Introduction
Cobalt oxide (Co3O4) is an
anti-ferromagnetic p-type semiconductor (with direct optical band
gaps at 1.48 and 2.19 eV), which is considered to be among the most
promising functional materials as it has gas-sensing, catalytic and
electrochemical properties. Co3O4 has
therefore been widely investigated for its potential application in
solid state sensors, electrochromic devices and heterogeneous
catalysts, as well as in lithium batteries (1–3).
Nanostructured Co3O4 materials were
demonstrated to have magnetic, optical, field emission and
electrochemical properties that are used in various devices
(2,4–7).
Notably, the shape and size-dependent properties of inorganic
nanomaterials have been the subject of numerous investigations,
with the aim of synthesizing Co3O4
nanomaterials with controlled size and shape (8–10).
Several techniques have been reported for the synthesis of
Co3O4 nanostructures with different
morphologies, including the nano-casting method for producing
Co3O4 nanowires (11), the surfactant-based template approach
for constructing Co3O4 nanoboxes (12), the mechano-chemical reaction method
for the synthesis of Co3O4 nanoparticles
(13), and the thermal decomposition
and oxidation method for the growth of Co3O4
nanorods (14). Cobalt nanowires are
converted to Co3O4 nanotubes by calcination
at 600°C in air for an extended period of time (15), followed by heating of a cobalt foil
for 12 h in air at 350°C which leads to the growth of
Co3O4 nanowalls (16). This nanostructured
Co3O4 has been investigated for a range of
shape and size-dependent properties.
The size reduction of inorganic materials into the
nanoscale facilitates numerous properties and technological
applications; however, the increase of surface area and reduction
in density of the nanoparticles poses a risk to the human body and
ecological environment compared with bulk materials. Cobalt
exposure occurs from industrial use, exposure from the environment,
and due to medical implants, and such exposure has been reported to
result in lung fibrosis and asthma (17–21). In
addition, cobalt is a known carcinogenic in animals (17,19,22).
However, the toxicity of cobalt nanoparticles is not well
understood. Furthermore, cobalt toxicity is complicated by the fact
that unlike other metals, the biological toxicity of cobalt
nanoparticles is not caused primarily by the cobalt ions released
(19). Cobalt ions and metals are
toxic, and studies that examined the genotoxic potential of
cobalt-based materials in vitro using cell culture systems
have shown that cobalt nanoparticles are highly genotoxic and
cytotoxic (19,23–28), and
are able to readily enter cells compared with cobalt ions. In
addition, these studies suggested that the genotoxic potential of
cobalt nanoparticles is contributed primarily by its ability to
generate/induce reactive oxygen species (ROS). Cobalt-chrome
nanoparticles have also been demonstrated to trigger
pro-inflammatory changes, including the secretion of tumor necrosis
factor-α (29).
Co3O4 nanoparticles
(Co3O4-NPs) have been shown to readily enter
cells and trigger ROS generation (30). Similarly, tungsten carbide-cobalt
nanoparticles are also genotoxic (31), inducing pro-inflammatory changes
including nuclear factor-κB and activator protein-1 activation
(32) and stimulating stress
signaling pathways such as mitogen-activated protein kinase. In
addition, cobalt-ferrite nanoparticles have been demonstrated to
possess genotoxic potential, as demonstrated by its interaction
with nucleic acids (33). It should
be noted that the majority of toxicity studies on cobalt-based
nanoparticles have been conducted using cell culture, and only
recently have investigations focused on biological toxicity in
vivo. A previous report on the toxicity of
Co3O4-NPs demonstrated that the compound was
cytotoxic, as determined by lactate dehydrogenase and acid
phosphatase release in microalgae and isolated cells from bivalve
gills (34). Phytoxicity of
Co3O4-NPs has also been demonstrated in
Allium cepa (35). These
studies suggest that Co3O4-NP is equally
toxic against plants and animals. A report by Falfushynska et
al (36) examined the in
vivo toxicity of cobalt from nanoscale composites in the fish
species Carassius auratus. Hepatic toxicity of cobalt was
demonstrated with metallothionein induction and apoptosis (36). Therefore, more in depth in
vivo studies are required to elucidate the mechanism underlying
the toxicity and biological responses to
Co3O4-NPs. It should be noted that toxicity
of nanoparticles is influenced by the shape (morphology), size, and
surface characteristics of the particle, in addition to their
interactions with one another and other molecules in vivo
(37). This may be important in
terms of the application of nanoparticles in biological systems.
With regards to cobalt-based nanomaterials, the characteristics
that are able to influence their toxicity have yet to be
elucidated. Therefore, the present study aimed to investigate the
influence of Co3O4-NPs morphology on its
toxicity using a zebrafish model.
Co3O4 nanoparticles with block
and sphere morphology were synthesized by changing the surfactants,
and the effect of morphology on toxicity in zebrafish was examined
by monitoring the biomarkers of the nanoparticles. The synthesized
nanoparticle structure, size and morphologies were verified by
powder X-ray diffraction pattern (PXRD) and field emission scanning
electron microscopy (FE-SEM). The use of tartarate as a
structure-controlling agent produced Co3O4
nanoparticles with block morphology (200 nm to 1 µm), whereas the
use of citrate produce spherical morphology (40–60 nm). Toxicity
studies revealed that Co3O4 morphology
influences the toxicity of the nanoparticles, with spherical
Co3O4 nanoparticles eliciting higher levels
of nitric oxide (NO) in the liver, and Co3O4
nanoparticles with block or plate morphology eliciting increased
inhibitory effects on the liver, and reducing glutathione and brain
acetylcholinesterase activity. These results suggested that
nanoparticle morphology, which often exhibits different material
properties, also exhibits various toxicity levels, and this could
have important implications for their biological applications.
Materials and methods
Chemicals
Cobalt chloride (CoCl2.6H2O),
sodium tartarate, and trisodium citrate were obtained from Sun
Pharmaceutical Industry, Ltd. (Mumbai, India). 2-Nitrobenzoic acid
(DTNB), acetylcholine iodide, epinephrine, napthylethylenediamine
dihydrochloride, and sulphanilamide were purchased from
Sigma-Aldrich (St. Louis, MO, USA). All other chemicals and
reagents were of the highest analytical grade and commercially
available.
Preparation of
Co3O4-NP block
(Co3O4-NP-B) and sphere
(Co3O4-NP-S) nanoparticles
In a typical experiment, 1.2 g
CoCl2·6H2O was dissolved in 10 ml distilled
water to form a 0.005 M solution. Organic structure-directing
agent, trisodium citrate (2.94 g, 0.01 M) was separately dissolved
in 10 ml distilled water. The solutions were mixed together and
vigorously agitated at room temperature for 6 h. The formed
precipitate was separated by centrifuging the solution at 1,872 × g
for 2 min. The product was washed with distilled water and dried
under vacuum (Rex RX-1S; SKU Vacuum Pump). Finally, 1.5 g dried
powder was calcined at 580–600°C in a high-temperature muffle
furnace (#05; ThermalTech Engineering, Inc., Chennai, India) in air
for 2 h, and stored in the furnace until cooled to room
temperature. The second sample was prepared by following a similar
protocol, but using sodium tartarate (0.01 M) as the organic
structure-directing agent instead of sodium citrate.
Nanoparticle characterization
Co3O4 morphology and sizes
were analyzed using FE-SEM (model JSM-6701F; JEOL Ltd., Tokyo,
Japan) with a 30 kV accelerating voltage and filament current of 20
mA for 45 sec. The samples adhered onto a double-face conducting
carbon tape mounted on a brass stub. The samples were then coated
with platinum which was sputtered at a current of 20 mA for 45 sec.
Powder X-ray diffraction measurements were conducted using a Bruker
diffractometer (XRD-Bruker D8 Advance XRD; Brucker Co., Billerica,
MA, USA) with Cu Kα radiation.
Animal acclimatization
A total of 60 adult zebrafish (Danio rerio)
irrespective of gender (length, 4–5 cm; weight, ~300 mg), were
purchased from Angel Aquarium (Thanjavur, India). The fish were fed
ad libitum, tanks cleaned and sterilized, and water replaced
periodically. Water quality was monitored regularly, and its
temperature was maintained at 25±2°C. The fish were allowed to
acclimatize for one week prior to nanoparticle exposure. The fish
were randomly assigned to seven groups: Control group, and groups
exposed to Co3O4-NPs at 1, 5, 10, 50, 100 and
200 ppm concentrations, with six fish in each group (200 ml/tank).
All fish studies were conducted in accordance with the
institutional animal ethics committee recommendations of SASTRA
University (Thanjavur, India).
Co3O4
exposure
Co3O4-NPs (1, 5, 10, 50, 100
and 200 ppm for Co3O4-NP-B and Co3O4-NP-S
morphologies, respectively) were prepared in tap water and
sonicated prior to addition to the exposure tanks, using an Oscar
Ultrasonics Pvt. Ltd. device. Six fish were used for each
concentration and the exposure period was 15 days. The exposure was
replicated twice with six fish/group. All assays were performed in
duplicate. Water and Co3O4 nanoparticles were
renewed every day. The water parameters were the following:
Dissolved oxygen, 8.5±1.3 mg/l; pH, 7.58; total hardness
(CaCO3), 145±8.5 mg/l; chlorides, 73±3 mg/l; calcium,
4.3±0.7 mg/l; magnesium, 2.5±0.4 mg/l; alkalinity, 352±9.6 mg/l;
total dissolved solids, 250±5 mg/l; and temperature, 25±2°C.
Tissue sample preparation
Following exposure (15 days), the fish were
anesthetized with 150 mM tricaine mesylate (Sigma-Aldrich) and
euthanized by decapitation. The skin was removed and the liver or
brain tissue samples from two fish of the same group were
homogenized in ice-cold buffer (Tris-HCl, 0.1 M, pH 7.4). The
tissue samples were then centrifuged (10,000 × g, 10 min, 4°C) and
the supernatant was collected and used for analyses. The brain
tissue samples were also homogenized for acetylcholinesterase
(AChE) assay. All assays were performed in duplicate.
Estimation of NO
NO was measured spectrophotometrically as previously
described (38), using an Evolution
201 spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). A total of 200 µl liver homogenate was mixed with Tris-HCl
(pH 7.4) to form 300 µl. To this, 100 µl 0.1%
napthylethylenediamine dihydrochloride and 100 µl 1% sulfanilamide
were added. The solution was incubated for 10 min at room
temperature prior to centrifugation (12,000 × g, 15 min, 4°C).
Optical density of the samples was measured at 540 nm against a
blank containing buffer instead of the homogenate. The activity
levels were expressed as µM nitrite.
Estimation of reduced glutathione
(GSH)
GSH levels were analyzed as previously described
(39). A total of 750 µl liver
homogenate was mixed with 0.5 ml 10% trichloroacetic acid and
centrifuged (11,000 × g, 15 min, 4°C). The protein-free supernatant
was added to 250 µl 0.2 M disodium phosphate (pH 8.0) and 1 ml 0.6
mM DTNB (Qualigens Fine Chemicals; Thermo Fisher Scientific, Inc.,
Prabhadevi, India). The absorbance of the resulting yellow colored
solution was read spectrophotometrically at 412 nm. GSH was
expressed as µM/g weight.
AChE assay
AChE activity levels were measured using the
Edmann's degradation method (40).
Briefly, 100 µl brain homogenate was added to 800 µl 100 mM sodium
phosphate buffer (pH 7.5). To this mixture 50 µl 10 mM DTNB
solution was added and the enzymatic reaction was initiated by
adding 50 µl 12.5 mM acetylthiocholine iodide. The samples were
incubated at room temperature for 5 min until the development of a
yellow color. Optical density of the samples was then measured at
400 nm against a blank containing buffer instead of sample. The
activity levels were expressed as µM acetylthiocholine
hydrolyzed/min.
Protein estimation
Protein was estimated using the method described by
Lowry et al (41).
Statistical analyses
All assays were performed in duplicates. Results
were expressed as the mean ± standard deviation of a minimum of six
experiments. All exposure experiments were replicated twice with
six fish in each group. The effects of an increase in nanoparticle
dose on biochemical parameters was analyzed using Pearson's
coefficient of correlation. One-way analysis of variance was
followed by Tukey's post-hoc test to evaluate the significant
difference between the control and
Co3O4-NPs-treated fish belonging to the same
group. Significant differences between the mean of the two types of
Co3O4-NPs (Co3O4-NP-B and
Co3O4-NP-S) were analyzed using a two-tailed
Student's t-test. P<0.05 was considered to indicate a
statistically significant result.
Results and Discussion
Co3O4
nanoparticle synthesis and characterization
Two organic structure-directing agents (sodium
tartarate and trisodium citrate) were used to synthesize
Co3O4 nanoparticles with different
morphologies. The calcinations of the cobalt-tartarate complex
produced block morphologies of Co3O4, whereas
those of cobalt-citrate complexes produced nanospheres. The change
in morphologies may be due to the varying coordination of organic
structure-directing ligands. Cobalt with citrate ligands form
water-soluble coordination complexes and display varied
coordination modes depending on the conditions (42). Conversely, the addition of tartarate
immediately produces water insoluble precipitates (43). The coordination differences of
organic ligands have previously been used to modulate the
morphologies of nanoparticles (44).
The crystal structures of the products were verified by PXRD.
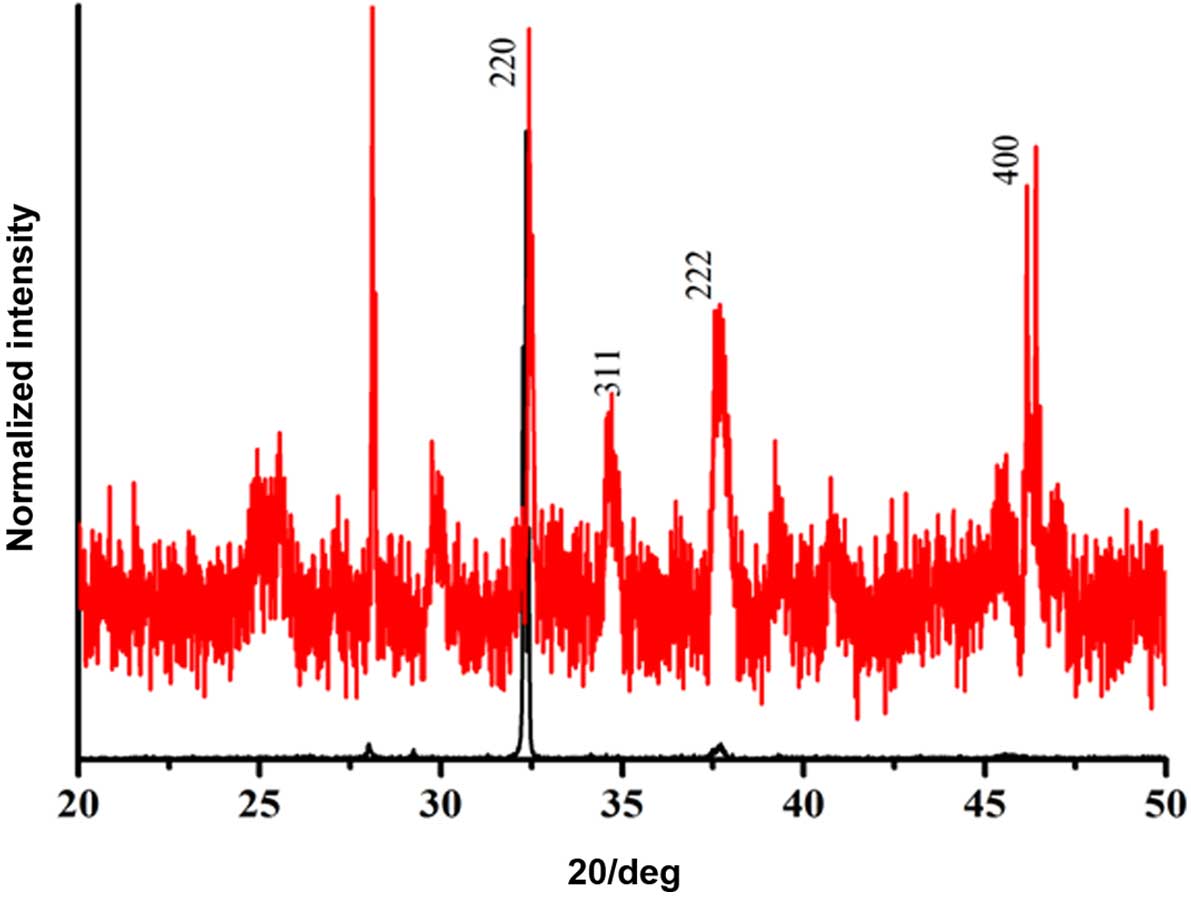
Fig. 1 shows the PXRD pattern of
Co3O4-NP-B and
Co3O4-NP-S. The diffraction peaks typical of
face-centered cubic Co3O4 are evident, and
are concordant with those of standard Co3O4
cubic structures (JCPDS card no. 42–1467) (45). The absence of impurity peaks
indicated the formation of pure Co3O4. The
marked intense single PXRD peak at 220 planes for
Co3O4-NP-B indicated adequate crystallinity
of the sample with flat morphology. The PXRD of
Co3O4-NP-S also determined the crystallinity,
although this was decreased compared with
Co3O4-NP-B, and the increased number of peaks
was due to the various crystallographic faces present in sphere
morphologies. Fig. 2 shows the
FE-SEM images of the Co3O4 products
Co3O4-NP-s and
Co3O4-NP-B synthesized by the calcination of
citrate (Fig. 2A–C) and tartarate
(Fig. 2D–F) cobalt complexes,
respectively. Co3O4-NP-S showed the presence
of aggregated spherical nanostructures. The high magnification
image shows the aggregation of many smaller spheres (40–60 nm)
(Fig. 2C).
Co3O4-NP-B exhibits clear blocks with 100–200
nm thickness and a length of ≥1 µm. The high magnification image
shows small pores on the sides of the blocks (Fig. 2F).
Morphology-dependent toxicity of
Co3O4-NPs
The present study aimed to examine the effects of
Co3O4-NP morphology on its toxicity using a
zebrafish model. Two forms of Co3O4-NPs were
successfully synthesized and characterized; one with block
morphology (Co3O4-NP-B) and the other with
spherical morphology (Co3O4-NP-S).
Although cobalt may be present in low quantities in
freshwater, cobalt concentrations can be high in water bodies near
ore and coal mining sites, as well as near textile industries
(46). Furthermore, environment
agencies such as Environment Canada and the United Nations
Environment Programme (UNEP), in addition to the Inter-Organization
Programme for the Sound Management of Chemicals, have primarily
examined cobalt and inorganic cobalt compounds (www.inchem.org/documents/cicads/cicads/cicad69.htm#10.2).
The report by Environment Canada presented the long-term toxicity
of cobalt in 15 species with values ranging from 2.9–59,000 µg/l
(http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=92F47C5D-1#a7).
The data compiled by Environment Canada also
demonstrated that invertebrates are more sensitive to cobalt than
fishes. The most sensitive fish was shown to be the zebrafish. The
data obtained from the Concise International Chemical Assessment
Documents compiled by the International Programme on Chemical
Safety of UNEP shows that in the case of water bodies close to
mining and agricultural areas, cobalt concentrations may range from
1–625,000 µg/l. In addition, cobalt concentrations in polluted lake
and river sediment are similar to those in the soil, and cobalt
concentration levels are higher in suspended sediment compared with
bottom sediment (47). This may be
due to the finer size of the suspended particles. This property
could also influence exposure patterns and subsequent
bioaccumulation in aquatic organisms. It should be noted that until
recently few studies have been conducted on the particle size or
morphology of cobalt in toxicity evaluations. However, recent
studies on cobalt nanoparticles used 0.0001–1 mg/l in the sperm
cells of sea urchins (48,49) and 1–40 µg/ml in human respiratory
cells (50). The high concentrations
of cobalt nanoparticles in polluted aquatic systems and the lack of
reliable data on dosage led to the examination of 1–200 ppm
Co3O4 nanoparticles in the present study.
Furthermore, to derive baseline data on the influence of morphology
on toxicity, two types of Co3O4 nanoparticles
were compared at the same concentrations.
In the present study, the toxicity profile of the
two types of Co3O4-NPs were evaluated by
analyzing three biomarkers of nanoparticle toxicity; NO, GSH and
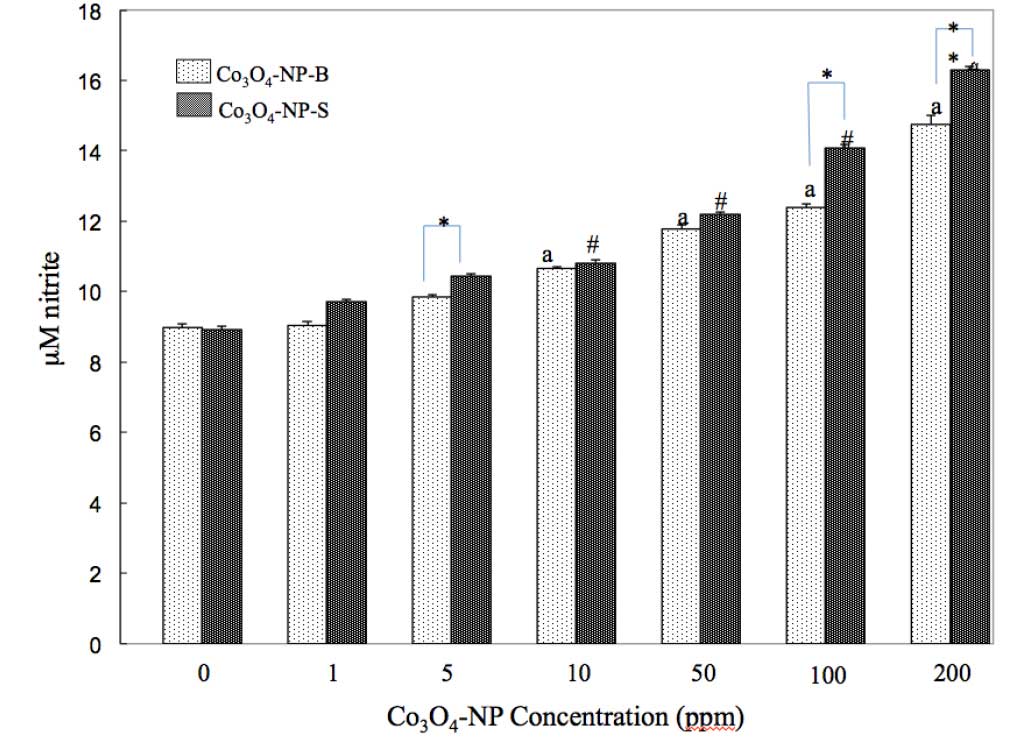
AChE. As shown in Fig. 3,
Co3O4-NP-B and
Co3O4-NP-S stimulated NO generation in the
liver of zebrafish, as compared with the control. NO levels
increased from 1–200 ppm in a dose-dependent manner following
exposure to both types of nanoparticle
(Co3O4-NP-B, r=0.953 and
Co3O4-NP-S, r=1.140). Both types of
nanoparticle induced a significant NO level increase at ≥5 ppm
(P<0.05), as compared with the control. In addition, the results
demonstrated that Co3O4-NP-S was more
effective (5–100 ppm, P<0.05; 200 ppm, P<0.01) at stimulating
NO generation, as compared with nanoparticles with block
morphologies (Co3O4-NP-B). The significant
difference in NO generation induced by the two types of
nanoparticles was most marked at 100 and 200 ppm. These results
suggested that Co3O4-NPs, like other
metal-based nanoparticles (51), are
capable of inducing ROS production in tissues, which may lead to
cellular damage. A previous study employing rodent and human cell
lines have demonstrated the genotoxicity of
Co3O4-NPs (30) and ROS has been suggested to be the
predominant cause of this genotoxic potential. The study also
reported that cobalt caused the rapid induction of ROS when
supplied as Co3O4-NPs compared with cobalt
ions (CoCl2), and the nano form had an increased ability
to enter into cells compared with the ionic form. In an in
vivo analysis using rats, subcutaneous implantation of
Co3O4-NPs was shown to result in nodule
formation as malignant mesenchymal tumors (52). Other studies employing in
vitro experiments have demonstrated superoxide and hydroxyl
radical generation by Co-Cr nanoparticles (27). Therefore,
Co3O4-NPs are capable of generating and/or
inducing ROS that, combined with the ability of
Co3O4-NPs to cross into cells (30), may result in widespread cellular
damage.
Co3O4-NPs are able to enter
cells and the nucleus, leading to cellular damage, as was
demonstrated by analyzing GSH activity levels in the liver tissue
samples of fish exposed to Co3O4-NPs. As
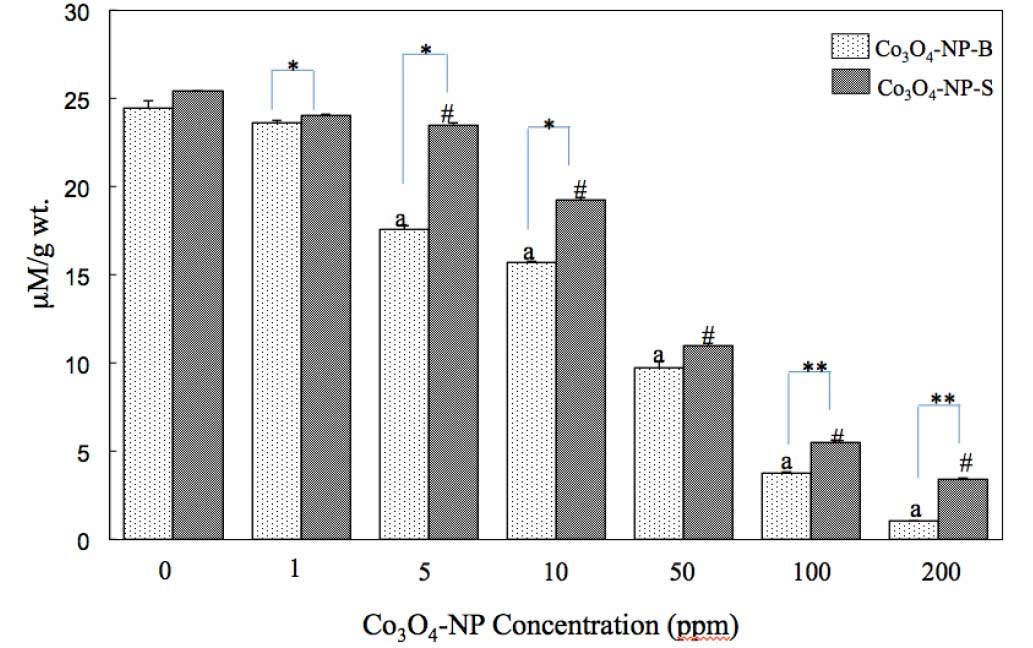
shown in Fig. 4, exposure to both
types of nanoparticle resulted in a reduction in liver GSH activity
levels in a dose-dependent manner
(Co3O4-NP-B, r=−0.359 and
Co3O4-NP-S, r=−0.429). However, when the
effect of morphology was analyzed, it appeared that
Co3O4-NP-B was a more potent inhibitor of
liver GSH activity compared with Co3O4-NP-S
(1, 5 and 10 ppm, P<0.05; 100 and 200 ppm, P<0.01).
Co3O4-NP-B was toxic at 5 ppm (P<0.05) and
GSH activity was almost entirely inhibited at 200 ppm. This was
unlike the response observed with NO generation, wherein
Co3O4-NP-S was more effective. These results
suggest that the morphology and concentration of the nanoparticles
may affect nanoparticle toxicity. Therefore,
Co3O4-NP-induced oxidative stress in cells
may be among the mechanisms underlying cobalt toxicity, and this
may result in loss of cell function.
Similarly to the results obtained with GSH, the two
types of Co3O4-NP proved to be toxic against
brain AChE (Co3O4-NP-B, r=−0.180 and
Co3O4-NP-S, r=−0.230), indicating that
synthesized Co3O4-NPs possessed the ability
to cross the blood-brain barrier and induce neuronal toxicity
(Fig. 5). This inhibition of AChE
could be attributed to the oxidative stress induced by
Co3O4-NPs. Between the two types of
nanoparticle, Co3O4-NP-B ameliorated the
inhibition of brain AChE activity, as compared with
Co3O4-NP-S (1, 5 and 10 ppm, P<0.05; 50,
100 and 200 ppm, P<0.01).
In the present study two types of
Co3O4-NPs were successfully synthesized, one
with a block morphology (≥100 and 500 nm) and the other spherical
(50–100 nm). The results of the present study demonstrated that
morphology of the nanoparticle influenced its toxicity in
vivo, as has been shown for other metal nanoparticles in
vitro (37).
Co3O4-NP-S was more effective at inducing NO
generation in the liver, whereas Co3O4-NP-B
was effective at inhibiting liver GSH and brain AChE activity.
These differences in toxicity show that distinct mechanisms may be
involved. In the case of spherical Co3O4-NPs,
it is possible that owing to their small size they are able to
easily cross the plasma membrane and accumulate inside cells,
resulting in ROS generation (30).
This increase in ROS generation may result in oxidative stress and
cellular damage such as inhibition of GSH and AChE. Conversely,
block or plate Co3O4-NPs were observed to be
more effective at causing inhibition of liver GSH and brain AChE
activity. This difference may be attributed to the fact that enzyme
inhibition by nanoparticles is primarily due to adsorption
(37). Although
Co3O4-NP-S has a larger surface area when
compared to Co3O4-NP-B, due to its smaller
size and high curvature Co3O4-NP-S helps to
preserve native protein structure and activity. For GSH, it is
possible that its adsorption onto Co3O4-NP-B
may result in its sequestration. These results are concordant with
those from a previous study which demonstrated that adsorption of
lysozyme onto larger sized spherical silica nanoparticles results
in increased loss of α-helicity, and thus unfolding, as compared
with smaller spheres (53). Similar
results have been obtained for soybean peroxidase (54), subtilisin (55), ribonuclease A (56), human carbonic anhydrase (57), α-chymotrypsin (58) and AChE (59).
Increased inhibition of GSH and AChE
Co3O4-NP-B may be a result of oxidative
stress, and may also be due to loss of native structure following
adsorption onto the ‘flat’ surface of the nanoparticle. Conversely,
the ability of spherical Co3O4-NPs to induce
higher levels of NO may be due to its ability to rapidly enter
cells. However, it should be noted that these effects of
nanoparticle morphology on enzyme adsorption and inactivation may
not be valid for all types of enzymes and nanoparticles (60), since concentration and surface
characteristics of nanoparticles may also influence their toxicity.
The toxicity of nanomaterials is dependent on its final state,
suggesting that nanoparticles may be modified by surface
modification, ion release, and interaction with biomolecules
(61). This ability of nanoparticles
to undergo changes makes it difficult to determine the mode of
toxicity. Nevertheless, nanoparticles are known to trigger
oxidative stress in cells (62).
This is also facilitated by their adverse effect on cellular
antioxidants, which further increases oxidative stress.
Nanoparticles such as nanosilver have been shown to interact with
proteins, leading to the formation of protein corona, protein
unfolding, and altered protein function (62). Therefore, it is possible to speculate
that a similar modification may occur with
Co3O4 nanoparticles and GSH, resulting in
loss of GSH activity. The importance of GSH in cellular homeostasis
is indicated by its role in numerous disease processes. GSH
supplies reducing equivalents (63),
and is therefore important for the regulation of cellular oxidative
stress (64). Cellular proteins
undergo irreversible modifications due to enhanced oxidative stress
(65), and thus affect protein
functions. Co3O4 nanoparticles may combine
with cellular proteins and interfere with GSH activity, leading to
loss of GSH activity and increase in cellular oxidative stress.
This may represent one of the mechanisms underlying nanoparticle
toxicity.
In conclusion, the present study demonstrated that
Co3O4-NPs exhibit toxicity, and the
morphology of Co3O4-NPs determined in part
their toxicity. Spherical morphology induced higher levels of ROS
and oxidative stress, while block or plate morphology were more
effective at inhibiting enzymes, which may have important
implications for their biological applications. This
morphology-dependent toxicity may be due to a change in the surface
area of the nanoparticles. Notably,
Co3O4-NP-S exhibited smaller-sized spherical
nanoparticles compared with micron-sized blocks of
Co3O4-NPs-B, and therefore has a higher
surface area. Furthermore, the micron-sized crystalline blocks of
Co3O4-NPs-B settle in water, whereas
Co3O4-NPs-S remains suspended in the water
column, which increases its chances of entering into the fish.
These results suggested that smaller-sized and low density
nanoparticles will have higher toxicity.
This study demonstrated that morphology influences
the biological toxicity of nanoparticles, with spherical
Co3O4 nanoparticles eliciting higher levels
of NO generation in the liver, and Co3O4
nanoparticles with block or plate morphologies exhibiting higher
inhibitory effects on GSH in the liver and AchE activity in the
brain. These results may have important implications for the
biological roles of these nanoparticles, and for the development of
strategies to inhibit the environmental toxicity of
nanoparticles.
Acknowledgements
The present study was supported by a grant from the
Science and Engineering Research Board of the Government of India,
(grant no. SERB/F/1266/2012-13, dated 31st May 2012). This study
was partially supported by the Russian Science Foundation (RSF-No
15-14-20032) which involved the participation of Dr Aristides
Michael Tsatsakis and Dr Kiril Sergeevich Golokhvast.
References
|
1
|
Ando M, Kobayashi T, Iijima S and Haruta
M: Optical recognition of CO and H2 by use of gas-sensitive
Au-Co3O4 composite films. J Mater Chem. 7:1779–1783. 1997.
View Article : Google Scholar
|
|
2
|
Li WY, Xu LN and Chen J: Co3O4
nanomaterials in lithium-ion batteries and gas sensors. Adv Funct
Mater. 15:851–857. 2005. View Article : Google Scholar
|
|
3
|
Ghosh M, Sampathkumaran EV and Rao CNR:
Synthesis and magnetic properties of CoO nanoparticles. Chem Mater.
17:2348–2352. 2005. View Article : Google Scholar
|
|
4
|
Wang RM, Liu CM, Zhang HZ, Chen CP, Guo L,
Xu HB and Yang SH: Porous nanotubes of Co3O4: Synthesis,
characterization, and magnetic properties. Appl Phys Lett.
85:2080–2082. 2004. View Article : Google Scholar
|
|
5
|
Wang X, Chen XY, Gao LS, Zheng HG, Zhang Z
and Qian YT: One-Dimensional arrays of Co3O4 nanoparticles:
Synthesis, characterization, and optical and electrochemical
properties. J Phys Chem B. 108:16401–16404. 2004. View Article : Google Scholar
|
|
6
|
Yang R, Wang Z, Liu J and Chen L: Nano
Co3O4 particles embedded in porous hard carbon spherules as anode
material for Li-ion batteries. Electrochem Solid-State Lett.
7:A496–A499. 2004. View Article : Google Scholar
|
|
7
|
Im Y, Lee C, Vasquez RP, Bangar MA, Myung
NV, Menke EJ, Penner RM and Yun M: Investigation of a single Pd
nanowire for use as a hydrogen sensor. Small. 2:356–358. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Zhuang J, Peng Q and Li Y: A
general strategy for nanocrystal synthesis. Nature. 437:121–124.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lou XWD, Archer LA and Yang Z: Hollow
Micro-/Nanostructures: Synthesis and Applications. Adv Mater.
20:3987–4019. 2008. View Article : Google Scholar
|
|
10
|
Li Y, Tan B and Wu Y: Mesoporous Co3O4
nanowire arrays for lithium ion batteries with high capacity and
rate capability. Nano Lett. 8:265–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
García-Pacheco G, Cabañas-Moreno JG,
Yee-Madeira H and Cruz-Gandarilla F: Co3O4 nanoparticles produced
by mechanochemical reactions. Nanotechnology. 17:2528–2535. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He T, Chen D, Jiao X and Wang Y: Co3O4
Nanoboxes: Surfactant-templated fabrication and microstructure
characterization. Adv Mater. 18:1078–1082. 2006. View Article : Google Scholar
|
|
13
|
Wang ZH, Chen XY, Zhang M and Qian YT:
Synthesis of Co3O4 nanorod bunches from a single precursor
Co(CO3)0.35Cl0.20(OH)1.10. Solid State Sci. 7:13–15. 2005.
View Article : Google Scholar
|
|
14
|
Salabaş EL, Rumplecker A, Kleitz F, Radu F
and Schüth F: Exchange anisotropy in nanocasted Co3O4 nanowires.
Nano Lett. 6:2977–2981. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li T, Yang S, Huang L, Gu B and Du Y: A
novel process from cobalt nanowire to Co3O4 nanotube.
Nanotechnology. 15:1479–1482. 2004. View Article : Google Scholar
|
|
16
|
Yu T, Zhu YW, Xu XJ, Shen ZX, Chen P, Lim
CT, Thong JTL and Sow CH: Controlled growth and field-emission
properties of cobalt oxide nanowalls. Adv Mater. 17:1595–1599.
2005. View Article : Google Scholar
|
|
17
|
Bhattacharya K, Cramer H, Albrecht C,
Schins R, Rahman Q, Zimmermann U and Dopp E: Vanadium
pentoxide-coated ultrafine titanium dioxide particles induce
cellular damage and micronucleus formation in V79 cells. J Toxicol
Environ Health A. 71:976–980. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lison D, Lauwerys R, Demedts M and Nemery
B: Experimental research into the pathogenesis of cobalt/hard metal
lung disease. Eur Respir J. 9:1024–1028. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lison D, De Boeck M, Verougstraete V and
Kirsch-Volders M: Update on the genotoxicity and carcinogenicity of
cobalt compounds. Occup Environ Med. 58:619–625. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Domingo JL: Metal-induced developmental
toxicity in mammals: A review. J Toxicol Environ Health.
42:123–141. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Magaye R, Zhao J, Bowman L and Ding M:
Genotoxicity and carcinogenicity of cobalt-, nickel- and
copper-based nanoparticles. Exp Ther Med. 4:551–561.
2012.PubMed/NCBI
|
|
22
|
Kuo CY, Wong RH, Lin JY, Lai JC and Lee H:
Accumulation of chromium and nickel metals in lung tumors from lung
cancer patients in Taiwan. J Toxicol Environ Health A.
69:1337–1344. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Boeck M, Kirsch-Volders M and Lison D:
Cobalt and antimony, Genotoxicity and carcinogenicity. Mutat Res.
533:135–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beyersmann D and Hartwig A: The genetic
toxicology of cobalt. Toxicol Appl Pharmacol. 115:137–145. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ponti J, Sabbioni E, Munaro B, Broggi F,
Marmorato P, Franchini F, Colognato R and Rossi F: Genotoxicity and
morphological transformation induced by cobalt nanoparticles and
cobalt chloride, An in vitro study in Balb/3T3 mouse fibroblasts.
Mutagenesis. 24:439–445. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Colognato R, Bonelli A, Ponti J, Farina M,
Bergamaschi E, Sabbioni E and Migliore L: Comparative genotoxicity
of cobalt nanoparticles and ions on human peripheral leukocytes in
vitro. Mutagenesis. 23:377–382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Papageorgiou I, Brown C, Schins R, Singh
S, Newson R, Davis S, Fisher J, Ingham E and Case CP: The effect of
nano- and micron-sized particles of cobalt-chromium alloy on human
fibroblasts in vitro. Biomaterials. 28:2946–2958. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Aker WG, Hwang HM, Yedjou CG, Yu H
and Tchounwou PB: A study of the mechanism of in vitro cytotoxicity
of metal oxide nanoparticles using catfish primary hepatocytes and
human HepG2 cells. Sci Total Environ. 409:4753–4762. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guildford AL, Poletti T, Osbourne LH, Di
Cerbo A, Gatti AM and Santin M: Nanoparticles of a different source
induce different patterns of activation in key biochemical and
cellular components of the host response. J R Soc Interface.
6:1213–1221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Papis E, Rossi F, Raspanti M, Dalle-Donne
I, Colombo G, Milzani A, Bernardini G and Gornati R: Engineered
cobalt oxide nanoparticles readily enter cells. Toxicol Lett.
189:253–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anard D, Kirsch-Volders M, Elhajouji A,
Belpaeme K and Lison D: In vitro genotoxic effects of hard metal
particles assessed by alkaline single cell gel and elution assays.
Carcinogenesis. 18:177–184. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ding M, Kisin ER, Zhao J, Bowman L, Lu Y,
Jiang B, Leonard S, Vallyathan V, Castranova V and Murray AR:
Size-dependent effects of tungsten carbide-cobalt particles on
oxygen radical production and activation of cell signaling pathways
in murine epidermal cells. Toxicol Appl Pharmacol. 241:260–268.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pershina AG, Sazonov AE, Novikov DV,
Knyazev AS, Izaak TI, Itin VI, Naiden EP, Magaeva AA and Terechova
OG: Study of DNA interaction with cobalt ferrite nanoparticles. J
Nanosci Nanotechnol. 11:2673–2677. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rebello V, Shaikh S and Desai PV: Toxicity
of cobalt oxide nanoparticles on microalgae (Navicula sp. and
Chetoceros sp.) and Bivalve (Meritrix meritrix) cells. 2010
International Conference on Environmental Engineering and
Applications. Singapore. 195–199. 2010.http://toc.proceedings.com/09360webtoc.pdf
View Article : Google Scholar
|
|
35
|
Ghodake G, Seo YD and Lee DS: Hazardous
phytotoxic nature of cobalt and zinc oxide nanoparticles assessed
using Allium cepa. J Hazard Mater. 186:952–955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Falfushynska H, Gnatyshyna L, Turta O,
Stoliar O, Mitina N, Zaichenko A and Stoika R: Responses of hepatic
metallothioneins and apoptotic activity in Carassius auratus
gibelio witness a release of cobalt and zinc from waterborne
nanoscale composites. Comp Biochem Physiol C Toxicol Pharmacol.
160:66–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu Z, Zhang B and Yan B: Regulation of
enzyme activity through interactions with nanoparticles. Int J Mol
Sci. 10:4198–4209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Manikandan R, Thiagarajan R, Beulaja S,
Sudhandiran G and Arumugam M: Effect of curcumin on
selenite-induced cataractogenesis in Wistar rat pups. Curr Eye Res.
35:122–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Manikandan R, Thiagarajan R, Beulaja S,
Sudhandiran G and Arumugam M: Curcumin prevents free
radical-mediated cataractogenesis through modulations in lens
calcium. Free Radic Biol Med. 48:483–492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ellman GL, Courtney KD, Andres V Jr and
Feather-Stone RM: A new and rapid colorimetric determination of
acetylcholinesterase activity. Biochem Pharmacol. 7:88–95. 1961.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
42
|
Matzapetakis M, Dakanali M, Raptopoulou
CP, Tangoulis V, Terzis A, Moon N, Giapintzakis J and Salifoglou A:
Synthesis, spectroscopic, and structural characterization of the
first aqueous cobalt(II)-citrate complex, Toward a potentially
bioavailable form of cobalt in biologically relevant fluids. J Biol
Inorg Chem. 5:469–474. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Haines RA, Kipp EB and Reimer M:
Cobalt(III) complexes containing optically active tartaric acid.
Inorg Chem. 13:2473–2476. 1974. View Article : Google Scholar
|
|
44
|
Theja GS, Lowrence RC, Ravi V, Nagarajan S
and Anthony SP: Synthesis of Cu2O micro/nanocrystals with tunable
morphologies using coordinating ligands as structure controlling
agents and antimicrobial studies. CrystEngComm. 16:9866–9872. 2014.
View Article : Google Scholar
|
|
45
|
Lou XW, Deng D, Lee JY, Feng J and Archer
LA: Self-supported formation of needlelike Co3O4 nanotubes and
their application as lithium-ion battery electrodes. Adv Mater.
20:258–262. 2008. View Article : Google Scholar
|
|
46
|
Diamond JM, Winchester EL, Mackler DG,
Rasnake WJ, Fanelli JK and Gruber D: Toxicity of cobalt to
fresh-water indicator species as a function of water hardness.
Aquat Toxicol. 22:163–179. 1992. View Article : Google Scholar
|
|
47
|
Gibbs RJ: Metals in the sediments along
the Hudson River estuary. Environ Int. 20:507–516. 1994. View Article : Google Scholar
|
|
48
|
Gambardella C, Aluigi MG, Ferrando S,
Gallus L, Ramoino P, Gatti AM, Rottigni M and Falugi C:
Developmental abnormalities and changes in cholinesterase activity
in sea urchin embryos and larvae from sperm exposed to engineered
nanoparticles. Aquat Toxicol 130-131. 77–85. 2013. View Article : Google Scholar
|
|
49
|
Gambardella C, Ferrando S, Morgana S,
Gallus L, Ramoino P, Ravera S, Bramini M, Diaspro A, Faimali M and
Falugi C: Exposure of Paracentrotus lividus male gametes to
engineered nanoparticles affects skeletal bio-mineralization
processes and larval plasticity. Aquat Toxicol. 158:181–191. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cavallo D, Ciervo A, Fresegna AM, Maiello
R, Tassone P, Buresti G, Casciardi S, Iavicoli S and Ursini CL:
Investigation on cobalt-oxide nanoparticles cyto-genotoxicity and
inflammatory response in two types of respiratory cells. J Appl
Toxicol. 35:1102–1113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang H, Liu C, Yang D, Zhang H and Xi Z:
Comparative study of cytotoxicity, oxidative stress and
genotoxicity induced by four typical nanomaterials, The role of
particle size, shape and composition. J Appl Toxicol. 29:69–78.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hansen T, Clermont G, Alves A, Eloy R,
Brochhausen C, Boutrand JP, Gatti AM and Kirkpatrick CJ: Biological
tolerance of different materials in bulk and nanoparticulate form
in a rat model, Sarcoma development by nanoparticles. J R Soc
Interface. 3:767–775. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vertegel AA, Siegel RW and Dordick JS:
Silica nanoparticle size influences the structure and enzymatic
activity of adsorbed lysozyme. Langmuir. 20:6800–6807. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Asuri P, Bale SS, Pangule RC, Shah DA,
Kane RS and Dordick JS: Structure, function, and stability of
enzymes covalently attached to single-walled carbon nanotubes.
Langmuir. 23:12318–12321. 2009. View Article : Google Scholar
|
|
55
|
Asuri P, Karajanagi SS, Vertegel AA,
Dordick JS and Kane RS: Enhanced stability of enzymes adsorbed onto
nanoparticles. J Nanosci Nanotechnol. 7:1675–1678. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shang W, Nuffer JH, Dordick JS and Siegel
RW: Unfolding of ribonuclease A on silica nanoparticle surfaces.
Nano Lett. 7:1991–1995. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lundqvist M, Sethson I and Jonsson BH:
Protein adsorption onto silica nanoparticles, Conformational
changes depend on the particles' curvature and the protein
stability. Langmuir. 20:10639–10647. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fischer NO, 5 CM, Simard JM and Rotello
VM: Inhibition of chymotrypsin through surface binding using
nanoparticle-based receptors. Proc Natl Acad Sci USA. 99:5018–5023.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang Z, Zhao J, Li F, Gao D and Xing B:
Adsorption and inhibition of acetylcholinesterase by different
nanoparticles. Chemosphere. 77:67–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gagner JE, Lopez MD, Dordick JS and Siegel
RW: Effect of gold nanoparticle morphology on adsorbed protein
structure and function. Biomaterials. 32:7241–7252. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sanford J and Venkatapathy R: State of the
Science Literature Review: Everything Nanosilver and More.
Scientific Technical, Research, Engineering, and Modeling Support
Final Report. Varner K: (Washington, DC). U. S. Environmental
Protection Agency, Office of Research and Development. 1–197.
2010.
|
|
62
|
Saptarshi SR, Duschl A and Lopata AL:
Interaction of nanoparticles with proteins, Relation to
bio-reactivity of the nanoparticle. J Nanobiotechnology. 11:262013.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Schafer FQ and Buettner GR: Redox
environment of the cell as viewed through the redox state of the
glutathione disulfide/glutathione couple. Free Radic Biol Med.
30:1191–1212. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gilbert HF: Redox control of enzyme
activities by thiol/disulfide exchange. Methods Enzymol.
107:330–351. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Klatt P and Lamas S: Regulation of protein
function by S-glutathiolation in response to oxidative and
nitrosative stress. Eur J Biochem. 267:4928–4944. 2000. View Article : Google Scholar : PubMed/NCBI
|