Introduction
The most common cause of female pseudo-hermaphrodism
is congenital adrenal hyperplasia (CAH); however this condition may
also be caused by an androgen-secreting adrenal tumor (1–3).
Androgen-secreting adrenal tumors, also known as virilizing adrenal
tumors, produce excessive amounts of androgen and are primarily
found in females. In pre-adolescent females, this presents as
pseudo-hermaphrodism (4), whilst for
adolescent and adult females this presents as varied degrees of
virilization. The majority of tumors are malignant. The benign
tumors may be normalized with surgery; virilization may be
alleviated and the menses of the patients may resume (5). Therefore, early detection is extremely
important for treatment. However, there are limited methods for
early detection. Thus, in the present study, a case of
pseudo-hermaphrodism caused by an adrenal adenoma in an adolescent
female is presented and the possible mechanism investigated, with
the hope that this may guide early diagnosis and treatment.
Case report
Case summary
A 15-year-old female was admitted to the Department
of Endocrinology, PLA General Hospital (Beijing, China) on August
10, 2011 ago due to abnormal external genitalia, discovered 12
years previously. Since childhood, the patient had dark skin, a
low, deep voice, excessive body hair and an enlarged clitoris. From
the age of 12 years, breast development started and scanty dark
hairs appeared above the patient's lip. The results from the
physical examination upon admission were as follows: height, 138
cm; body weight, 36 kg; blood pressure, 170/120 mmHg; and heart
rate, 108 bpm. The breasts were symmetrical, Tanner Stage II.
Hypertrophy of the clitoris was observed and the pubic hairs were
dark and thick, Tanner stage IV. The karyotype was 46, XX. As shown
in Table I, there was no abnormality
in the biochemical profile of the patient, nor were there
abnormalities in her growth hormone and insulin-like growth factor
1 (IGF-1) levels. Testosterone was observed to be elevated (3.35
nmol/l), and dehydroepiandrosterone (DHEAS) and
17-hydroxyprogesterone levels were slightly elevated. Circadian
rhythm of adrenocorticotropic hormone (ACTH) and cortisol, as well
as serum and urinary aldosterone levels and renin activities, were
normal.
 | Table I.Laboratory results prior to and
following surgery. |
Table I.
Laboratory results prior to and
following surgery.
| Variable | Prior to surgery | Following
surgery | Normal range |
|---|
| Serum potassium
(mmol/l) | 3.54 | 4.29 | 3.5–5.5 |
| Urinary free cortisol
(nmol/l) | 440.5 | 185.5 | 98–500 |
| Serum cortisol at
8:00 a.m. (nmol/l) | 245.9 | – | 198.7–797.5 |
| Cortisol
(nmol/l) | 25.6 | – | – |
| Serum ACTH at 8:00
a.m. (pmol/l) | 7.64 | – | <10 |
| ACTH (pmol/l) | 1.11 | – | – |
| Testosterone
(nmol/l) | 3.35 | 0.34 | 0.5–2.6 |
| LH (mIU/ml) | 14.63 | 11.9 | 0.5–76.3 |
| FSH (mIU/ml) | 6.42 | 4.14 | 1.5–33.4 |
| E2 (pmol/l) | 221.3 | 343.2 | 48.2–1531.8 |
|
17-hydroxyprogesterone (ng/ml) | 3.45 | 0.38 | 0.6–3.34 |
| DHEAS (µg/dl) | 464 | 15.0 | 35–430 |
X-ray imaging revealed closed epiphyses. The
ultrasound (U/S) investigation showed normal ovaries and a small
uterus. A non-uniform mass was detected at the right adrenal area
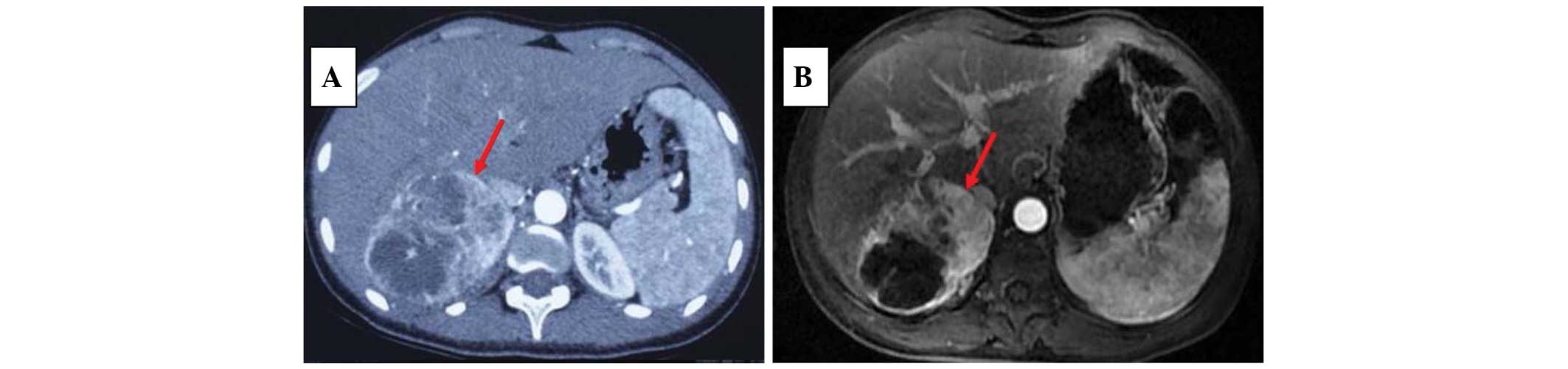
by U/S, which was ~11.4×6.9×9.4 cm in size. Abdominal computed
tomography (CT) scans indicated that it was a mixed cystic and
solid occupying mass in the right adrenal area, with non-uniform
density and enhancement with contrast (Fig. 1A). This is consistent with the
magnetic resonance imaging (MRI) scan, in which a large solid mass,
rich in lipids and vessels, with local necrosis was observed
(Fig. 1B).
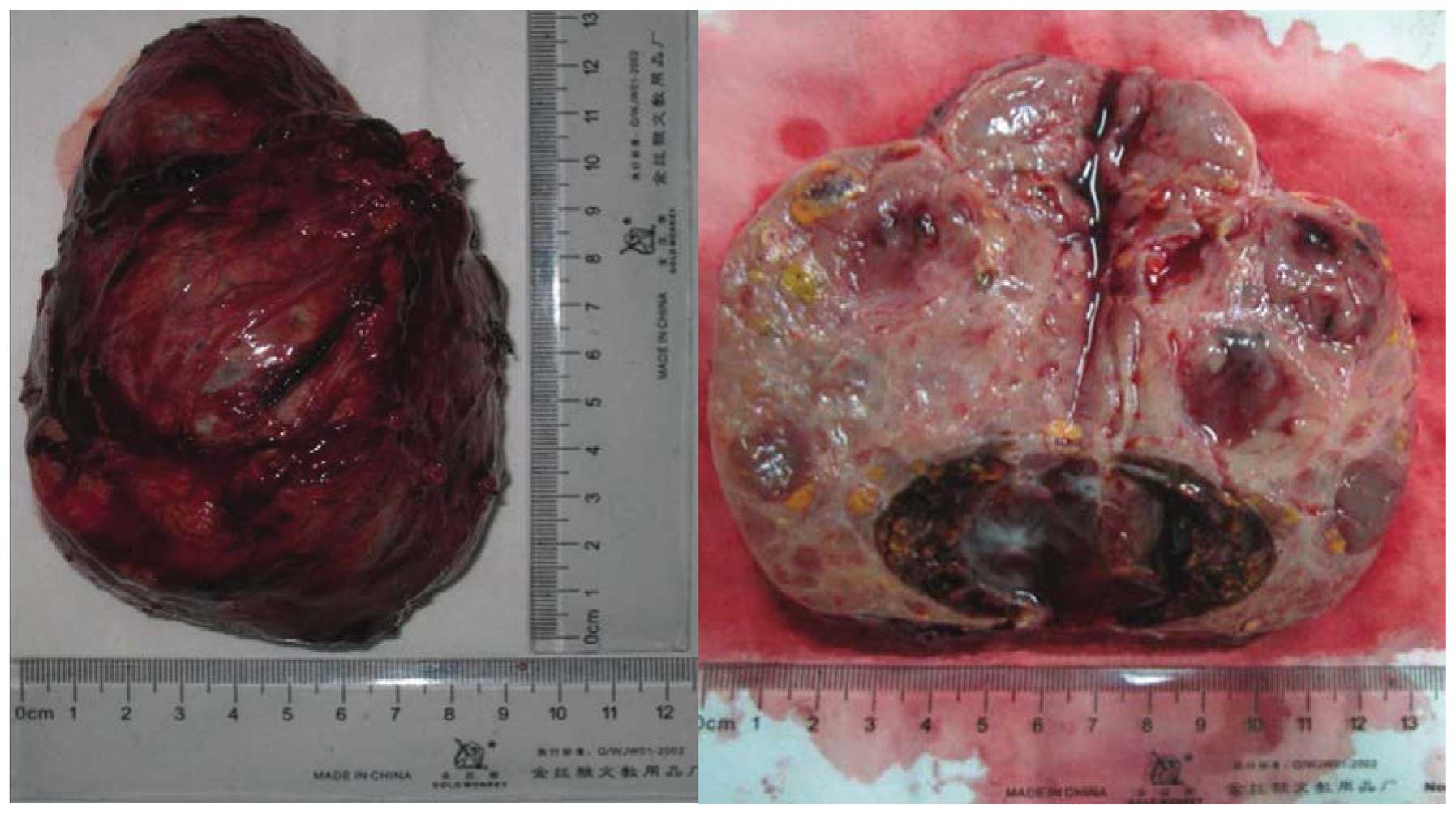
The patient was diagnosed with an androgen-secreting
adrenal adenoma. The tumor was removed with an intact envelope
(Fig. 2). Pathology confirmed that
it was an adrenal adenoma (Fig. 3).
Four days following the surgery, testosterone, DHEAS and
17-hydroxyprogesterone levels dropped (Table I). After three months, the patient
experienced her menarche, the amount of body hair was reduced and
there was no further development of the breasts. The blood pressure
of the patient remained at 130–140/90–100 mmHg without
antihypertensive medication.
Materials and methods
Tissue samples
Tumor tissues were obtained from the adrenal adenoma
and control samples were obtained from normal adrenal tissues.
Immediately upon removal, the tissues were stored in liquid
nitrogen for further analysis. The present study was approved by
the Ethics Committee of the PLA general hospital and written
informed consent was obtained from the patient prior to
participation in the study.
Immunohistochemical staining
Sections were immunostained for luteinizing
hormone/human chorionic gonadotrophin (LH/hCG) receptors using the
avidin-biotin immunoperoxidase method and detected with
3,3′-diaminobenzidine. Rabbit anti-human LH receptor (LHR)
polyclonal antibody was used as the primary antibody and
biotin-labeled goat anti-rabbit immunoglobulin G (IgG) as the
secondary antibody (Bioss, Inc., Woburn, MA, USA).
Under a microscope, brown staining of the membrane
was regarded as positive expression. Ten fields were randomly
selected for each slice. The results were graded according to the
percentage of positive cells among the same type of cells as
follows: (−), no positive cells visible or the number of positive
cells was <25%; (+), number of positive cells was 25–50%; (++),
number of positive cells was 50–75%; (+++) number of positive cells
was >75%.
Enzyme-linked immunosorbent assay (ELISA)
The ABC ELISA kits were purchased from Shanghai
XiTang Biotechnology Co., Ltd. (Shanghai, China). The standard
solutions were prepared and the assays of tissue sample homogenates
were conducted according to the manufacturer's instructions. The
integrated optical density was measured at 450 nm for
3β-hydroxysteroid dehydrogenase 2 (HSD2), cytochrome P450
17α-hydroxylase (CYP17) and 17β-hydroxysteroid dehydrogenase 3
(HSD3) to determine the activities of these enzymes.
Quantitative polymerase chain reaction
(qPCR)
qPCR was performed to detect the relative quantities
of the mRNA of GAPDH (control) 3β-HSD2, 17β-HSD3, CYP17 and LH/hCGR
genes using SYBR Green RT-qPCR reagents kit (Applied Biosystems™,
Foster City, CA, USA) according to the instructions of the
manufacturer and as previously described (6). The primers were as follows: GAPDH
forward, 5′-caatgaccccttcattgacc-3′ and reverse,
5′-GACAAGCTTCCCGTTCTCAG-3′; 3β-HSD2 forward,
5′-AGCTTCCTACTCAGCCCAAT-3′ and reverse, 5′-TACCCACATGCACATCTCTG-3′;
17β-HSD3 forward, 5′-GGCTGCTCCTGACACACTAT-3′ and reverse,
5′-TTCAGCGGACTAGGTTGAAG-3′; CYP17 forward,
5′-ACATGCTGGACACACTGATG-3′ and reverse, 5′-CAGGGTCCATTTAACCACAG-3′;
LH/hCGR forward, 5′-GCCAATCCATTTCTGTATGC-3′ and reverse,
5′-GTGCAATGTGGACAACTTCA-3′.
Statistical analysis
IBM SPSS statistics software, version 19.0, was used
to conduct the statistical analyses (IBM Corp., Armonk, NY, USA)
and a t-test was performed for comparison between the groups. Data
are presented as the mean ± standard deviation, P<0.05 was
considered to indicate a statistically significant difference.
Results
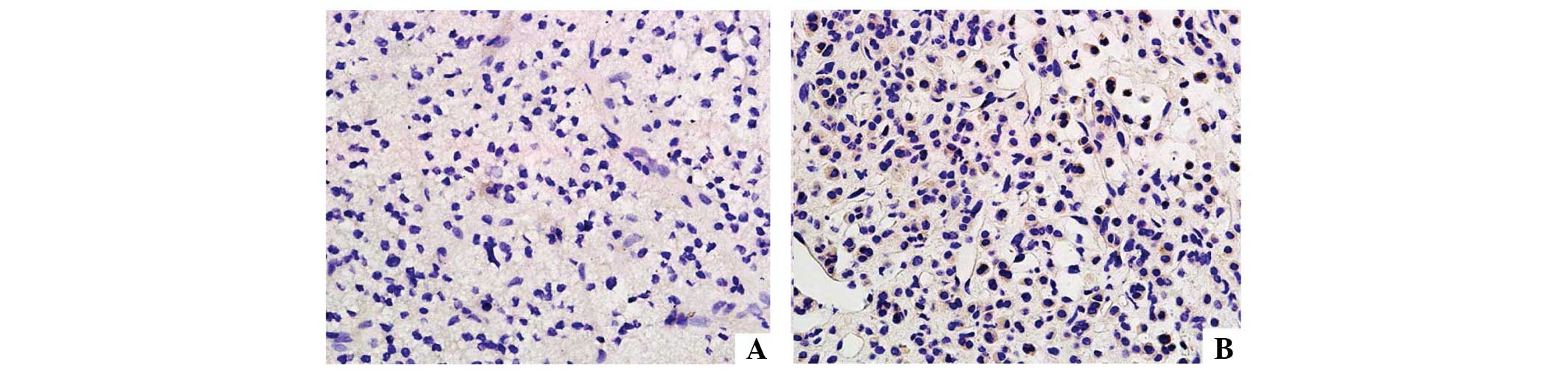
Immunohistochemistry for the LH/hCG receptor
As shown in Fig. 4,
the LH/hCG receptor was not expressed in tumor tissues (<25%
positive cells). However, in normal tissue high expression levels
were observed (+++, >75% positive cells).
3β-HSD2, 17β-HSD3 and CYP17 enzyme
activities
3β-HSD2, CYP17 and 17β-HSD3 concentrations in tissue
homogenates were detected by ABC-ELISA. The levels of 3β-HSD2
(1,819±10.8 pg/ml) and CYP17 (93.5±2.73 µmol/ml) in the tumor
tissue were higher than those of the normal tissues (1,458±29.6
pg/ml and 58.6±1.96 µmol/ml, respectively; P<0.01), while the
level of 17β-HSD3 (2,639±70.6 pg/ml) in the tumor tissue was lower
compared with that of normal tissues (9,148±383 pg/ml;
P<0.01).
Relative mRNA levels of 3β-HSD2, 17β-HSD3, CYP17
and LH/hCG receptor genes
As shown in Table
II, the mRNA levels of 3β-HSD2 and CYP17 were higher
(P<0.01), while those of 17β-HSD3 and LH/hCG receptor genes were
lower (P<0.01) in the tumor tissues than in the normal
tissues.
 | Table II.Relative mRNA levels of 3β-HSD2,
17β-HSD3, CYP17 and LH/hCG receptor genes in tissue
homogenates. |
Table II.
Relative mRNA levels of 3β-HSD2,
17β-HSD3, CYP17 and LH/hCG receptor genes in tissue
homogenates.
| mRNA | Normal tissue | Tumor tissue |
|---|
| 3β-HSD2 | 3.501±0.312 |
13.960±3.977a |
| 17β-HSD3 | 4.517±0.225 |
0.962±0.288a |
| CYP17 | 0.995±0.208 |
6.173±0.982a |
| LH/hCG receptor | 4.209±0.585 |
1.098±0.087a |
Discussion
Androgen-secreting adrenal tumors are rare. The
tumors are usually malignant; however, certain tumors may be benign
(7). It has reported that only 11
patients with purely androgen-secreting tumors were identified at
Mayo Clinic (Rochester, MN, USA) for more than >50 years
(8). In the past 18 years in the PLA
General Hospital (Beijing, China), only two cases (0.13%) have been
diagnosed as androgen-secreting adrenal tumors among 1,494 cases of
adrenal tumors.
In the present case, virilization, hormone levels,
CT and MRI scans all supported the diagnosis of an
androgen-secreting adrenal tumor, which was further confirmed by
the pathological analysis. Following surgical removal of the tumor,
the testosterone level of the patient returned to normal,
suggesting that excessive amounts of testosterone had been secreted
by the tumor.
Testosterone levels were significantly elevated in
the patient due to the androgen-secreting adrenal adenoma. The
DHEAS and urinary 17-ketosteroid (17-KS) levels were increased
significantly but not suppressed by dexamethasone, suggesting their
autonomous and independent secretion without ACTH. Since the levels
of CYP21 and CYP11 are normal or reduced, the aldosterone, cortisol
and urinary metabolite 17-hydroxycorticosteroid (17-OHCS) levels
are usually normal (9,10). Thus, only a quarter of virilizing
adrenal tumors are accompanied by Cushing's syndrome and the
majority of them are malignant (11). In the present case, circadian rhythm
of ACTH and cortisol, urinary free cortisol, serum and urinary
aldosterone levels and renin activity were all normal, indicating
that the adrenal mass did not secrete excessive amounts of cortisol
and aldosterone.
Previous studies have demonstrated that 3β-HSD and
17β-HSD3 but not 11β-HSD, are highly expressed in
androgen-secreting adrenal tumors, suggesting that the abnormal
secretion of androgen may be associated with changes of enzyme
activities during steroid synthesis (12,13). The
elevated enzyme activity of 3β-HSD and CYP17 drives the synthesis
towards androgen production, and subsequently to the production of
DHEA and 17-hydroprogesterone, some of which is then converted into
estradiol and testosterone (14).
This may explain the serum levels observed in the patient of the
present study, and suggest a simple method for the early detection
of androgen-secreting adrenal tumors.
In addition, the results of present study
demonstrated that LH/hCG receptors were expressed in adrenal cortex
cells. These receptors regulate steroid synthesis and secretion,
and may stimulate cortisol or androgen secretion when LH/hCG is
present. Numerous studies have confirmed that Cushing's syndrome
and hyperaldosteronism are closely associated with the
overexpression of LH/hCG receptors (15,16).
Although a few cases of an association between the adrenal
overexpression of the LH/hCG receptor and virilization have been
reported (17,18), studies investigating the association
between androgen synthesis and the LH/hCG receptor are limited.
The results of the present study showed there was no
expression LH/hCG receptor in normal adrenal tissue, suggesting
negative feedback from accelerated steroid synthesis caused by
elevated 3β-HSD and CYP17 enzyme activities. Furthermore, other
hormones, including ACTH, cortical androgen-stimulating hormone,
pro-opiomelanocortin, IGF-1 and prolactin regulate androgen
secretion (19–21). However, this requires further
study.
In the present report, a rare case of an
androgen-secreting adrenal tumor is presented. Due to the limited
clinical samples and materials, this study focused on the tissue
from a single case and investigated the possible mechanism;
however, it may not be representative of all cases.
In conclusion, the results from the present study
suggest that virilization in adrenal adenoma may be associated with
the overexpression of 3β-HSD and CYP17, resulting in elevated
enzymatic activities, but not the expression of the LH/hCG receptor
in adrenal cells. To the best of our knowledge, this is the first
study indicating this association, and it may represent a potential
target for early diagnosis and treatment.
Acknowledgements
The authors would like to thank the patient who
consented to this study and all the staff involved in this
study.
References
|
1
|
Rohana AG, Ming W, Norlela S and Norazmi
MK: Functioning adrenal adenoma in association with congenital
adrenal hyperplasia. Med J Malaysia. 62:158–159. 2007.PubMed/NCBI
|
|
2
|
Bédrine H D.M..Mangeot JP and Bégueri F:
Familial male pseudo-hermaphrodism of karyotype XY and ambiguous
morphology. Gynecol Obstet (Paris). 67:15–28. 1968.(In French).
PubMed/NCBI
|
|
3
|
Ben Charfeddine I, Riepe FG, Kahloul N,
Kulle AE, Adala L, Mamaï O, Amara A, Mili A, Amri F, Saad A,
Holterhus PM and Gribaa M: Two novel CYP11B1 mutations in
congenital adrenal hyperplasia due to steroid 11β hydroxylase
deficiency in a Tunisian family. Gen Comp Endocrinol. 175:514–518.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choukair D, Beuschlein F, Zwermann O, Wudy
SA, Haufe S, Holland-Cunz S and Bettendorf M: Virilization of a
young girl caused by concomitant ectopic and intra-adrenal adenomas
of the adrenal cortex. Horm Res Paediatr. 79:318–322. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakamura A, Schimizu C, Nagai S, Taniguchi
S, Umetsu M, Atsumi T, Wada N, Yoshioka N, Ono Y, Sasano H and
Koike T: Unilateral adrenalectomy improves insulin resistance and
polycystic ovaries in a middle-aged woman with virilizing
adrenocortical adenoma complicated with Cushing's syndrome. J
Endocrinol Invest. 30:65–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang S, Kamat A, Pergola P, Swamy A, Tio F
and Cusi K: Metabolic factors in the development of hepatic
steatosis and altered mitochondrial gene expression in vivo.
Metabolism. 60:1090–1099. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanaka S, Tanabe A, Aiba M, Hizuka N,
Takano K, Zhang J and Young WF: Glucocorticoid- and
androgen-secreting black adrenocortical adenomas, unique cause of
corticotropin-independent Cushing syndrome. Endocr Pract.
17:e73–e78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cordera F, Grant C, van Heerden J,
Thompson G and Young W: Androgen-secreting adrenal tumors. Surgery.
134:874–880. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lipsett MB, Hertz R and Ross GT: Clinical
and pathophysiologic aspects of adrenocortical carcinoma. Am J Med.
35:374–383. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukushima DK and Gallagher TF: Steroid
Production in ‘Nonfunctioning’ Adrenal Cortical Tumor: J Clin
Endocrinol Metab. 23:923–927. 1963.
|
|
11
|
Kim BY, Chun AR, Kim KJ, Jung CH, Kang SK,
Mok JO and Kim CH: Clinical characteristics and metabolic features
of patients with adrenal incidentalomas with or without subclinical
Cushin's syndrome. Endocrinol Metab (Seoul). 29:457–463. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rodríguez-Gutiérrez R, Bautista-Medina MA,
Teniente-Sanchez AE, Zapata-Rivera MA and Montes-Villarreal J: Pure
androgen-secreting adrenal adenoma associated with resistant
hypertension. Case Rep Endocrinol. 2013:3560862013.PubMed/NCBI
|
|
13
|
Petersons CJ and Burt MG: The utility of
adrenal and ovarian venous sampling in the investigation of
androgen-secreting tumours. Intern Med J. 41:69–70. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nguyen PT, Lee RS, Conley AJ, Sneyd J and
Soboleva TK: Variation in 3β-hydroxysteroid dehydrogenase activity
and in pregnenolone supply rate can paradoxically alter
androstenedione synthesis. J Steroid Biochem Mol Biol. 128:12–20.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Groot JW, Links TP, Themmen AP,
Looijenga LH, de Krijger RR, van Koetsveld PM, Hofland J, van den
Berg G, Hofland LJ and Feelders RA: Aberrant expression of multiple
hormone receptors in ACTH-independent macronodular adrenal
hyperplasia causing Cushing's syndrome. Eur J Endocrinol.
163:293–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rask E, Schvarcz E, Hellman P, Hennings J,
Karlsson FA and Rao CV: Adrenocorticotropin-independent Cushing's
syndrome in pregnancy related to overexpression of adrenal
luteinizing hormone/human chorionic gonadotropin receptors. J
Endocrinol Invest. 32:313–316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morris LF, Park S, Daskivich T, Churchill
BM, Rao CV, Lei Z, Martinez DS and Yeh MW: Virilization of a female
infant by a maternal adrenocortical carcinoma. Endocr Pract.
17:e26–e31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujieda K and Ito Y: Inactivating and
activating mutations of the human LH/hCG receptor leading to male
pseudohermaphroditism and familial male-limited precocious puberty.
Nihon Rinsho. 60:265–271. 2002.(In Japanese). PubMed/NCBI
|
|
19
|
Lai KP, Huang CK, Fang LY, Izumi K, Lo CW,
Wood R, Kindblom J, Yeh S and Chang C: Targeting stromal androgen
receptor suppresses prolactin-driven benign prostatic hyperplasia
(BPH). Mol Endocrinol. 27:1617–1631. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mieritz MG, Sorensen K, Aksglaede L,
Mouritsen A, Hagen CP, Hilsted L, Andersson AM and Juul A: Elevated
serum IGF-I, but unaltered sex steroid levels, in healthy boys with
pubertal gynaecomastia. Clin Endocrinol (Oxf). 80:691–698. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Monroe WE, Panciera D and Zimmerman KL:
Concentrations of noncortisol adrenal steroids in response to ACTH
in dogs with adrenal-dependent hyperadrenocorticism,
pituitary-dependent hyperadrenocorticism, and nonadrenal illness. J
Vet Intern Med. 26:945–952. 2012. View Article : Google Scholar : PubMed/NCBI
|