Introduction
Hepatitis C virus (HCV) is a major cause of chronic
liver disease, and ~170 million people are infected with this virus
worldwide. Patients with persistent HCV infection are at high risk
of developing hepatocellular carcinoma, chronic liver diseases and
cirrhosis (1). HCV is an enveloped
flavivirus, which contains a positive single-stranded RNA of ~9,600
nucleotides (2,3). These nucleotides encode a single
polypeptide of ~3,000 amino acids, which is divided into structural
(core, E1 and E2) and nonstructural [p7, nonstructural protein
(NS)2, NS3, NS4A, NS5A and NS5B] proteins through proteolysis
(2,3).
HCV induces cell damage via different mechanisms
that remain poorly understood. The generation of reactive oxygen
species (ROS) and oxidative stress have been suggested to play
major roles in the pathogenesis of chronic HCV infection (4). HCV infection is associated with
increasing levels of various oxidative stress markers, including
mitochondrial injury, iron overload and chronic inflammation, which
are thought to be induced by HCV proteins (1–5). Until
recently, the standard of care (SOC) for patients with chronic HCV
infection consisted of a combination of pegylated interferon-α and
ribavirin (6). At present, a new SOC
is used for genotype 1 patients, which includes one protease
inhibitor, such as telaprevir, boceprevir or simeprevir, in
combination with pegylated interferon-α and ribavirin treatment
(7).
A number of studies have reported the beneficial
effects of antioxidants, such as glycyrrhizin, catechin, silymarin,
phytosterols, N-acetylcysteine and phytochemicals, which are able
to decrease HCV replication and liver damage (8). In our previous study, we reported that
acetylsalicylic acid reduces the levels of HCV-RNA and viral
protein by decreasing cellular oxidative stress and modifying the
Cu/Zn superoxide dismutase expression (9). Furthermore, another study reported that
carotene, vitamin D2 and linoleic acid inhibited HCV-RNA expression
(10).
Gallic acid (GA), also known as
3,4,5-trihydroxybenzoic acid, is a phenolic compound obtained from
plants, fruits and vegetables (11).
Currently, GA is used in various sectors, for instance as a
pharmaceutical, an industrial compound (12,13) and
a food additive (14). Previous
studies have reported that GA has certain biological effects, such
as anti-inflammatory, antibiotic, antiviral, anticancer and
cardiovascular protection effects (15,16).
These effects result from the fact that GA is a potent antioxidant
that is involved in absorbing and neutralizing free radicals
produced by cells (17).
GA has also been found to significantly decrease the
viability, proliferation and invasion of cancer cells (18–20). In
addition, GA isolated from Woodfordia fruticosa flowers
exhibited a higher anti-enterovirus 71 activity (21). Another study has demonstrated that GA
possess anti-herpes simplex virus type 1 (HSV-1) and anti-human
immunodeficiency virus activities (22).
Based on the aforementioned observations, the
present study aimed to explore the effect of GA on HCV-RNA
expression and further investigate the underlying mechanisms by
measuring oxidative stress markers using an HCV subgenomic replicon
cell culture system. Furthermore, a potent antioxidant, pyrrolidine
dithiocarbamate (PDTC), was used as a control since its effect as
an antioxidant has already been reported (23).
Materials and methods
Chemicals
GA, PDTC and H2O2 were
purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Huh7 hepatocarcinoma cells (donated by Dr.
Koromilas; McGill University, Montreal, Canada) were cultured in
advanced Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Grand Island, NY, USA) supplemented with 1%
nonessential amino acids, 2% heat-inactivated fetal bovine serum
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA), 1%
antibiotics (100 U penicillin G and 100 µg/ml streptomycin; Thermo
Fisher Scientific, Inc.) and 1% glutamine, in a humidified
atmosphere with 5% CO2 at 37°C. In addition, a genotype
1b HCV subgenomic replicon cell culture system was established
(24), which was maintained at the
same conditions, but in the presence of 500 µg/ml G418 (Geneticin;
Thermo Fisher Scientific, Inc.). Cells grown to 80–85% confluence
were trypsinized with 2.5 ml trypsin diluted with fresh medium and
counted using a hematocytometer (Marienfield-Superior,
Lauda-Königshofen, Germany) with trypan blue (Gibco).
GA treatment and cytotoxic assay
Huh7 parental and HCV replicon cells were seeded
onto 96-well plates (2×104 cells/well) and cultured for
24 h. Next, the medium was changed and the cells were treated with
different concentrations of GA (100, 300 and 600 µM) dissolved in
sterile phosphate buffered saline (PBS) and incubated for 0, 24, 48
and 72 h (25). Following
incubation, cell viability was evaluated using an MTT [also known
as 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
assay, according to the standard experimental protocol (26,27).
Total protein extraction
Huh7 HCV replicon cells (~6×105
cells/well) were seeded onto 6-well plates, cultured for 24 h and
then treated with 300 µM GA for a duration between 0 and 72 h.
After each time point, the cells were harvested and total protein
extraction was performed. Briefly, the cells were washed twice with
ice-cold 1X PBS/0.5 M EDTA, and proteins were extracted with 1X
lysis buffer containing 10 mM Tris-HCl (pH 7.5), 50 mM KCl, 2 mM
MgCl2, 1% Triton X-100, 1 mM dithiothreitol, 1 mM
phenylmethylsulfonyl fluoride, 3 µg/ml aprotinin, 1 µg/ml leupeptin
and 1 µg/ml pepstatin. Upon incubation, the cell lysates were
centrifuged at 16,438 × g for 5 min at 4°C (28). The supernatant was recovered, the
protein concentration was measured using the Bradford method with a
Bio-Rad Protein Assay kit (500-0006; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), and a standard curve was obtained using bovine
serum albumin (Amresco LLC, Solon, OH, USA).
Western blot analysis
Total cellular protein extracts were resolved by 12%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Subsequently, the samples were transferred to Hybond-P
polyvinylidene difluoride membranes (GE Healthcare Life Sciences,
Little Chalfont, UK), activated with methanol, washed with pure
water and equilibrated with 1X transfer buffer. The electrotransfer
was performed at 100 V for 1 h at 4°C, and then the membrane was
blocked with mouse anti-HCV NS5A monoclonal antibody (MAb;
dilution, 1:1,000; AB20342; Abcam, Cambridge, UK) and anti-actin
MAb (β-actin; dilution, 1:1,000; MAB1501; EMD Millipore, Billerica,
MA, USA). Immunocomplexes on the membranes were detected using an
enhanced chemiluminescence assay (Luminol, ImmunoCruz; Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) (10). The expression of NS5A relative to
β-actin protein was quantified using ImageJ software, version 1.46r
(http://imagej.nih.gov/ij/docs/guide/146.html).
RNA extraction
Huh7 HCV replicon cells (~2×105
cells/well) were seeded onto 24-well plates, cultured for 24 h and
then treated with 300 µM GA for a duration between 0 and 72 h.
Cells were harvested after each time point and total RNA was
extracted using TRIzol reagent (Ambion; Thermo Fisher Scientific,
Inc.) according to the manufacturer's specifications. RNA
precipitates were washed with 75% alcohol and resuspended in 12 µl
RNase-free water, and then the samples were stored at −80°C
(29).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for HCV-RNA quantification
The total RNA extracted was subjected to RT to
obtain complementary DNA (cDNA) using a SuperScript III RT kit
according to the manufacturer's specifications (Applied Biosystems;
Thermo Fisher Scientific, Inc., Foster City, CA, USA).
Subsequently, 200 ng cDNA was amplified by qPCR to quantify the
levels of HCV and GAPDH mRNA using an ABI-7500 Fast Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
primers used were as follows: HCV forward (75–93 nt),
5′-GCGTCTAGCCATGGCGTTA-3′, and reverse (138–157 nt),
5′-GGTTCCGCAGACCACTATGG-3′; GAPDH forward,
5′-GTGTTCCTACCCCCAATGTGT-3′, and reverse,
5′-ATTGTCATACCAGGAAATGAGCTT-3′; and the TaqMan probe (94–110 nt),
5′-FAM-CTGCACGACACTCATAC-NFQ-3′. For each PCR reaction, the
following were used: 1 µl assay mix, 9 µl cDNA diluted in
RNase-free water and 10 µl TaqMan PCR Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermal cycling
conditions were as follows: Initial setup at 50°C for 2 min, then
95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C
for 60 sec. GAPDH-RNA expression was used to normalize the cDNA
concentration and the amplification plots were analyzed using the
ABI-7500 Real-Time PCR System software, version 2.0.1, according to
the manufacturer's specifications (Applied Biosystems; Thermo
Fisher Scientific, Inc.) (30). The
2−ΔΔCq method was used to calculate relative changes in
gene expression determined from RT-qPCR experiments.
ROS measurement
To evaluate the effect of GA on the oxidative stress
level, ROS production was measured. Briefly, Huh7 HCV replicon
cells (~2×104 cells/well) were seeded onto 96-well
plates, cultured for 24 h and then treated with 300 µM GA, 2 µM
H2O2 as a damage control or 5 µM PDTC as an
antioxidant control, for 0.5, 1, 3, 6, 12 and 24 h at 37°C. Next, 2
µl of 2′,7′-dichlorodihydrofluorescein diacetate
(H2DCFDA; Invitrogen Molecular Probes, Eugene, OR, USA)
were added 30 min before the end of the treatment. The fluorescence
of cells was measured at room temperature, using 485 nm and 528 nm
as the excitation and emission wavelengths, respectively (BioTek
Synergy H5; BioTek Instruments, Inc., Winooski, VT, USA) (31,32).
Statistical analysis
All variables were tested in triplicate, and
experiments were repeated at least three times. Values were
presented as the mean ± standard deviation. Statistically
significant differences between control and treated groups were
determined by Student's t-test. Differences were considered to be
statistically significant for values of P<0.05.
Results
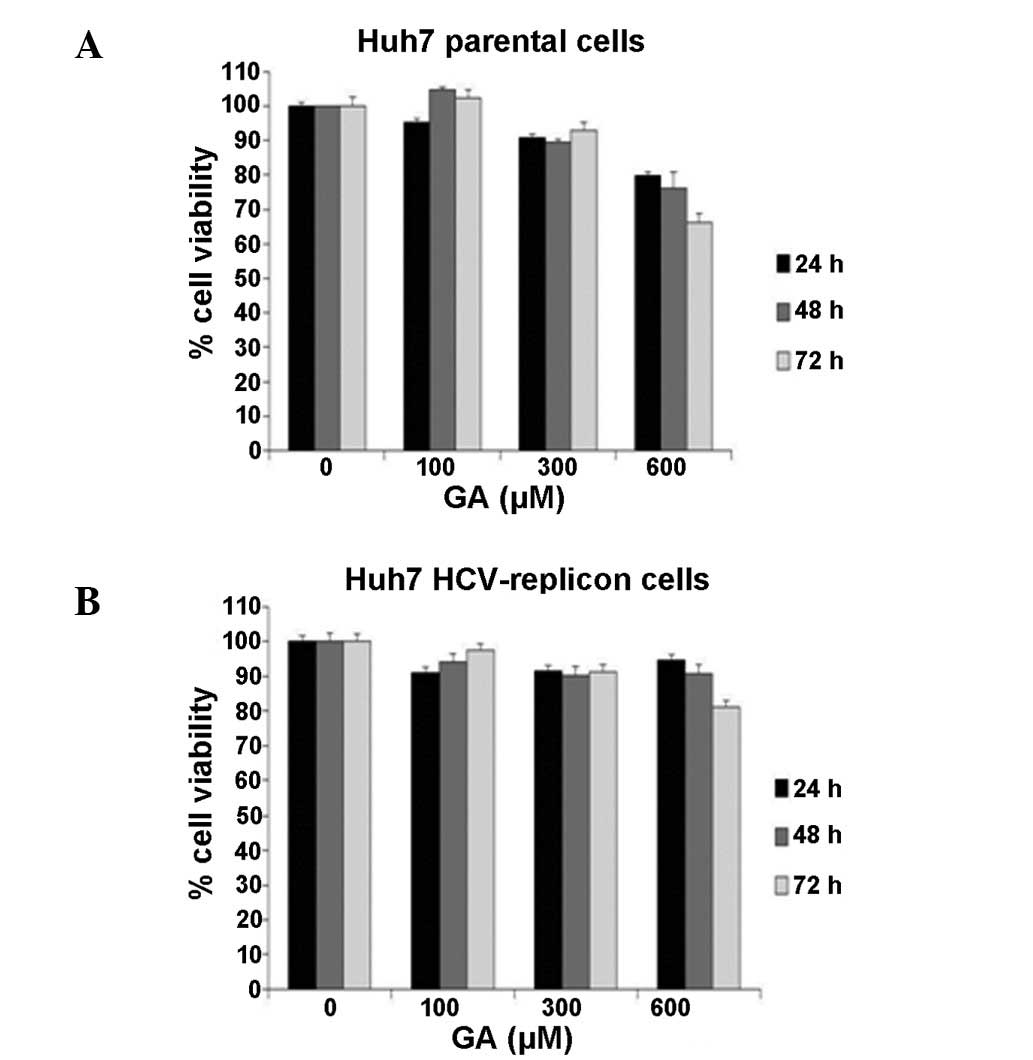
Viability of hepatoma Huh7 cells
treated with GA
Initially, the study investigated whether GA induces
a cytotoxic effect on treated cells. Huh7 parental and Huh7 HCV
replicon cells were exposed to three different concentrations of GA
(100, 300 and 600 µM) and incubated for a time between 24 and 72 h.
Next, total cell count and viability determinations were performed
using an MTT assay. Fig. 1 shows
that after 72 h of treatment no statistically significant
differences in cell number and viability were detectable between
the untreated (cell viability, 100%) and treated cell lines
(Fig. 1A, parental cells; Fig. 1B, HCV replicon cells) when using the
100 and 300 µM GA concentrations (cell viability, ~98 and 95%,
respectively). By contrast, cells treated with 600 µM GA showed a
lower cell survival rate in the two cell lines at all times of
exposure. Based on this finding, we selected the concentration of
300 µM GA in all subsequent experiments.
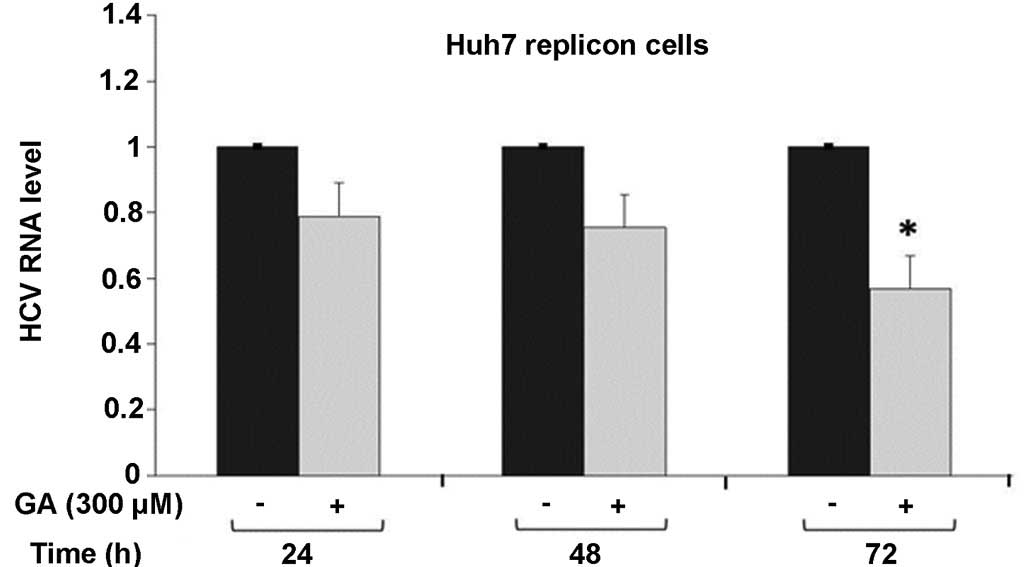
GA decreases HCV-RNA replication
To evaluate the effect of GA on HCV-RNA expression
in HCV replicon-containing cells, these cells were incubated with
300 µM GA for three different durations (24, 48 and 72 h).
Subsequently, the total cellular RNA was extracted and subjected to
RT-qPCR for HCV-RNA quantification, as described in the Materials
and methods. GA was found to inhibit HCV-RNA expression in a
time-dependent manner compared with the untreated cells, showing a
higher effect at 72 h post-treatment, at which the lowest HCV-RNA
expression was observed (22% inhibition at 48 h and 44% inhibition
at 72 h, P<0.01; Fig. 2).
Collectively, these results reveal that 300 µM GA is able to
decrease HCV expression at the transcriptional level in the HCV
replicon cell culture system.
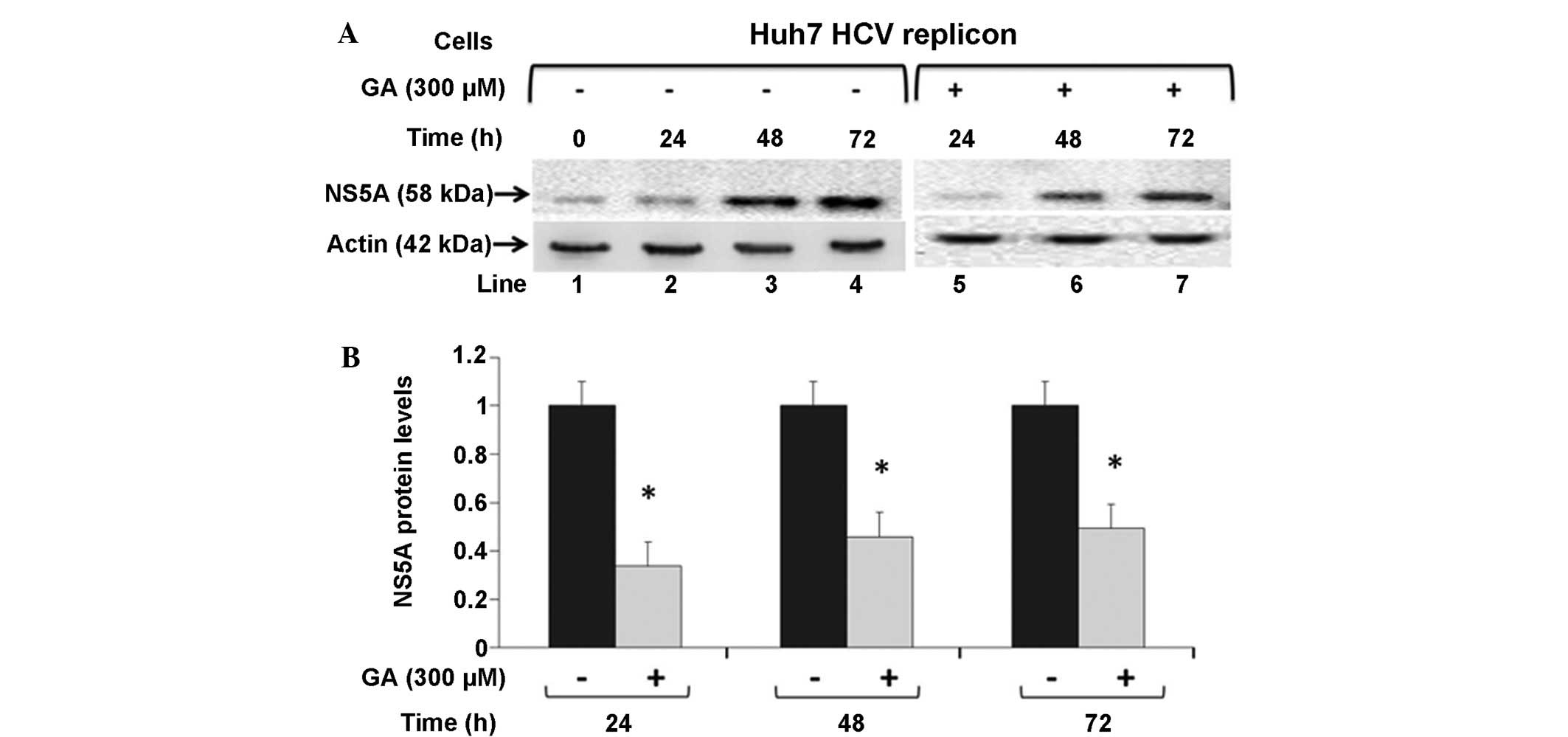
NS5A protein levels are downregulated
by GA in Huh7 HCV replicon cells
To investigate whether GA can influence the
synthesis of HCV viral proteins, NS5A and actin protein levels were
examined by western blot analysis in untreated HCV replicon cells
or cells treated with 300 µM GA, and incubated for 24, 48 and 72 h
(Fig. 3A). Cells treated with 300 µM
GA expressed lower levels of NS5A-HCV proteins, as shown by the
lower ratio of NS5A-HCV protein at the three time points after
exposure compared with the untreated cells (0.33, 0.45 and 0.49 for
the time points 24, 48 and 72 h, respectively, compared with the
control value; P<0.05; Fig. 3B).
These data suggest that GA treatment may diminish the translational
rate of viral proteins or decrease viral protein stability, in
addition to the negative effect on HCV-RNA levels.
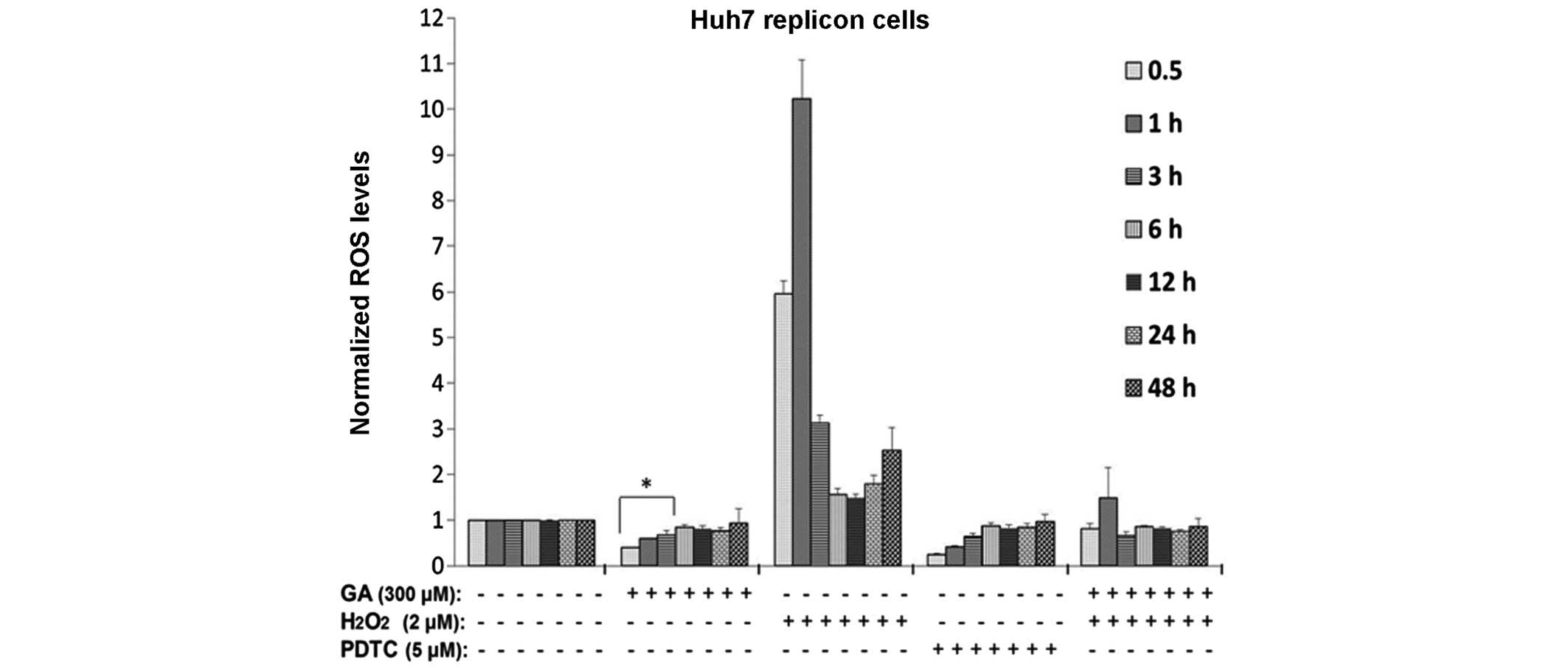
GA decreases ROS production in HCV
replicon cells
It has been reported that HCV promotes ROS
production in infected hepatocytes, which further promote lipid and
protein oxidation, leading to cell death (33). To determine whether GA treatment has
an antioxidant activity in HCV replicon cells, ROS levels were
evaluated using the H2DCF-DA assay. HCV replicon cells
were incubated in the presence or absence of 300 µM GA and
incubated for 24, 48 and 72 h, following which the ROS levels were
quantified. GA was found to reduce ROS levels in a time-dependent
manner (Fig. 4), with a greater
effect at earlier time points, including 0.5, 1 and 3 h after
treatment (~50, 30 and 20% reduction, respectively; P<0.05). In
addition, at 6, 12 and 24 h post-treatment, ROS production was
maintained <20% compared with the control cells. As a positive
control of antioxidant activity, HCV replicon cells were treated
with 5 µM PDTC. Fig. 4 shows that
PDTC decreased ROS levels starting at 0.5 h and showing a similar
effect as that observed at 3 h after GA treatment (P<0.05). In
addition, the present study investigated whether GA is able to
decrease the levels of ROS generated by a strong oxidizing agent,
such as H2O2. Huh7 replicon cells were
treated simultaneously with 2 µM H2O2 and GA,
and incubated between 0.5 and 48 h. Subsequently, ROS levels were
measured at the end of each time point. It was observed that GA was
able to abate the increased ROS levels induced by
H2O2, reaching levels similar to those of
cells without the oxidizing agent (Fig.
4). This suggested that GA acid was able to mitigate the
cellular oxidative stress induced by an oxidizing agent. Therefore,
these results confirm that GA was able to decrease oxidative stress
markers in the same way the antioxidant agent, PDTC (5 µM).
Discussion
Chronic HCV infection is a cause of liver disease
worldwide that leads to progressive fibrosis, and may result in
cirrhosis, hepatocellular carcinoma, liver failure and mortality
(34). The current treatment for HCV
is not effective for all patients and causes severe side effects.
Therefore, investigations continue to identify alternative
therapies for hepatitis C (35).
Oxidative stress plays an important role in various diseases,
including viral infection and chronic inflammation. HCV gene
expression can increase the levels of ROS. Therefore, antioxidants
have been found to exert antiviral activities against a variety of
viruses by decreasing the oxidative stress generated by the viruses
(36,37). In addition, previous studies have
demonstrated that GA has an antiviral activity due to its strong
radical scavenging activity (38).
Numerous studies have shown that interference with the generation
of ROS through the use of antioxidants can drastically reduce
replication of various viruses (35).
In the present study, we evaluated the GA effect on
HCV-RNA and protein expression in a Huh7 replicon cell system. GA
was not found to induce cytotoxicity at the concentration used in
the present study. These results are in agreement with those of
other reports using natural compounds such as silymarin, in which
cell viability was not affected upon treatment (35,39).
The results revealed the downregulation of HCV-RNA
and viral protein levels that was induced by GA, demonstrating that
GA may diminish the translational rate of viral proteins or
decrease the stability of viral protein or RNA (Fig. 3). The current results are in
agreement with those of other reports showing the antiviral
activity of natural compounds with an antioxidant activity
(40–42). In addition, a previous study
demonstrated that GA has an effect on HSV-1 gD, gC and VP5 viral
proteins of HSV-1 in Vero cells, showing that GA suppressed the
expression of these proteins (22).
Another natural compound, silymarin, has been reported to have an
anti-HCV activity by inducing downregulation (80%) of HCV-RNA, core
and NS5A viral proteins in the CON1 subgenomic genotype 1b HCV cell
line (35).
Recently, an antiviral assay demonstrated that GA
possessed good antiviral spectrum against other viruses, including
human rhinoviruses in HeLa cells, without inducing cytotoxicity at
the concentration used (21). The
present study is in agreement with previously published results
mentioning that GA is a strong antiviral antioxidant (43–46). The
antiviral effect of GA and its derivatives has also been
demonstrated in certain RNA viruses, including the vesicular
stomatitis virus (Rhabdoviridae family), influenza virus
(Orthomyxoviridae family) and poliovirus
(Picornaviridae family). Thus, GA inhibits the
multiplication of these RNA viruses, which have a different
structure, such as enveloped or nonenveloped, and positive- or
negative-stranded genome RNA (47).
According to the GA chemical structure, the observed
virucidal activity of GA may be due to the hydrophobic interaction
between the functional group (hydroxyl) and virion components,
providing GA with the capacity to bind free radicals and exert an
antioxidative effect. This antioxidant property of GA may explain
the antiviral effect observed against HCV in replicon cells, and
may be proposed as an alternative therapy for antiviral
treatment.
In conclusion, GA treatment was found to diminish
the cellular oxidative stress by decreasing ROS production, which
in turn was unfavorable for HCV. Thus, GA is suggested to be a
promising adjuvant in HCV therapy. Further research is required to
elucidate the underlying mechanism(s) of the GA effect on HCV
replication.
Acknowledgements
This study was supported in part by grants from
CONACYT (no. CB2010-01155082 and SALUD-2008-C01-86996; awarded to
Ana M. Rivas-Estilla) and FONCYT-COECYT (no. COAH-2002-C08-C37;
awarded to Jesus A. Morlett-Chávez).
Glossary
Abbreviations
Abbreviations:
|
GA
|
gallic acid
|
|
HCV
|
hepatitis C virus
|
|
RT-PCR
|
reverse transcription polymerase chain
reaction
|
|
cDNA
|
complementary DNA
|
|
ROS
|
reactive oxygen species
|
|
H2DCFDA
|
2′,7′-dichlorodihydrofluorescein
diacetate
|
|
NS5A
|
nonstructural protein 5A
|
|
MTT
|
[3-(4,5-dimethlthiazol-2-yl)-2,
5-diphenyl-tetrazolium bromide]
|
References
|
1
|
Pahl HL: Signal transduction from the
endoplasmic reticulum to the cell nucleus. Physiol Rev. 79:683–701.
1999.PubMed/NCBI
|
|
2
|
Warris G and Siddiqui A: Hepatitis C virus
stimulates the expression of cyclooxygenase-2 via oxidative stress:
Role of prostaglandin E2 in RNA replication. J Virol. 79:9725–9734.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang H, Chen Y and Ye J: Inhibition of
hepatitis C virus replication by peroxidation of arachidonate and
restoration by vitamin E. Proc Natl Acad Sci USA. 104:18666–18670.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gong G, Waris G, Tanveer R and Siddiqui A:
Human hepatitis C virus NS5A protein alters intracellular calcium
levels, induces oxidative stress, and activates STAT-3 and NF-kappa
B. Proc Natl Acad Sci USA. 98:9599–9604. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burra P and Hepatitis C: Semin Liver Dis.
29:53–65. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
do Kim Y, Ahn SH and Han KH: Emerging
therapies for hepatitis C. Gut Liver. 8:471–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poordad F, McCone J Jr, Bacon BR, Bruno S,
Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai
N, et al: Boceprevir for untreated chronic HCV genotype 1
infection. N Engl J Med. 364:1195–1206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patrick L and Hepatitis C: epidemiology
and review of complementary/alternative medicine treatments. Altern
Med Rev. 4:220–238. 1999.PubMed/NCBI
|
|
9
|
Trujillo-Murillo K, Rincón-Sánchez AR,
Martinez-Rodriguez H, Bosques-Padilla F, Ramos-Jiménez J,
Barrera-Saldaña HA, Rojkind M and Rivas-Estilla AM: Acetylsalicylic
acid inhibits hepatitis C virus RNA and protein expression through
cyclooxygenase 2 signaling pathways. Hepatology. 47:1462–1472.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yano M, Ikeda M, Abe K, Dansako H, Ohkoshi
S, Aoyagi Y and Kato N: Comprehensive analysis of the effects of
ordinary nutrients on hepatitis C virus RNA replication in cell
culture. Antimicrob Agents Chemother. 51:2016–2027. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taitzoglou IA, Tsantarliotou M, Zervos I,
Kouretas D and Kokolis NA: Inhibition of human and ovine acrosomal
enzymes by tannic acid in vitro. Reproduction. 121:131–137.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haslam E: Practical polyphenolics, From
structure to molecular recognition and physiological action.
Cambridge University Press Cambridge. 84–177. 1998.
|
|
13
|
Lekha PK and Lonsane BK: Production and
application of tannin acyl hydrolase: State of the art. Adv Appl
Microbiol. 44:215–260. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hocman G: Chemoprevention of cancer: P
henolic antioxidants (BHT, BHA). Int J Biochem. 20:639–651. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharma S, Wyatt GP and Steele VE: A
carcinogen-DNA binding assay as a biomarker screen for identifying
potential chemopreventive agents. Methods Cell Sci. 19:45481997.
View Article : Google Scholar
|
|
16
|
Kim YJ: Antimelanogenic and antioxidant
properties of gallic acid. Biol Pharm Bull. 30:1052–1055. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji BC, Hsu WH, Yang JS, Hsia TC, Lu CC,
Chiang JH, Yang JL, Lin CH, Lin JJ, Suen LJ, et al: Gallic acid
induces apoptosis via caspase-3 and mitochondrion-dependent
pathways in vitro and suppresses lung xenograft tumor growth
in vivo. J Agric Food Chem. 57:7596–7604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inoue M, Suzuki R, Sakaguchi N, Li Z,
Takeda T, Ogihara Y, Jiang BY and Chen Y: Selective induction of
cell death in cancer cells by gallic acid. Biol Pharm Bull.
18:1526–1530. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen HM, Wu YC, Chia YC, Chang FR, Hsu HK,
Hsieh YC, Chen CC and Yuan SS: Gallic acid, a major component of
Toona sinensis leaf extracts, contains a ROS-mediated
anti-cancer activity in human prostate cancer cells. Cancer Lett.
286:161–171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
You BR and Park WH: The effects of
mitogen-activated protein kinase inhibitors or small interfering
RNAs on gallic acid induced HeLa cell death in relation to reactive
oxygen species and glutathione. J Agric Food Chem. 59:763–771.
2001. View Article : Google Scholar
|
|
21
|
Choi HJ, Song JH, Bhatt LR and Baek SH:
Anti-Human Rhinovirus activity of gallic acid possessing
antioxidant capacity. Phytother Res. 24:1292–1296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kratz JM, Andrighetti-Frohner CR, Kolling
DJ, Leal PC, Cirne-Santos CC, Yunes RA, Nunes RJ, Trybala E,
Bergström T, Frugulhetti IC, et al: Anti-HSV-1 and anti-HIV-1
activity of gallic acid and pentyl gallate. Mem Inst Oswaldo Cruz.
103:437–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cuzzocrea S, Chatterjee PK, Mazzon E,
Serraino I, Britti D, Dugo L, Mazzullo G, Caputi AP and Thiemermann
C: Pyrrolidine dithiocarbamate attenuates the development of acute
and chronic inflammation. Br J Pharmacol. 135:496–510. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lohmann V, Körner F, Koch J, Herian U,
Theilmann L and Bartenschlager R: Replication of subgenomic
hepatitis C virus RNAs in a hepatoma cell line. Science.
285:110–113. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Devasagayam TP, Maurya DK and Nandakumar
N: Anticancer property of gallic acid in A549, a human lung
adenocarcinoma cell line and possible mechanisms. J Clin Biochem
Nutr. 48:85–90. 2011.PubMed/NCBI
|
|
26
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: A pplications to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yedjou CG and Tchounwou PB: In
vitro cytotoxic and genotoxic effects of arsenic trioxide on
human leukemia (HL-60) cells using the MTT and alkaline single cell
gel electrophoreis (Comet) assays. Mol Cell Biochem. 301:123–130.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rivas-Estilla AM, Svitkin Y, Lastra Lopez
M, Hatzoglou M, Sherker A and Koromilas AE: PKR-dependent
mechanisms of gene expression from a subgenomic hepatitis C virus
clone. J Virol. 76:10637–10653. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rivas-Estilla AM, Bryan-Marrugo OL,
Trujillo-Murillo K, Pérez-Ibave D, Charles-Niño C, Pedroza-Roldan
C, RíosIbarra C, Ramírez-Valles E, Ortíz-López R, Islas-Carbajal
MC, et al: Cu/Zn superoxide dismutase (SOD1) induction is
implicated in the antioxidative and antiviral activity of
acetylsalicylic acid in HCV-expressing cells. Am J Physiol
Gastrointest Liver Physiol. 302:G1264–G1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kopff M, Kopff A and Kowalczyk E: The
effect of nonsteroidal anti-inflammatory drugs on
oxidative/antioxidative balance. Pol Merkur Lekarski. 23:184–187.
2007.(In Polish). PubMed/NCBI
|
|
31
|
Polat A and Emre MH: Effects of melatonin
or acetylsalicylic acid on gastric oxidative stress after bile duct
ligation in rats. J Gastroenterol. 41:433–439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chatel-Chaix L, Baril M and Lamarre D:
Hepatitis C Virus NS3/4A Protease inhibitors, A light at the end of
the Tunnel. Viruses. 2:1752–1765. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ivanov AV, Bartosh B, Smirnova OA,
Isaguliants MG and Kochetkov SN: HCV and oxidative stress in the
liver. Viruses. 5:439–469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mehrab-Mohseni M, Sendi H, Steuerwald N,
Ghosh S, Schrum LW and Bonkovsky HL: Legalon-SIL down regulates HCV
core and NS5A in human hepatocytes expressing full-length HCV.
World J Gastroenterol. 17:1694–1700. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi HJ, Song JH, Park KS and Baek SH:
In vitro anti-enterovirus 71 activity of gallic acid from
Woodfordia fruticosa flowers. Lett Appl Microbiol.
50:438–440. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sriwilaijaroen N, Fukumoto S, Kumagai K,
Hiramatsu H, Odagiri T, Tashiro M and Suzuki Y: Antiviral effect of
Psidium guajava Linn (guava) tea on the growth of clinical
isolated H1N1 viruses: Its role in viral hemagglutination and
neuraminidase inhibition. Antiviral Res. 94:139–146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
de Oliveira A, Adams SD, Lee LH, Murray
SR, Hsu SD, Hammond JR, Dickinson D, Chen P and Chu TC: Inhibition
of herpes simplex virus type 1 with the modified green tea
polyphenol palmitoyl-epigallocatechin gallate. Food Chem Toxicol.
52:207–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
You BR, Moon HJ, Han YH and Park WH:
Gallic acid inhibits the growth of HeLa cervical cancer cells via
apoptosis and/or necrosis. Food Chem Toxicol. 48:1334–1340. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Polyak SJ, Morishima C, Lohmann V, Pal S,
Lee DYW, Liu Y, Graf TN and Oberlies NH: Identification of
hepatoprotective flavonolignans from silymarin. Proc Natl Acad Sci
USA. 107:5595–5599. 2010. View Article : Google Scholar
|
|
40
|
Lee JC, Chen WC, Wu SF, Tseng CK, Chiou
CY, Chang FR, Hsu SH and Wu YC: Anti-hepatitis C virus of Acacia
confusa extract via suppressing cyclooxygenase-2. Antiviral
Res. 89:35–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ravikumar YS, Ray U, Nandhitha M, Perween
A, Naika Raja HR, Khanna N and Das S: Inhibition of hepatitis C
virus replication by herbal extract. Phyllanthus amarus as potent
natural source. Virus Res. 158:89–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Manvar D, Mishra M, Kumar S and Pandey VN:
Identification and evaluation of anti Hepatitis C virus
phytochemicals from Eclipta alba. J Ethnopharmacology.
144:545–554. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ahn MJ, Kim CY, Lee JS, Kim TG, Kim SH,
Lee CK, Lee BB, Shin CG, Huh H and Kim J: Inhibition of HIV-1
integrase by galloyl glucoses from Terminalia chebula and
flavonol glycoside gallates from Euphorbia pekinensis.
Planta Med. 68:457–459. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Duan D, Li Z, Luo H, Zhang W, Chen L and
Xu X: Antiviral compounds from traditional Chinese medicines Galla
Chinese as inhibitors of HCV NS3 protease. Bioorg Med Chem Lett.
14:6041–6044. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Uosaki M, Yamasaki H, Katsuyama Y, Higuchi
M, Higuti T and Koyama AH: Antiviral effect of octyl gallate
against DNA and RNA viruses. Antiviral Res. 73:85–91. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nutan Modi M, Goel T, Das T, Malik S, Suri
S, Rawat AK, Srivastava SK, Tuli R, Malhotra S and Gupta SK:
Ellagic acid & gallic acid from Lagerstroemia speciosa
L. inhibit HIV-1 infection through inhibition of HIV-1 protease
& reverse transcriptase activity. Indian J Med Res.
137:540–548. 2013.PubMed/NCBI
|
|
47
|
Liu G, Xiong S, Xiang YF, Guo CW, Ge F,
Yang CR, Zhang YJ, Wang YF and Kitazato K: Antiviral activity and
possible mechanisms of action of pentagalloylglucose (PGG) against
influenza A virus. Arch Virol. 156:1359–1369. 2011. View Article : Google Scholar : PubMed/NCBI
|