardiovascular disease and chronic inflammation, amongst
other diseases that have previously been associated with immune
dysfunction. A complete antioxidant defense system is well-evolved,
and includes antioxidant enzymes, antioxidants and a variety of
other mechanisms tasked with damage repair and re-synthesis
(4). The coordination and
complementation of the various antioxidant defense systems in
vivo guarantee their stable and effective involvement in the
antioxidative stress effect. At present, the pathogenesis of RA
remains to be elucidated, but it has previously been indicated that
oxidative stress has an important role in the pathology of the
disease (5).
RA is an inflammatory form of arthritis that may be
caused by a variety of factors, including genetic or environmental
causes and microbial invasion, amongst others. Tumor necrosis
factor-α (TNF-α) and interleukin-1β (IL-1β) are pro-inflammatory
cytokines that are pivotal in the pathogenesis of RA (6). Cyclooxygenase (COX) is an enzyme
necessary for the synthesis of prostaglandins, and a key
rate-limiting enzyme in the initial steps of prostaglandin
synthesis. In a previous study, COX-1 was suggested not to be
directly involved in inflammation (7). However, another study has reported that
COX-1 is not only involved in inflammation, but that it also
aggravates inflammation, while COX-2 appears to be mainly involved
in the early inflammatory processes, but has an anti-inflammatory
effect during chronic inflammation (8).
Paeoniflorin is the main active constituent of
peonies, used in traditional Chinese medicine, and is a monoterpene
glycoside compound. Previous investigations of the pharmacological
effects of paeoniflorin have revealed that paeoniflorin has
multiple roles, which include the attenuation of free radical
damage, the inhibition of intracellular calcium overload and the
abrogation of neurotoxicity (9).
In vivo experiments indicate that this has numerous
biological effects, including a reduction in blood viscosity and
platelet aggregation, dilation of blood vessels, improvement of
microcirculation, inhibition of oxidation and action as an
anti-convulsive, with low toxicity and few side effects (10). However, the mechanisms underlying the
protective effects of paeoniflorin upon RA remain unclear. The
present study therefore aimed to investigate the delayed protective
effects of paeoniflorin in a rat model of RA, and to reveal the
signaling pathways involved in the actions of paeoniflorin.
Materials and methods
Experimental rat model
Healthy, male, Sprague-Dawley rats weighing 250–300
g were obtained from the Animal Resource Center of the First
Affiliated Hospital of Dalian Medical University (Dalian, China).
The rats were maintained in individual cages under standard
conditions (12:12-h light-dark cycle, 40–60% humidity and 22–24°C),
and provided with food and water ad libitum. All study
protocols employed were in accordance with the guidelines of the
Animal Care and Use Committee of the First Affiliated Hospital of
Dalian Medical University.
Model establishment
The RA rat model was established as described
previously (11). The experimental
rats were placed in a cage with a fan in a high position (12:12-h
light-dark cycle, 80–90% humidity, 4–8°C) for 20 days. On the 21st
day of the experiment, rats were anesthetized with an
intraperitoneal (i.p.) injection of 50 mg/kg sodium pentobarbital.
Freund's complete adjuvant (10 mg/ml; F-5881; Sigma-Aldrich, St.
Louis, MO, USA) was injected subcutaneously between the 2nd and 3rd
toes of the right foot. The experimental rats were observed for 3
days and the right ankle demonstrated acute inflammatory swelling
within 24 h. Secondary, widespread arthritis occurred within 24 h,
manifesting in the forelimbs and contralateral limbs as red
swellings or inflamed nodes; arthritis also spread to the ear and
tail, indicating a successful model.
Grouping and treatment
The experimental rats were randomly divided into 5
groups. In the control (Con; n=8) and RA rat model (RA; n=8)
groups, the rats received sodium pentobarbital (10 mg/ml, i.p.),
while in the paeoniflorin(5),
(10) and (20) groups [Pae(5), Pae(10)
and Pae(20), respectively; n=8 in
each], the rats were treated with 5, 10 or 20 mg/kg paeoniflorin
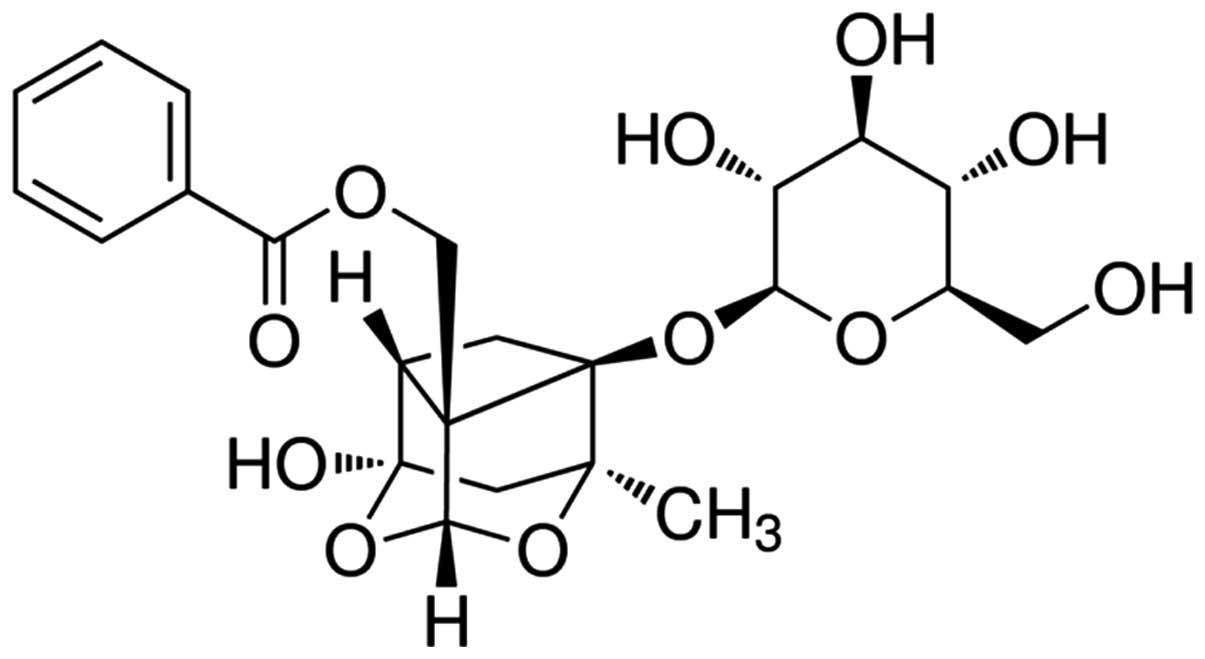
(i.p.), respectively, all for 3 weeks (12). The chemical structure of paeoniflorin
(purity >98%; Nanjing University of Traditional Chinese
Medicine, Institute of Chinese Material Medica, Nanjing, China) is
indicated in Fig. 1.
Measurement of pain thresholds of the
RA rat model
After a 3-week treatment with paeoniflorin, the
pressure pain threshold (g) was detected three times each session
with an interval of 20 min between sessions, using an electronic
pressure pain detector (Somedic AB, Hörby, Sweden), as previously
described (13). The mean value was
used to indicate the pressure pain threshold.
Clinical arthritic scoring of RA
rats
After the 3-week paeoniflorin treatment, the rats
were evaluated for arthritis using a macroscopic scoring system, as
follows: Severe arthritis of the entire paw and digits, 11–15
points; >2 joints involved, 6–10 points; 2 joints involved, 1–5
points; and no signs of arthritis, 0 points.
Measurement of oxidative stress of RA
rats
After the 3-week paeoniflorin treatment, peripheral
blood was collected. The blood samples were centrifuged at 3,000 ×
g for 10 min at 4°C, and were analyzed to detect the concentration
of malondialdehyde (MDA) and the activity of superoxide dismutase
(SOD), catalase (CAT) and glutathione peroxidase (GSH-Px),
following the manufacturer's protocol (Beijing Boaosen
Biotechnology, Ltd., Beijing, China).
Measurement of inflammatory effects on
RA rats
Peripheral blood samples were processed as
aforementioned, and the activity of nuclear factor (NF)-κB p65
unit, TNF-α, IL-1β and IL-6 were analyzed, following the
manufacturer's protocol (Beijing Boaosen Biotechnology, Ltd.).
Western blot analysis of COX-2 in RA
rats
Following treatment with paeoniflorin for 3 weeks,
~10-mg RA tissue samples were removed and incubated on ice for 30
min with 100 µl tissue lysis buffer. Homogenates were centrifuged
at 3,000 × g for 10 min at 4°C and protein concentration was
measured using a bicinchoninic acid kit (Fermentas, Beijing,
China). Equal protein was loaded onto 12% sodium dodecyl
sulfate-polyacrylamide gels and transferred to polyvinylidene
fluoride membranes (Millipore, Billerica, MA, USA). The following
antibodies were used for the western blot analysis: Monoclonal
anti-COX-2 (1:1,000; sc-376861) and anti-β-actin (1:500; sc-7210;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C.
Membranes were incubated with anti-rabbit immunoglobulin G (IgG)
horseradish peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology, Inc.) at 37°C for 1 h. The relative band intensity
was detected using the Amersham ECL Western Blotting Detection kit
(GE Healthcare Buchler GmbH & Co. KG, Braunschweig,
Germany).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Differences in arthritic score were evaluated by Student's t-test,
and these were considered significant at P<0.05.
Results
Effect of paeoniflorin on the pain
thresholds of the RA rat model
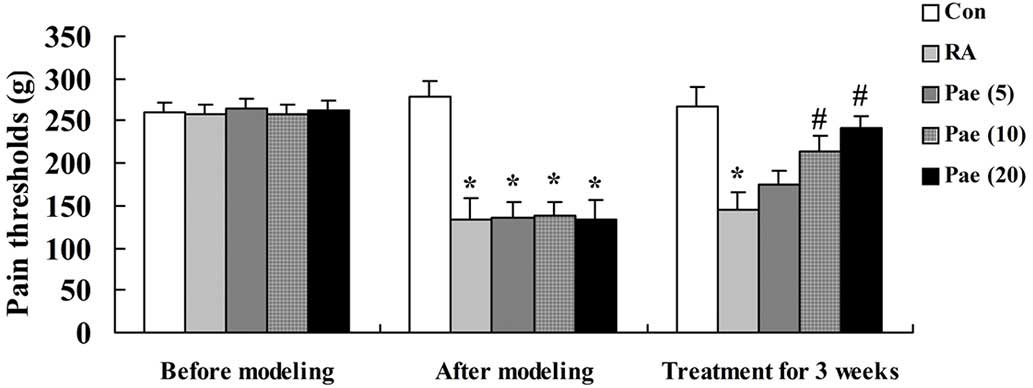
The data in Fig. 2
demonstrates that 3 weeks of RA markedly reduced the pain threshold
in the rats when compared with the control group. After the 3-week
paeoniflorin treatment, 10 and 20 mg/kg of paeoniflorin were
demonstrated to significantly recover the pain thresholds when
compared with the RA rats (P<0.01; Fig. 2).
Effect of paeoniflorin on the clinical
arthritic score of RA rats
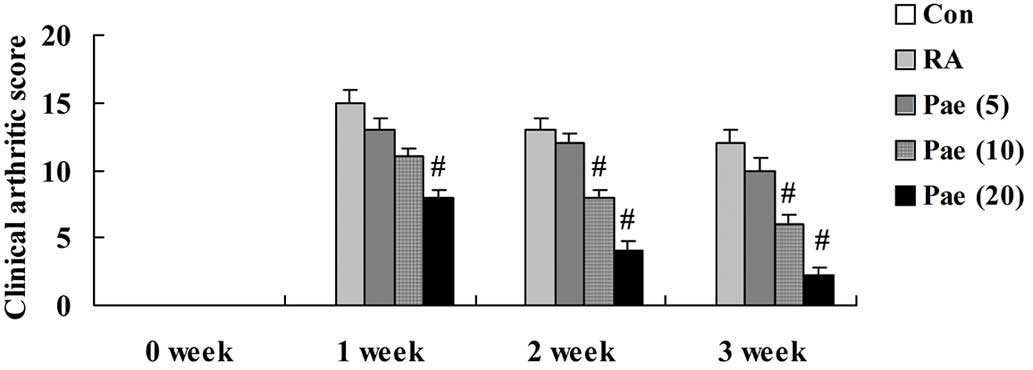
The clinical arthritic score of the RA model rats
was markedly increased in comparison to the control group (Fig. 3). Treatment with 20 mg/kg of
paeoniflorin significantly decreased the clinical arthritic score
at 1, 2 and 3 weeks of treatment when compared with that of the RA
rats (P<0.01; Fig. 3). Clinical
arthritic score also significantly decreased following treatment
with 10 mg/kg paeoniflorin for 2 and 3 weeks when compared with the
RA rats (P<0.01; Fig. 3).
Effect of paeoniflorin on the
concentration of MDA and the SOD, CAT and GSH-Px activity in RA
rats
To elucidate the antioxidant effects of paeoniflorin
treatment in the RA model rats, the concentration of MDA, and the
SOD, CAT and GSH-Px activity were measured. After the 3-week
treatment period, the MDA concentration increased, and SOD, CAT and
GSH-Px activity were reduced in the RA rats when compared with the
control group (Fig. 4A–D). However,
this effect was rescued following treatment with 10 and 20 mg/kg
paeoniflorin (Fig. 4A–D).
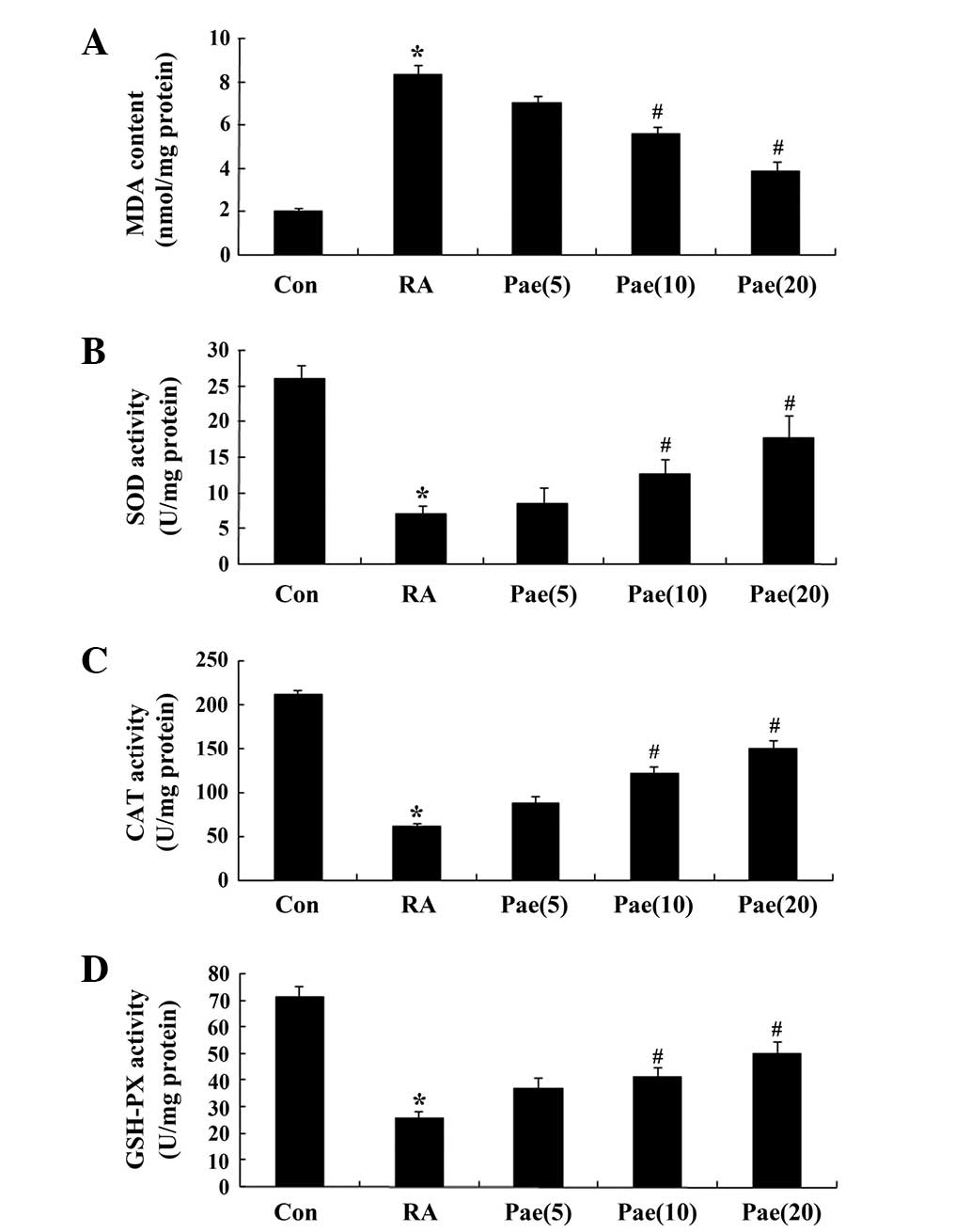 | Figure 4.Effect of treatment with paeoniflorin
on the concentration of (A) MDA, and activity of (B) SOD, (C) CAT
and (D) GSH-Px in the RA rat model. *P<0.01 compared with the
control group; #P<0.01 compared with the RA group.
Con, control; RA, rheumatoid arthritis; Pae, paeoniflorin;
Pae(5), 5 mg/kg-treated;
Pae(10), 10 mg/kg-treated;
Pae(20), 20 mg/kg-treated. SOD,
superoxide mutase; CAT, catalase; GSH-Px, glutathione peroxidase;
MDA, malondialdehyde. |
Effect of paeoniflorin on NF-κB p65
unit, TNF-α, IL-1β and IL-6 activity in RA rats
To elucidate the anti-inflammatory effects of
paeoniflorin treatment in RA rats, NF-κB p65 unit, TNF-α, IL-1β and
IL-6 activity were analyzed; activity was found to be significantly
increased when compared with the control group (Fig. 5A–D). After a 3-week treatment with
paeoniflorin (10 and 20 mg/kg), NF-κB p65 unit, TNF-α, IL-1β and
IL-6 activity was reduced in comparison with that of the RA rats
(P<0.01; Fig. 5A–D).
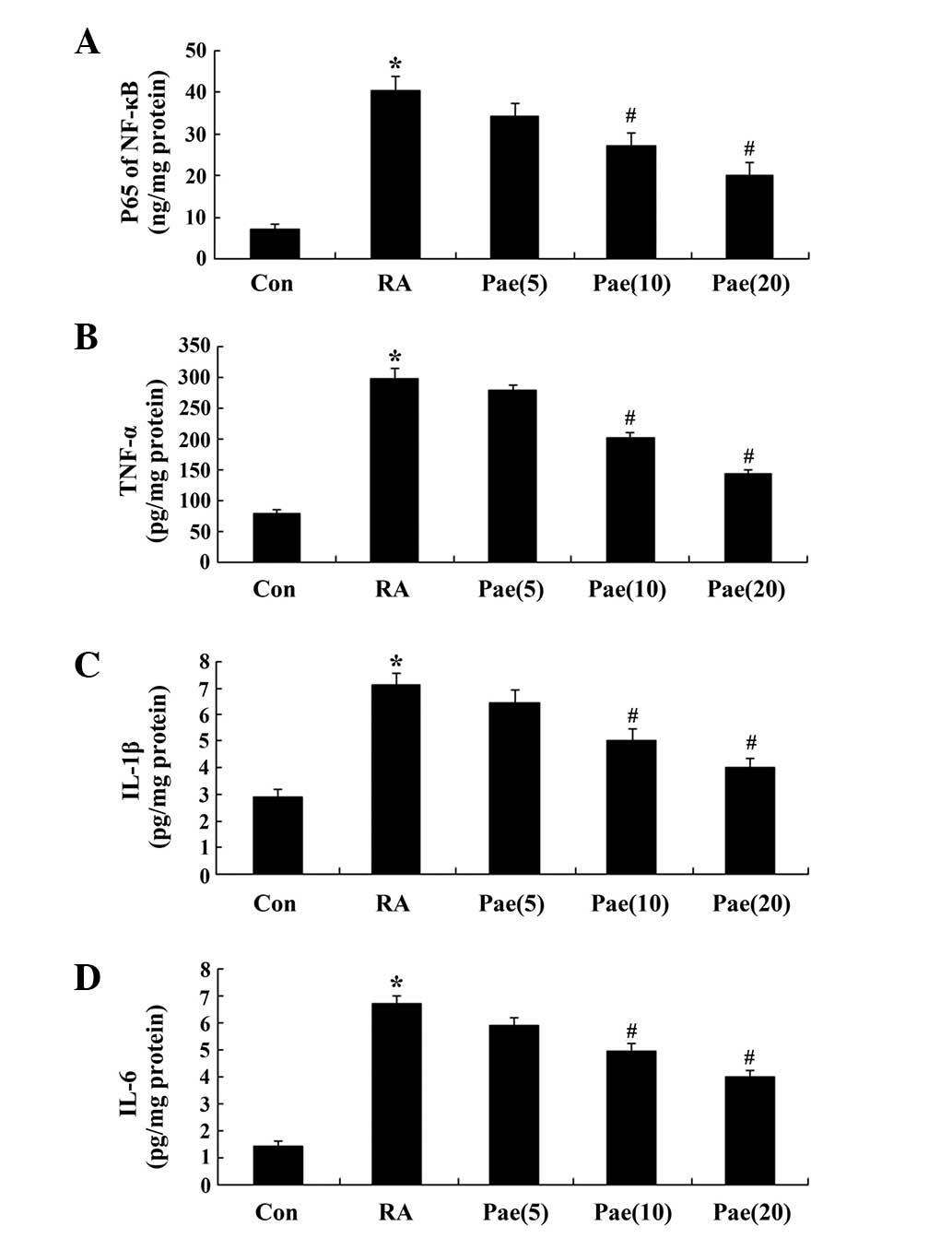 | Figure 5.Effect of paeoniflorin treatment on
the activity of (A) NF-κB p65 unit, (B) TNF-α, (C) IL-1β and (D)
IL-6 of the RA rat model. *P<0.01 compared with the control
group; #P<0.01 compared with the RA group. Con,
control; RA, rheumatoid arthritis; Pae, paeoniflorin; Pae(5), 5 mg/kg-treated; Pae(10), 10 mg/kg-treated; Pae(20), 20 mg/kg-treated.; TNF-α, tumor
necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6. |
Effect of paeoniflorin on COX-2 in RA
rats
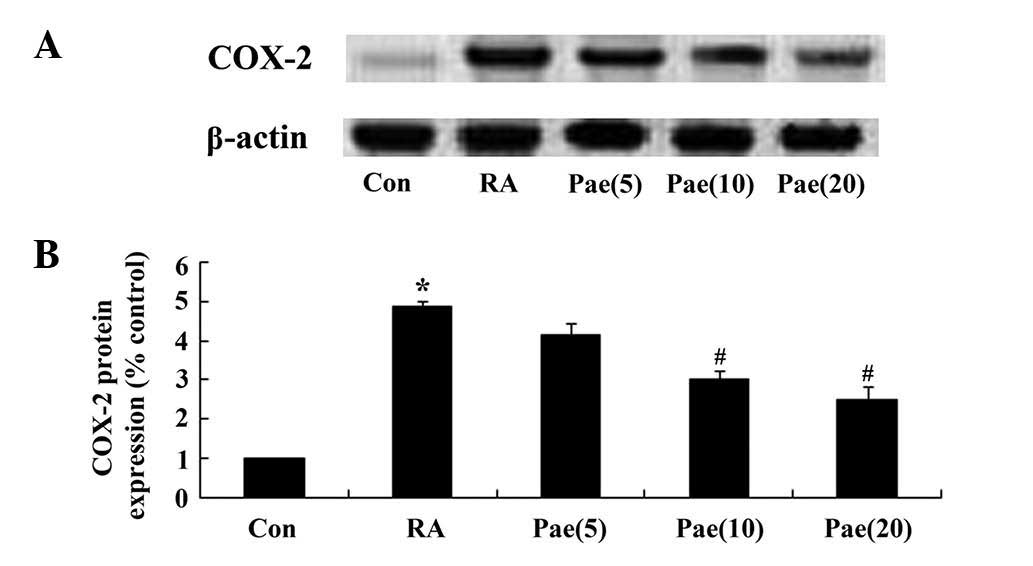
As COX-2 has crucial roles in inflammation, the
regulatory effects of paeoniflorin on the inflammatory response in
the RA rats was examined. COX-2 protein expression was elevated in
the RA rats compared with that of the control group (Fig. 6). Notably, paeoniflorin
administration at 10 and 20 mg/kg significantly reduced COX-2
protein expression in the RA rat model (P<0.01; Fig. 6).
Discussion
RA is a symmetrical, chronic inflammatory disease
primarily affecting multiple small peripheral joints, with possible
extra-articular systemic damage (14). RA patients may suffer from pain,
numbness, weight gain, difficulty in joint flexion and extension,
joint swelling and a burning sensation in the muscles, bones and
joints (15). In the present study,
paeoniflorin significantly improved pain thresholds and reduced
arthritic symptoms in an RA rat model. This is consistent with the
results of a previous study by Zheng et al, which indicated
that paeoniflorin suppressed arthritis in a rat model through its
effects on synoviocytes, and by reducing COX-2 expression in the
synovium (12). Paeoniflorin may
therefore represent a potential therapeutic agent for the treatment
of RA.
Within a normal mammalian body, the production and
clearance of active oxygen are in a state of dynamic equilibrium.
When the antioxidant system is dysfunctional, excessive
accumulation of reactive oxygen species and associated metabolites
occurs, causing tissue damage (16,17).
Previous studies have indicated that oxidative stress and RA are
associated with elevated serum levels of lipid peroxidation
reactant, a reduced level of SOD and the abnormal activity of
antioxidant enzymes (18). In the
present study, treatment with paeoniflorin decreased the MDA
concentration and increased the SOD, CAT and GSH-Px activity in
rats with RA. Similarly, Wankun et al demonstrated that
paeoniflorin induces cellular apoptosis and protects ARPE-19 cells
through repression of oxidative stress (19), and Zhao et al revealed that
paeoniflorin protects against α-naphthylisothiocyanate-induced
cholestasis by ameliorating oxidative stress in rats (20).
Collagen-induced arthritis is primarily
characterized by an early local inflammatory reaction, and
secondary lesions are manifested as contralateral hindlimb and
forelimb swellings. When the synovial macrophages of RA patients
are activated, the overexpression of inflammatory cytokines
(including IL-1β and TNF-α), chemokines (including IL-8 and
macrophage inflammatory protein-1) and matrix metalloproteinases
follows (21). The symptoms and
degree of joint damage in RA are closely associated with the number
of macrophages present, and with the levels of IL-1β and TNF-α
(22). TNF-α induces endothelial
cells to express adhesion molecules, and to promote leukocyte
endothelial adhesion and tissue infiltration, resulting in local
inflammation. In addition, TNF-α is able to promote cartilage cells
to secrete plasminogen activator, transforming plasminogen into
plasmin and thus accelerating arthritic damage. Furthermore, TNF-α
can also induce synovial cells, macrophages, fibroblasts and
chondrocytes to secrete IL-1 and IL-8, which increases tissue
damage (23). The present study
similarly demonstrated that paeoniflorin modulated the activity of
NF-κB p65 unit, TNF-α, IL-1β and IL-6 in the RA rat model. In
previous associated studies, paeoniflorin induced anti-inflammatory
effects in asthmatic mice (24) and
inhibited the inflammatory response in mice presenting with
allergic contact dermatitis (25).
COX-2 is an inducible chemical enzyme that is not
typically expressed in numerous tissues (26). When the body is stimulated by
proinflammatory cytokines, certain cells, including endothelial
cells, vascular smooth muscle cells, monocytes macrophages and
fibroblasts, are induced to express COX-2, such that COX-2 protein
levels are rapidly upregulated between 8- and 10-fold. COX-2
overexpression induces the synthesis and accumulation of
prostaglandins, the inflammatory cytokines, in the damaged tissues,
and promotes local inflammation and tissue damage (27). Overexpression of COX-2 can also
promote cell proliferation, and inhibit apoptosis and the immune
response, thereby evading immune surveillance, finally resulting in
disruption of the balance between cell proliferation and apoptosis
(28). In the current study,
paeoniflorin significantly inhibited COX-2 protein expression.
Similarly, a previous study also reported that paeoniflorin
suppressed arthritis in a rat model by reducing COX-2 expression in
the synovium (12), and another
study revealed that paeoniflorin protected against ischemia-induced
brain damage by inhibiting COX-2-mediated activity in rats
(29).
In conclusion, the present findings demonstrate that
the protective effect of paeoniflorin in RA treatment may occur
through anti-oxidative and anti-inflammatory effects, and through
the suppression of COX-2. Paeoniflorin may thus be considered a
potential therapeutic agent in the treatment of RA, but more
in-depth study is required to fully elucidate its mechanism and
clinical effects.
Acknowledgements
The present study was supported by grants from the
Research Foundation of Dalian Technology Bureau, China (grant no.
2012E15SF166) and Liaoning Province Science and Technology Plan
Projects (grant no. 2013225002).
Glossary
Abbreviations
Abbreviations:
|
RA
|
rheumatoid arthritis
|
|
COX
|
cyclooxygenase
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
CAT
|
catalase
|
|
GSH-Px
|
glutathione peroxidase
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL
|
interleukin
|
References
|
1
|
Dhaouadi T, Sfar I, Abelmoula L,
Jendoubi-Ayed S, Aouadi H, Ben Abdellah T, Ayed K, Zouari R and
Gorgi Y: Role of immune system, apoptosis and angiogenesis in
pathogenesis of rheumatoid arthritis and joint destruction, a
systematic review. Tunis Med. 85:991–998. 2007.PubMed/NCBI
|
|
2
|
Wang K, Zhao L, Liu X, Hao Z, Zhou Y, Yang
C and Li H: Differential co-expression analysis of rheumatoid
arthritis with microarray data. Mol Med Rep. 10:2421–2426.
2014.PubMed/NCBI
|
|
3
|
Liu H and Pope RM: The role of apoptosis
in rheumatoid arthritis. Curr Opin Pharmacol. 3:317–322. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Radhakrishnan A, Tudawe D, Chakravarthi S,
Chiew GS and Haleagrahara N: Effect of γ-tocotrienol in
counteracting oxidative stress and joint damage in collagen-induced
arthritis in rats. Exp Ther Med. 7:1408–1414. 2014.PubMed/NCBI
|
|
5
|
Shahmohamadnejad S, Vaisi-Raygani A,
Shakiba Y, Kiani A, Rahimi Z, Bahrehmand F, Shakiba E and
Pourmotabbed T: Association between butyrylcholinesterase activity
and phenotypes, paraoxonase192 rs662 gene polymorphism and their
enzymatic activity with severity of rheumatoid arthritis,
Correlation with systemic inflammatory markers and oxidative
stress, preliminary report. Clin Biochem. 48:63–69. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu QY, Han QH, Li X, Li ZC, Pan YT, Liu L
and Fu QG: Analysis of differentially expressed genes between
rheumatoid arthritis and osteoarthritis based on the gene
co-expression network. Mol Med Rep. 10:119–124. 2014.PubMed/NCBI
|
|
7
|
Mederle K, Meurer M, Castrop H and Hocherl
K: Inhibition of COX-1 attenuates the formation of thromboxane A2
and ameliorates the acute decrease in glomerular filtration rate in
endotoxemic mice. Am J Physiol Renal Physiol. 309:F332–F340. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi YJ, Lee WS, Lee EG, Sung MS and Yoo
WH: Sulforaphane inhibits IL-1β-induced proliferation of rheumatoid
arthritis synovial fibroblasts and the production of MMPs, COX-2,
and PGE2. Inflammation. 37:1496–1503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong H, Li R, Yu C, Xu T, Zhang X and Dong
M: Paeoniflorin inhibition of 6-hydroxydopamine-induced apoptosis
in PC12 cells via suppressing reactive oxygen species-mediated
PKCδ/NF-κB pathway. Neuroscience. 285:70–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi EM, Suh KS, Rhee SY and Kim YS:
Inhibitory effect of paeoniflorin on methylglyoxal-mediated
oxidative stress in osteoblastic MC3T3-E1 cells. Phytomedicine.
21:1170–1177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo L, Hu L, He L, Tang ZL, Song XG,
Dirckinck-Holmfeld L and Cai RL: Effect of moxibustion on
ultrastructure of synovial cells in rheumatoid arthritis rats. Zhen
Ci Yan Jiu. 36:105–109. 2011.(In Chinese). PubMed/NCBI
|
|
12
|
Zheng YQ, Wei W, Zhu L and Liu JX: Effects
and mechanisms of Paeoniflorin, a bioactive glucoside from peony
root, on adjuvant arthritis in rats. Inflamm Res. 56:182–188. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng B, Hu L, Song X, Wu Z, Cai R, He L,
Zhang C and Yu Q: Analgesic effect of different moxibustion
durations in rheumatoid arthritis rats. J Tradit Chin Med.
34:90–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng G, Wang L, Jia X, Li F, Yan Y, Yu Z,
Li L, Wei Q and Zhang F: Application of high frequency color
Doppler ultrasound in the monitoring of rheumatoid arthritis
treatment. Exp Ther Med. 8:1807–1812. 2014.PubMed/NCBI
|
|
15
|
Almoallim HM and Alharbi LA: Rheumatoid
arthritis in Saudi Arabia. Saudi Med J. 35:1442–1454.
2014.PubMed/NCBI
|
|
16
|
Baldeiras I, Santana I, Proença MT,
Garrucho MH, Pascoal R, Rodrigues A, Duro D and Oliveira CR:
Peripheral oxidative damage in mild cognitive impairment and mild
Alzheimer's disease. J Alzheimers Dis. 15:117–128. 2008.PubMed/NCBI
|
|
17
|
Hitchon CA and El-Gabalawy HS: Oxidation
in rheumatoid arthritis. Arthritis Res Ther. 6:265–278. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toyokuni S: Molecular mechanisms of
oxidative stress-induced carcinogenesis: F rom epidemiology to
oxygenomics. IUBMB Life. 60:441–447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wankun X, Wenzhen Y, Min Z, Weiyan Z, Huan
C, Wei D, Lvzhen H, Xu Y and Xiaoxin L: Protective effect of
paeoniflorin against oxidative stress in human retinal pigment
epithelium in vitro. Mol Vis. 17:3512–3522. 2011.PubMed/NCBI
|
|
20
|
Zhao Y, Zhou G, Wang J, Jia L, Zhang P, Li
R, Shan L, Liu B, Song X, Liu S and Xiao X: Paeoniflorin protects
against ANIT-induced cholestasis by ameliorating oxidative stress
in rats. Food Chem Toxicol. 58:242–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klein K, Kabala PA and Grabiec AM: The
bromodomain protein inhibitor I-BET151 suppresses expression of
inflammatory genes and matrix degrading enzymes in rheumatoid
arthritis synovial fibroblasts. Ann Rheum Dis Dec. 2:2014.(Epub
ahead of print).
|
|
22
|
Ichihara H, Yamasaki S, Hino M, Ueoka R
and Matsumoto Y: Therapeutic effects of hybrid liposomes with
downregulation of inflammatory cytokine for model mice of
rheumatoid arthritis in vivo. Bioorg Med Chem Lett. 25:2686–2689.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Chen L, Delzell E, Muntner P,
Hillegass WB, Safford MM, Millan IY, Crowson CS and Curtis JR:
Republished: The association between inflammatory markers, serum
lipids and the risk of cardiovascular events in patients with
rheumatoid arthritis. Postgrad Med J. 90:722–729. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun J, Wu J, Xu C, Luo Q, Li B and Dong J:
Paeoniflorin attenuates allergic inflammation in asthmatic mice.
Int Immunopharmacol. 24:88–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang C, Yuan J, Wu HX, Chang Y, Wang QT,
Wu YJ, Liu LH and Wei W: Paeoniflorin inhibits inflammatory
responses in mice with allergic contact dermatitis by regulating
the balance between inflammatory and anti-inflammatory cytokines.
Inflamm Res. 62:1035–1044. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoon HY, Lee EG, Lee H, Cho IJ, Choi YJ,
Sung MS, Yoo HG and Yoo WH: Kaempferol inhibits IL-1β-induced
proliferation of rheumatoid arthritis synovial fibroblasts and the
production of COX-2, PGE2 and MMPs. Int J Mol Med. 32:971–977.
2013.PubMed/NCBI
|
|
27
|
Dubois RN, Abramson SB, Crofford L, Gupta
RA and Simon LS: VanD e Putte LB and Lipsky PE: Cyclooxygenase in
biology and disease. FASEB J. 12:1063–1073. 1998.PubMed/NCBI
|
|
28
|
Markosyan N, Chen EP, Evans RA, Ndong V,
Vonderheide RH and Smyth EM: Mammary carcinoma cell derived
cyclooxygenase 2 suppresses tumor immune surveillance by enhancing
intratumoral immune checkpoint activity. Breast Cancer Res.
15:R752013. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo RB, Wang GF, Zhao AP, Gu J, Sun XL and
Hu G: Paeoniflorin protects against ischemia-induced brain damages
in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses.
PLoS One. 7:e497012012. View Article : Google Scholar : PubMed/NCBI
|