Introduction
Uterine fibroids, which have an incidence rate of
20–25%, are the most common benign tumors in females (1). The clinical manifestations of uterine
fibroids mainly include menorrhagia, menostaxis, dysmenorrhea and
anemia. Clinically, uterine fibroids are mainly treated with
surgical therapy (hysterectomy and myomectomy) and hormone therapy
(2,3); however, the recurrence rate of uterine
fibroids subsequent to these treatments is relatively high, and the
trauma caused by these treatments can be extensive. Uterine artery
embolization (UAE) is a minimally invasive procedure that offers an
alternative to the traditional surgical removal of the uterine
fibroids (4). During the procedure,
the blood supply of the fibroids is blocked, which leads to the
shrinkage of the fibroids. UAE has been widely used in recent years
for the treatment of uterine fibroids.
Insulin-like growth factor 1 (IGF-1) and vascular
endothelial growth factor (VEGF) play important roles in the growth
of uterine fibroids (5). IGF-1, a
tumor autocrine growth factor, is highly expressed in numerous
types of tumor and has a key role in stimulating cell growth and
proliferation (6–8). VEGF is an important growth factor that
stimulates the proliferation of vascular endothelial cells. It has
been reported that VEGF promotes cell migration and proliferation
by binding to the associated receptors (9), thereby affecting angiogenesis (7). Although uterine fibroids are benign
tumors, angiogenesis is also critical for its occurrence and
development. UAE treatment can induce ischemia and hypoxia of
uterine fibroids by obstructing their blood supply (10). It has been found that
hypoxia-inducible factor-1α, an important factor regulating oxygen
balance, can induce VEGF gene transcription and increase the
stability of VEGF mRNA (11), thus
elevating the expression level of VEGF and stimulating uterine
fibroid angiogenesis.
It has yet to be elucidated whether IGF-1 and VEGF
could be used as prognostic factors for patients with uterine
fibroids following UAE treatment. In the present study, the serum
levels of IGF-1 and VEGF prior to and following UAE treatment were
measured, and the effects of IGF-1 and VEGF on progression-free
survival were analyzed, in order to provide experimental evidence
to enable the evaluation of the prognosis of uterine fibroid
patients following UAE treatment.
Patients and methods
Clinical data
A total of 70 patients with uterine fibroids, who
were hospitalized at the Department of Intervention, the First
Affiliated Hospital of Baotou Medical College (Baotou, China)
between January 2012 and December 2014, were included in the UAE
group and received UAE treatment. The inclusion criteria were as
follows: i) Uterine fibroids were diagnosed through clinical
gynecological, type-B ultrasound and other auxiliary examinations;
ii) patients exhibited a normal coagulation function; iii) patients
were free from underlying diseases, comorbidities or complications;
and iv) the age of the patients was ≤60 years. The exclusion
criteria were as follows: i) Patients not meeting the inclusion
criteria; ii) presence of lesions in other parts of the body or
intraoperative complications; iii) patients with cervical and broad
ligament fibroids; and iv) patients with abnormal coagulation
function. The age of the 70 patients in the UAE group ranged from
27 to 60 years, with an average age of 39.6±7.69 years. Among the
patients in this group, 25 were single and 45 were married. A total
of 47 patients exhibited menorrhagia and menostaxis, and 22
patients suffered from pain in the lower abdomen and waist.
Compression symptoms, such as urinary frequency, urinary urgency
and constipation, were found in 54 cases, and anemia was found in
39 cases. A total of 20 healthy individuals booked in for random
health checks were additionally included in the study as a control
group. The Karnofsky Performance Scale score of patients was
evaluated following admission to the hospital (12).
The patients were followed-up at 1 week and 3, 6 and
18 months after UAE treatment. The size of the fibroids and uterus
were evaluated by ultrasound, computed tomography or magnetic
resonance imaging examination. Progression-free survival referred
to the period between the diagnosis of the tumor and the end of the
follow-up in patients without recurrence and regeneration of
fibroids. A total of 21 patients did not complete the study, as a
result of being lost to follow-up at the time of last contact or
prior to the study cut-off or due to succumbing to an unrelated
cause. These cases were censored.
Prior written and informed consent was obtained from
every patient, and the study was approved by the Ethics Review
Board of the First Affiliated Hospital of Baotou Medical
College.
Sample preparation
Peripheral blood (4 ml) was collected from cubital
veins prior to UAE treatment and at 1 week and 3 and 6 months after
UAE treatment. Serum was isolated by centrifugation at 1,411 × g
for 15 min and then stored at −40°C until further analysis.
UAE treatment
UAE treatment was performed by two physicians as
previously described (13). Briefly,
catheterization was performed through the right femoral artery
under local anesthesia (5 ml lidocaine hydrochloride; Tianjin Jin
Yao Amino Acid Co., Ltd., Tianjin, China). Then, contralateral
iliac artery angiography and uterine artery angiography was
performed (Artis dTC Angiography; Siemens AG, Munich, Germany).
Following the elimination of key branches of the uterine artery,
the emulsified mixture of lipiodol (5 ml; Guerbet, Villepinte,
France) and pingyangmycin (4 mg; Shanghai Yansheng Shiye Co., Ltd.
Shanghai, China) was injected into the uterine artery. After the
majority of the uterus was dyed and the blood flow reduced, gelatin
sponge (10×2×2 mm) was used to embolize the uterine artery. The
embolization was verified by angiography. The embolization of the
ipsilateral uterine artery was performed accordingly.
ELISA
ELISA assay was performed using IGF-1 (YM-S2154) and
VEGF165 (YM-10632) ELISA kits (Shanghai YuanMu Biological
Technology Co., Ltd., Shanghai, China) according to the
manufacturer's instructions. Briefly, serum was added to the
pre-coated microplate and incubated at 4°C overnight. The plate was
then washed 5 times with phosphate-buffered saline and the
detection antibody was added to the wells. Following incubation at
room temperature for 1 h, the microplate was washed again, and
horseradish peroxidase-conjugated antibody was added and incubated
at room temperature for 30 min. All antibodies were from the IGF-1
and VEGF165 ELISA kits. After 30 min, the plate was re-washed 5
times, o-phenylenediamine (OPD) substrate solution was added
and the plate was incubated at room temperature for a further 15
min. OPD was part of the IGF-1 and VEGF165 ELISA kits. Finally,
stop solution was added to terminate color development and the
plate was read at 450 nm on a microplate reader (SpectraMax M2;
Molecular Devices LLC, Sunnyvale, CA, USA). The standard curve was
generated using 2-fold serial dilutions of the standard samples.
Levels of IGF-1 and VEGF were calculated according to the standard
curve.
Statistical analysis
Results are expressed as the mean ± standard
deviation. All statistical analyses were performed with SPSS
version 17.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Comparisons between groups and analyses of paired data were
conducted using the Student's t-test. Multiple-factor analysis was
performed to analyze the association between levels of IGF-1 or
VEGF and certain clinical characteristics, including adenomyosis
and the age of the patients, as well as fibroid size, location and
number. The Kaplan-Meier method was used to assess the
progression-free survival of the patients. The differences in
survival time were analyzed using a Log-Rank test. P<0.05 was
considered to indicate a statistically significantly
difference.
Results
General data of patients with uterine
fibroids
The general data of the patients with uterine
fibroids are shown in Table I. Among
the 70 patients, 53 were unable or not willing to undergo surgery
and 17 were patients who had recurrent uterine fibroids following
surgical removal. There were 31 patients with a solitary fibroid
and 39 with multiple fibroids. The fibroids were located as
follows: 42, intramural; 17, submucosal; and 11, subserosal. Eight
patients with uterine fibroids exhibited concurrent adenomyosis. In
all 70 patients, the Karnofsky score was ≥90.
 | Table I.Clinical data of patients with uterine
fibroids. |
Table I.
Clinical data of patients with uterine
fibroids.
| Clinical
features | Average (range) | Cases, n |
|---|
| Age, years | 39.6±7.69
(25–60) |
|
| ≥50 |
| 7 |
|
<50 |
| 63 |
| Adenomyosis of
uterus |
|
|
| Yes |
| 8 |
| No |
| 62 |
| Location of
fibroids |
|
|
|
Intramural | 42 |
|
|
Submucosal | 17 |
|
|
Subserosal | 11 |
|
| Size of fibroids,
cm | 3.97±1.48
(1.5–6.5) |
|
| ≥1.5 |
| 58 |
|
<6.5 |
| 12 |
| Number of
fibroids |
|
|
|
Solitary |
| 31 |
|
Multiple |
| 39 |
Serum levels of IGF-1 and VEGF prior
to and following UAE treatment
To determine the levels of IGF-1 and VEGF in the
serum, ELISA was performed prior to and following the UAE
treatment. As shown in Table II,
the serum levels of IGF-1 and VEGF in the UAE group prior to
treatment were 134.5±45.1 and 144.0±56.6 pg/ml, respectively; these
levels were significantly higher than those in the control group
(122.1±46.4 pg/ml for IGF-1 and 77.9±34.8 pg/ml for VEGF)
(P<0.05). At 1 week after UAE treatment, the serum levels of
IGF-1 and VEGF in the patients of the UAE group were 70.2±20.4 and
86.2±33.3 pg/ml, respectively. Compared with the levels prior to
UAE treatment, the serum levels of IGF-1 and VEGF at 1 week after
UAE treatment were significantly lower (P<0.05). At 1 month
after UAE treatment, the serum levels of IGF-1 (118.3±48.8 pg/ml)
and VEGF (109.5±42.7 pg/ml) were significantly higher than those at
1 week after UAE treatment (P<0.05). Similarly, the serum levels
of IGF-1 (131.3±43.1 pg/ml) and VEGF (136.7±52.6 pg/ml) at 3 months
after UAE treatment were significantly higher than those at 1 week
after UAE treatment (P<0.05).
 | Table II.Levels of IGF-1 and VEGF in patients
with uterine fibroids before and after UAE treatment. |
Table II.
Levels of IGF-1 and VEGF in patients
with uterine fibroids before and after UAE treatment.
| Groups | IGF-1 (pg/ml) | P-value | VEGF (pg/ml) | P-value |
|---|
| Control | 122.1±46.4 |
| 77.9±34.8 |
|
| UAE |
|
|
|
|
| Before
UAE |
134.5±45.1a | 0.025 |
144.0±56.6a | <0.001 |
| 1 week
after UAE |
70.2±20.4b | 0.001 |
86.2±33.3b | 0.001 |
| 1 month
after UAE |
118.3±48.8c | <0.001 |
109.5±42.7c | <0.001 |
| 3 months
after UAE |
131.3±43.1c | <0.001 |
136.7±52.6c | 0.015 |
Association between the serum levels
of IGF-1 and VEGF and the clinical characteristics of patients with
uterine fibroids
To investigate the association between the serum
levels of IGF-1 and VEGF at 1 week after UAE treatment and the
clinical characteristics of the patients with uterine fibroids,
multiple-factor analysis was performed. The analyzed clinical
characteristics included the age of the patient, adenomyosis and
the size, location and number of fibroids. As shown in Table III, the serum IGF-1 level at 1 week
after UAE treatment significantly differed according to the
clinical characteristics of adenomyosis and the size, location and
number of fibroids (P<0.05). No significant difference was found
in the serum IGF-1 level between different age groups (P>0.05).
The serum VEGF level at 1 week after UAE treatment significantly
differed according to the clinical characteristics of adenomyosis
and the size and location of the fibroids (P<0.05). No
significant differences in serum VEGF levels were found between
patients with different numbers of fibroids or different ages
(P>0.05). These results suggest that IGF-1 and VEGF may be used
for predicting the prognosis of patients with uterine fibroids.
 | Table III.Association between the serum levels
of IGF-1 or VEGF and clinical characteristics of uterine
fibroids. |
Table III.
Association between the serum levels
of IGF-1 or VEGF and clinical characteristics of uterine
fibroids.
| Clinical
characteristics | Cases | IGF-1 (pg/ml) at 1
week after UAE treatment | P-value | VEGF (pg/ml) at 1
week after UAE treatment | P-value |
|---|
| Adenomyosis of
uterus |
|
| 0.002 |
| 0.048 |
| Yes | 8 | 96.2±8.51 |
| 77.6±13.6 |
|
| No | 62 | 66.9±23.7 |
| 87.4±35.0 |
|
| Location of
fibroids |
|
| 0.041 |
| 0.032 |
|
Intramural | 42 | 60.5±21.4 |
| 71.5±21.5 |
|
|
Submucosal | 17 | 81.3±25.5 |
| 104.7±33.8 |
|
|
Subserosal | 11 | 90.3±10.3 |
| 114.1±39.9 |
|
| Number of
fibroids |
|
| 0.043 |
| 0.093 |
|
Solitary | 31 | 84.3±17.9 |
| 86.1±27.6 |
|
|
Multiple | 39 | 60.2±22.7 |
| 86.4±37.6 |
|
| Size of fibroids,
cm |
|
| 0.006 |
| 0.005 |
| ≥1.5 | 58 | 65.6±23.7 |
| 79.8±25.2 |
|
|
<6.5 | 12 | 92.8±11.6 |
| 117.3±49.0 |
|
| Age, years |
|
| 0.054 |
| 0.201 |
| ≥50 | 7 | 88.1±141 |
| 95.3±16.2 |
|
|
<50 | 63 | 68.3±24.5 |
| 85.2±33.3 |
|
Effect of serum IGF-1 and VEGF levels
on progression-free survival of patients with uterine fibroids
To investigate the effect of IGF-1 and VEGF serum
levels on the prognosis of patients with uterine fibroids, the
Kaplan-Meier method was performed to analyze the progression-free
survival of the patients. The patients were followed-up for 18
months. Progression-free survival curves were constructed based on
the serum levels of IGF-1 and VEGF at 1 week after UAE treatment
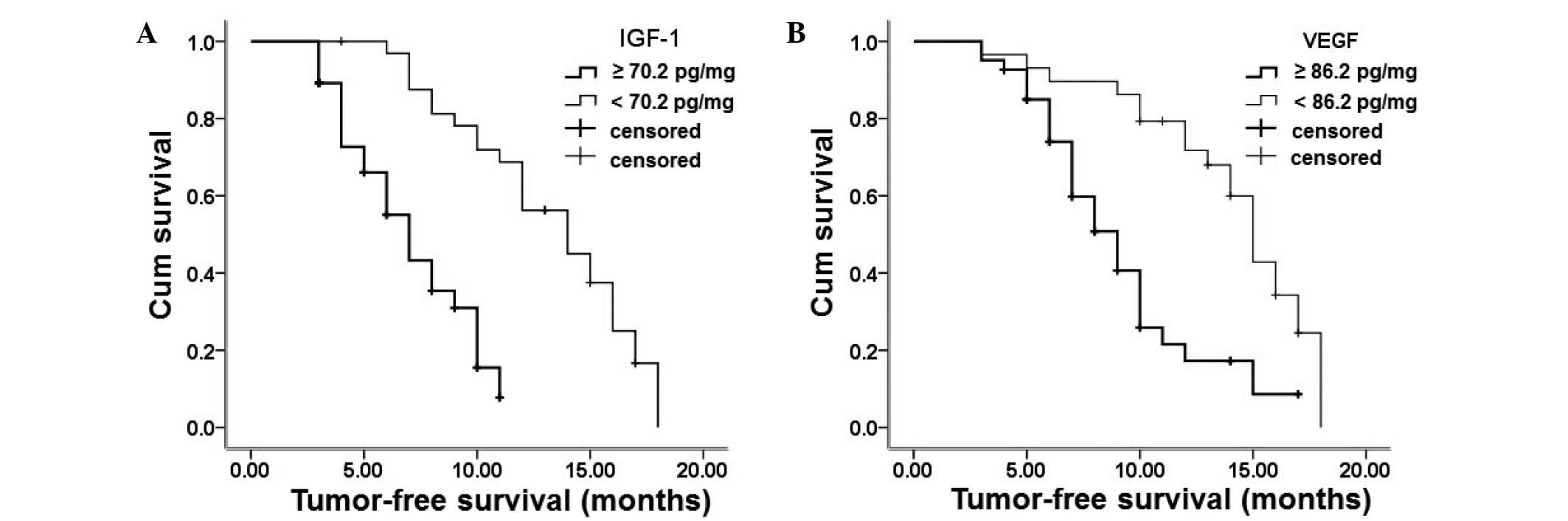
(70.2 and 86.2 pg/ml, respectively) and are shown in Fig. 1. A total of 37 patients had a serum
IGF-1 level ≥70.2 pg/ml and 33 patients had a serum IGF-1 level
<70.2 pg/ml. The progression-free survival of the patients with
a serum IGF-1 level ≥70.2 pg/ml was 1.5 months, which was
significantly shorter than that of patients with a serum IGF-1
level <70.2 pg/ml (3 months). The number of patients with serum
VEGF levels ≥86.2 and <86.2 pg/ml was 29 and 41, respectively.
The progression-free survival of patients with a serum VEGF level
≥86.2 pg/ml was 2 months, which was significantly shorter than that
of patients with a serum IGF-1 level <86.2 pg/ml (4 months).
These results indicate that patients with higher levels of IGF-1
and VEGF have a poorer prognosis than those with lower IGF-1 and
VEGF levels.
Discussion
The main blood supply of uterine fibroids is from
the uterine artery and its branches. UAE treatment can induce
ischemia and atrophy of the uterine fibroids (14) and is less invasive than the
traditional surgical approach. Furthermore, UAE treatment can
successfully preserve the uterus and maintain its normal
physiological functions (15,16).
IGF-1 and VEGF are important factors involved in the growth of
uterine fibroids (5).
In the present study, the serum levels of IGF-1 and
VEGF in the UAE group prior to UAE treatment were significantly
higher than those in the control group. At 1 week after UAE
treatment, the serum IGF-1 and VEGF levels in the UAE group were
significantly decreased compared with those prior to UAE treatment;
however, at 1 and 3 months after UAE treatment, the serum IGF-1 and
VEGF levels were significantly increased compared with those at 1
week after UAE treatment. These results were consistent with the
findings of a previous study by Ji et al (17). The decrease in the serum level of
IGF-1 at 1 week after UAE treatment may have been due to the
temporary inhibition of IGF-1 expression. At 1 and 3 months after
UAE treatment, the serum level of IGF-1 was increased. These
results suggest that IGF-1 plays an important role in the growth of
fibroids. The decrease in the serum level of VEGF following UAE
treatment may have been due to the ischemia and hypoxia after the
embolization. These findings therefore indicate that IGF-I and VEGF
can be used as indicators of prognosis following UAE treatment.
This study further analyzed the association between
serum levels of IGF-1 and VEGF and the clinical factors associated
with the therapeutic effect of uterine fibroid treatment, including
the size, location and number of uterine fibroids, as well as the
age of the patient and the presence of adenomyosis. The serum
levels of IGF-1 and VEGF were significantly different among
patients with different sizes and locations of uterine fibroids, as
well as between patients with and without adenomyosis. These data
suggest that IGF-1 and VEGF have an important effect on the
prognosis associated with uterine fibroids.
To further verify the association between serum
IGF-1 and VEGF levels and the prognosis of patients with uterine
fibroids, the progression-free survival of the patients was
analyzed using the Kaplan-Meier method. The median levels of IGF-1
and VEGF were calculated according to the serum levels of IGF-1 and
VEGF at 1 week after UAE treatment. Patients were divided into two
groups according to whether their serum IGF-1 (or VEGF) level was
higher or lower than the median level. The results showed that, at
the end of follow-up, the progression-free survival of patients
with serum levels of IGF-1 and VEGF lower than the median level was
significantly enhanced. These data suggest that lower levels of
IGF-1 and VEGF in the serum following UAE treatment indicate a good
prognosis.
In conclusion, the serum levels of IGF-1 and VEGF
exhibited significant changes prior to and following UAE treatment.
Furthermore, the serum levels of IGF-1 and VEGF at 1 week after UAE
treatment were significantly different among patients with
different uterine fibroid-related clinical characteristics.
Additionally, patients with lower levels of IGF-1 and VEGF in the
serum following UAE treatment had significantly longer
progression-free survival. Based on these results, IGF-1 and VEGF
may be used as prognostic factors for evaluating the prognosis of
patients with uterine fibroids following UAE treatment.
Acknowledgements
This study was supported by a grant from Baotou
Science and Technology Bureau Intellectual Property (no.
BK2008123).
References
|
1
|
Okogbo FO, Ezechi OC, Loto OM and Ezeobi
PM: Uterine Leiomyomata in South Western Nigeria, A clinical study
of presentations and management outcome. Afr Health Sci.
11:271–278. 2011.PubMed/NCBI
|
|
2
|
Zhang Y, Sun L, Guo Y, Cheng J, Wang Y,
Fan S and Duan H: The impact of preoperative gonadotropin-releasing
hormone agonist treatment on women with uterine fibroids, A
meta-analysis. Obstet Gynecol Surv. 69:100–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang A and Yin S: Controversy and
challenge in the treatment methods of uterine fibroids. Zhong Guo
Quan Ke Yi Xue. 12:14–15. 2009.(In Chinese).
|
|
4
|
Singh C, Gupta M, Tripathi R and Tyagi S:
Successful use of transcatheter embolisation in an emergent
life-threatening situation of bleeding from uterine arteriovenous
malformation. BMJ Case Rep. 2013:2013.
|
|
5
|
Mathonnet M, Descottes B, Valleix D,
Labrousse F, Truffinet V and Denizot Y: Quantitative analysis using
ELISA of vascular endothelial growth factor and basic fibroblast
growth factor in human colorectal cancer, liver metastasis of
colorectal cancer and hepatocellular carcinoma. World J
Gastroenterol. 12:3782–3783. 2006.PubMed/NCBI
|
|
6
|
Fan ZR, Yang DH, Zuo HR, Xu Z and Qiu QL:
Expressions of IGF-I, IGF-II and their receptor mRNAs in
hepatocellular carcinomas and adjacent non-tumor tissues. Ai Zheng.
19150–152. (155)2000.(In Chinese).
|
|
7
|
Pourgholami MH and Morris DL: Inhibitors
of vascular endothelial growth factor in cancer. Cardiovasc Hematol
Agents Med Chem. 6:343–347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dai B, Wang DS, Zhang Y, Zhou L, Liu J and
Sun W: The role of insulin-like growth factors in tumor. Zhong Hua
Lin Chuang Yi Shi Za Zhi (Dian Zi Ban). 7:7099–7102. 2013.(In
Chinese).
|
|
9
|
Ding ZX, Zhang LZ and Ling L: Levels of
matrix metalloproteinase and vascular endothelial growth factor in
patients with primary liver cancer. Gan Zang. 10:214–215. 2005.(In
Chinese).
|
|
10
|
Liao D, Fan Z and Huang R: Clinical Report
of treating uterine fibroids and uterine adenomyosis with uterine
artery embolization. Wei Chuang Yi Xue. 7:256–258. 2012.(In
Chinese).
|
|
11
|
Gu J, Guan Q, Ji W and Ren W: The
influence of serum HIF-1a and VEGF content on the prognosis in
patients with hepatocellular carcinoma after TACE. Jie Ru Fang She
Xue Za Zhi. 23:142–146. 2014.(In Chinese).
|
|
12
|
Marina O, Suh JH, Reddy CA, Barnett GH,
Vogelbaum MA, Peereboom DM, Stevens GH, Elinzano H and Chao ST:
Treatment outcomes for patients with glioblastoma multiforme and a
low Karnofsky Performance Scale score on presentation to a tertiary
care institution. Histopathology. J Neurosurg. 115:220–229. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahmad A, Qadan L, Hassan N and Najarian K:
Uterine artery embolization treatment of uterine fibroids, effect
on ovarian function in younger women. J Vasc Interv Radiol.
13:1017–1020. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pelage JP, Cazejust J, Pluot E, Le Dref O,
Laurent A, Spies JB, Chagnon S and Lacombe P: Uterine fibroid
vascularization and clinical relevance to uterine fibroid
embolization. Radiographics. 25((Suppl 1)): S99–S117. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L: Clinical study of treating uterine
fibroids with uterine artery embolization. Zhong Guo Yi Yao.
2:238–241. 2012.(In Chinese).
|
|
16
|
Qu W, Guo P and Wu Y: Clinical research of
uterine artery embolization for symptomatic hysteromyoma. J Pract
Med Tech. 18:573–576. 2011.
|
|
17
|
Ji W, Xin X and Chen B: The influence of
insulin-like growth factor 1 and insulin-like growth factor 1
receptor on the growth of uterine leiomyoma. Zhong Guo Zhong Liu
Lin Chuang Yu Kang Fu. 10:24–26. 2003.(In Chinese).
|