Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common type of cancer and the third most common cause of
cancer-related mortality worldwide. The International Agency for
Research on Cancer estimates that 748,300 new cases of liver cancer
and 695,900 cancer-related mortalities occur worldwide every year
(1). Despite improvements in
surgical techniques and perioperative management, as well as the
development of non-surgical treatments such as radiofrequency
ablation and transarterial chemoembolization (TACE), the prognosis
of HCC remains poor due to the advanced tumor stage accompanied by
chronic liver disease (CLD) at diagnosis (2). The identification of biomarkers that
correlate with the outcome of patients with HCC may be useful in
determining the prognosis of this disease, identifying the patients
most likely to benefit from particular treatments and assisting
clinicians in the design of personalized treatment strategies
(3).
Insulin-like growth factor-1 (IGF-1) is a potent
survival factor involved in the development and progression of
various cancers (4). Studies have
suggested that high circulating levels of IGF-1 are associated with
increased risk of different types of cancers, such as prostate,
breast and colon cancers, due to activation of the downstream
cascade of the IGF axis (5–7). However, the association between IGF-1
and HCC is somewhat different from that of other cancers. Since
IGF-1 is synthesized primarily by the liver, circulating IGF-1
levels reflect liver function and decrease significantly in
patients with hepatitis C virus infection (8), liver steatosis (9), liver cirrhosis (10), non-alcoholic steatohepatitis
(9) and HCC (11). A recent study has demonstrated
significant associations between IGF-1 expression and liver
cirrhosis and survival following resection in patients with HCC,
which is independent from the underlying liver disease (12). Moreover, low baseline serum levels of
IGF-1 were found to be associated with low disease control rate and
poor progression-free survival and overall survival (OS) of
patients with advanced HCC treated with systemic antiangiogenic
therapy (13). Furthermore, since
HCC is a hypervascular tumor and vascular endothelial growth factor
(VEGF)-induced angiogenesis plays a major role in tumor progression
and metastasis, TACE is an effective nonsurgical treatment for HCC
(14). Therefore, it may be
speculated that low baseline levels of IGF-1 in the serum may also
independently associated with poor outcomes in patients receiving
TACE as their initial treatment. Thus, the aim of the present study
was to investigate the prognostic value of baseline serum IGF-1
levels in patients with HCC undergoing TACE.
Materials and methods
Patients
Between May 2007 and June 2011, 145 consecutive
patients with HCC who underwent TACE as initial treatment at the
Department of Interventional Radiology and Nuclear Medicine,
Yuhuangding Hospital (Yantai, China) were enrolled in this study.
HCC was reconfirmed in all patients on the basis of the American
Association for the Study of Liver Diseases practice guidelines
(15). The study inclusion criterion
was HCC classified as Barcelona Clinic Liver Cancer (BCLC) stage B
or C, which is generally not regarded as an indication for
treatment with curative intent. Patients with early-stage HCC (BCLC
stage 0 or A) who were unsuitable for surgical resection or
ablation because of liver function, comorbidity or technical
infeasibility were also included. Exclusion criteria were
extrahepatic metastasis, Child-Pugh class C and the concurrent
presence of another primary liver cancer (such as fibrolamellar HCC
or cholangiocarcinoma) or other types of cancers. The study
protocol was conducted in accordance with the ethical guidelines of
the Declaration of Helsinki and was approved by Institutional
Review Board of Yuhuangding Hospital. Written informed consent was
obtained from all patients.
TACE procedure
All patients were treated using the same TACE
procedure conducted by the same team. Briefly, angiographic
examination was performed using a 5-Fr catheter inserted through
the femoral artery. Using arteriography, the hepatic or superior
mesenteric artery was selected on the basis of tumor arterial blood
supply and the tip of the catheter was superselected into the
tumor-feeding branches, with the use of a microcatheter if
necessary. Once the target tumor-feeding artery had been
identified, chemoembolization was achieved as selectively as
possible for all targeted lesions in the left and right lobes of
the liver with 2–20 ml emulsion comprising cisplatin and lipiodol
(1:1). Polyvinyl alcohol or gelatin sponge particles were injected
if required to embolize tumor-feeding vessels to guarantee that
there was no longer any tumor staining following repeat
angiography. Patients were subsequently managed for potential
postembolization syndrome (such as fever, nausea, vomiting and
abdominal pain). Dynamic liver computed tomography (CT) or magnetic
resonance (MR) imaging was conducted 4–8 weeks after the procedure.
If the presence of residual viable tumors was confirmed or new
lesions developed in patients with adequate liver function, further
TACE procedures were carried out.
Baseline serum IGF-1 and VEGF
determination
Peripheral venous blood samples (5 ml) were
collected prior to the first TACE procedure and centrifuged at 4°C
for 25 min (400 × g). Serum samples were then collected and stored
at −20°C until used. Serum IGF-1 and VEGF levels were tested by an
enzyme-linked immunosorbent assay (ELISA) method using Human IGF-1
and VEGF Quantikine ELISA kits (R&D Systems, Minneapolis, MN,
USA) according to the manufacturer's instructions.
Statistical analysis
Baseline continuous variables were presented as the
mean ± standard deviation (SD). Categorical variables were
expressed as frequencies (percentages). The primary endpoint was
time to progression (TTP), which was measured from the time of
enrollment until tumor progression was first documented in imaging
studies, in accordance with the modified Response Evaluation
Criteria in Solid Tumors (mRECIST). The secondary endpoint was OS,
which was defined as the time from enrollment to mortality. The
association of clinical variables with TTP or OS was identified by
univariate analysis with hazard ratios (HRs) and 95% confidence
intervals (CIs), and variables with a P-value less than 0.05 in the
univariate analysis were then entered into stepwise Cox regression
multivariate models. The Kaplan-Meier method was used to plot the
TTP and OS curves and the log-rank test was used for comparison.
Statistical analyses were performed using SPSS software, version
19.0 (IBM Corporation, Armonk, NY, USA). All tests were two-sided
and P<0.05 was considered statistically significant.
Results
Baseline characteristics and treatment
response
Between May 2007 and June 2011, 145 patients with
HCC who underwent TACE as initial treatment were eligible for this
study. Baseline serum samples were available for 128 (88.3%) of
these patients. Various characteristics of the patients are listed
in Table I. Of the 128 patients, 96
(75.0%) were male, 103 (80.5%) were positive for hepatitis B virus,
79 (61.7%) had clinical liver cirrhosis, 88 (68.8%) had Child-Pugh
class A disease, and 122 (95.3%) had elevated serum α-fetoprotein
(AFP) levels above the normal upper limit (>20 ng/ml). The
median age at the time of diagnosis was 55 years (range, 38–76
years). The median size of the largest measurable lesion was 3.5 cm
(range, 1.8–10.4 cm). According to the BCLC staging system, 7, 23,
67 and 31 patients were at stages 0, A, B and C, respectively.
During a median follow-up period of 47 months (range, 10.6–69.3
months), 98 patients (76.6%) experienced disease progression with a
median TTP of 7.5 months (range, 1.6–29.8 months). The overall
cumulative progression rate in patients with HCC following TACE was
54.5, 69.3 and 78.4% after 1, 2 and 3 years, respectively. The
median survival time was 34.5 months (range, 5.8–69.3 months), with
59 of 128 patients (46.1%) succumbing during the follow-up. The
overall cumulative mortality rate was 19.3% after 1 year, 36.8%
after 2 years and 44.7% after 3 years.
 | Table I.Serum levels of IGF-1 and VEGF
according to clinical characteristics of 128 patients with HCC
undergoing TACE. |
Table I.
Serum levels of IGF-1 and VEGF
according to clinical characteristics of 128 patients with HCC
undergoing TACE.
|
|
| IGF-1 (ng/ml)
| VEGF (pg/ml)
|
|---|
| Characteristics | No. of patients
(%) | Mean ± SD | P-value | Mean ± SD | P-value |
|---|
| Age, years |
|
| 0.385 |
| 0.077 |
|
<60 | 73 (57.0) | 61.3±33.8 |
| 269.5±77.8 |
|
| ≥60 | 55 (43.0) | 56.4±37.4 |
| 281.6±99.4 |
|
| Gender |
|
| 0.276 |
| 0.316 |
|
Female | 32 (25.0) | 62.1±38.1 |
| 267.4±79.8 |
|
| Male | 96 (75.0) | 55.2±43.1 |
| 279.3±83.2 |
|
| Hepatitis infection
status |
|
| 0.044 |
| 0.059 |
| HBV | 97 (75.8) | 56.7±36.2 |
| 289.7±103.6 |
|
| HCV | 14 (10.9) | 58.4±39.4 |
| 278.1±85.4 |
|
| HBV and
HCV | 6 (4.7) | 50.1±22.8 |
| 290.2±97.2 |
|
| None | 11 (8.6) | 63.0±27.5 |
| 265.4±81.1 |
|
| Clinical
cirrhosis |
|
| 0.093 |
| 0.184 |
|
Present | 79 (61.7) | 54.8±36.5 |
| 284.6±88.9 |
|
|
Absent | 49 (38.3) | 60.2±39.7 |
| 270.3±81.2 |
|
| Child-Pugh class |
|
| 0.003 |
| 0.023 |
| A | 88 (68.7) | 63.4±41.2 |
| 264.3±77.2 |
|
| B | 40 (31.3) | 54.9±33.8 |
| 289.5±83.5 |
|
| Bilirubin level,
µmol/l |
|
| 0.032 |
| 0.458 |
| ≤34 | 92 (71.9) | 64.1±38.6 |
| 271.6±73.4 |
|
|
>34 | 36 (28.1) | 55.3±29.1 |
| 280.3±79.8 |
|
| Serum AFP level,
ng/ml |
|
| 0.087 |
| 0.376 |
|
<200 | 97 (75.8) | 60.4±37.8 |
| 268.2±75.6 |
|
| ≥200 | 31 (24.2) | 56.2±33.6 |
| 284.1±80.3 |
|
| Tumor size, cm |
|
| 0.005 |
| 0.009 |
| <5
cm | 86 (67.2) | 61.5±40.7 |
| 265.7±75.3 |
|
| ≥5
cm | 42 (32.8) | 52.9±30.6 |
| 288.3±82.1 |
|
| Tumor nodularity |
|
| 0.025 |
| 0.784 |
|
Uninodular | 90 (70.3) | 60.3±38.9 |
| 275.1±76.2 |
|
|
Multinodular | 38 (29.7) | 54.8±33.7 |
| 277.5±74.9 |
|
| Vascular
invasion |
|
| 0.017 |
| 0.459 |
| No | 104 (81.2) | 61.7±36.4 |
| 271.7±71.2 |
|
| Yes | 24 (18.8) | 53.5±30.8 |
| 279.0±78.5 |
|
| Lymph node
involvement |
|
| 0.064 |
| 0.036 |
| No | 99 (77.3) | 60.3±39.1 |
| 273.3±72.5 |
|
| Yes | 29 (22.7) | 55.8±34.3 |
| 289.1±82.1 |
|
| BCLC stage |
|
| 0.011 |
| 0.034 |
| 0 | 7 (5.5) | 61.8±22.6 |
| 267.1±66.1 |
|
| A | 23 (18.0) | 59.4±32.7 |
| 277.5±76.4 |
|
| B | 67 (52.3) | 52.7±35.4 |
| 287.3±81.5 |
|
| C | 31 (24.2) | 53.3±31.2 |
| 292.7±79.6 |
|
Association between clinical factors
and expression of IGF-1 and VEGF
The associations between clinical factors and serum
levels of IGF-1 and VEGF are presented in Table I. The serum IGF-1 level was found to
be significantly associated with hepatitis infection status,
Child-Pugh class, bilirubin level, tumor size and nodularity,
vascular invasion and BCLC stage, with the Child-Pugh score having
the strongest association (P=0.003). The serum VEGF level was found
to be significantly associated with the Child-Pugh class, tumor
size, lymph node involvement and BCLC stage, with tumor size having
the strongest association (P=0.009).
Association of clinical factors with
TTP and OS
Cut-off values for each of the clinical factors
(including age and gender; Table
II) were selected and univariate analysis was carried out to
identify the factors significantly associated with TTP and OS. The
results showed that high Child-Pugh score, larger tumor size,
multiple tumors, vascular invasion, lymph node involvement and
advanced BCLC stage were significantly associated with shorter TTP
(all P<0.05). All the other selected factors (including age,
gender and hepatitis virus infection) were not found to be
significantly associated with TTP (P>0.05). In the multivariate
analysis, BCLC stage and vascular invasion were independent risk
factors for disease progression (Table
II). When clinical factors were examined as potential
independent risk factors for OS, only advanced BCLC stage was found
to be significantly associated with poorer OS (Table III).
 | Table II.Univariate and multivariate Cox
analyses of clinical variables for time to progression. |
Table II.
Univariate and multivariate Cox
analyses of clinical variables for time to progression.
|
| Univariate analysis
| Multivariate
analysis
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (≥60 vs. <60
years) | 0.882
(0.596–1.125) | 0.652 |
|
|
| Gender (male vs.
female) | 1.086
(0.814–1.447) | 0.874 |
|
|
| Hepatitis virus
infection |
| 0.348 |
|
|
|
HBV | 1.245
(0.782–1.841) |
|
|
|
|
HCV | 1.208
(0.695–1.918) |
|
|
|
| HBV and
HCV | 1.336
(0.816–1.976) |
|
|
|
|
None | 1.000 |
|
|
|
| Child-Pugh class (A
vs. B) | 0.775
(0.559–0.978) | 0.043 | 0.897
(0.653–1.130) | 0.692 |
| Bilirubin (>34
vs. ≤34 µmol/l) | 1.122
(0.846–1.492) | 0.890 |
|
|
| AFP (≥200 vs.
<200 ng/ml) | 1.250
(0.912–1.534) | 0.649 |
|
|
| Tumor size (≥5 vs.
<5 cm) | 1.543
(1.184–1.932) | 0.003 | 1.352
(0.997–1.761) | 0.054 |
| Tumor nodularity
(uninodular vs. multinodular) | 1.327
(1.095–1.570) | 0.022 | 1.287
(0.952–1.534) | 0.061 |
| Vascular
invasion | 1.384
(1.134–1.594) | 0.015 | 1.319
(1.024–1.694) | 0.043 |
| Lymph node
involvement | 1.461
(1.125–1.738) | 0.009 | 1.245
(0.895–1.585) | 0.082 |
| BCLC stage |
| <0.001 |
|
|
| 0 | 1.000 |
| 1.000 |
|
| A | 1.934
(1.447–2.586) |
| 1.467
(1.186–1.952) | 0.005 |
| B | 2.213
(1.602–2.884) |
| 1.787
(1.356–2.127) | <0.001 |
| C | 2.675
(1.937–3.361) |
| 2.175
(1.743–2.685) | <0.001 |
| Serum IGF-1
(ng/ml) | 0.874
(0.816–0.952) | 0.002 | 0.892
(0.853–0.937) | <0.001 |
| Serum VEGF
(pg/ml) | 1.136
(1.067–1.218) | 0.025 | 1.118
(1.048–1.192) | 0.031 |
 | Table III.Univariate and multivariate Cox
analyses of clinical variables for overall survival. |
Table III.
Univariate and multivariate Cox
analyses of clinical variables for overall survival.
|
| Univariate analysis
| Multivariate
analysis
|
|---|
| Variables | HR (95%CI) | P-value | HR (95%CI) | P-value |
|---|
| Age (≥60 vs. <60
years) | 0.769
(0.603–1.092) | 0.348 |
|
|
| Gender (male vs.
female) | 1.143
(0.786–1.549) | 0.864 |
|
|
| Hepatitis virus
infection |
| 0.273 |
|
|
|
HBV | 1.291
(0.659–1.754) |
|
|
|
|
HCV | 1.180
(0.783–1.651) |
|
|
|
| HBV and
HCV | 1.381
(0.832–1.903) |
|
|
|
|
None | 1.000 |
|
|
|
| Child-Pugh class (A
vs. B) | 0.832
(0.595–1.087) | 0.083 |
|
|
| Bilirubin (>34
vs. ≤34 µmol/l) | 1.069
(0.896–1.221) | 0.290 |
|
|
| AFP (≥200 vs.
<200 ng/ml) | 1.145
(0.917–1.365) | 0.184 |
|
|
| Tumor size (≥5 vs.
<5 cm) | 1.476
(1.189–1.841) | 0.004 | 1.362
(0.982–1.754) | 0.0593 |
| Tumor nodularity
(uninodular vs. multinodular) | 1.293
(0.974–1.615) | 0.059 |
|
|
| Vascular
invasion | 1.384
(1.022–1.795) | 0.041 | 1.185
(0.883–1.506) | 0.136 |
| Lymph node
involvement | 1.473
(1.085–1.890) | 0.013 | 1.297
(0.944–1.672) | 0.087 |
| BCLC stage |
| <0.001 |
|
|
| 0 | 1.000 |
| 1.000 |
|
| A | 1.592
(1.225–1.931) |
| 1.424
(1.116–1.758) | 0.015 |
| B | 1.874
(1.416–2.385) |
| 1.692
(1.285–2.136) | <0.001 |
| C | 2.136
(1.775–2.587) |
| 1.921
(1.547–2.378) | <0.001 |
| Serum IGF-1
(ng/ml) | 0.752
(0.606–0.914) | 0.005 | 0.824
(0.705–0.949) | 0.009 |
| Serum VEGF
(pg/ml) | 1.195
(1.092–1.287) | 0.013 | 1.125
(1.058–1.196) | 0.037 |
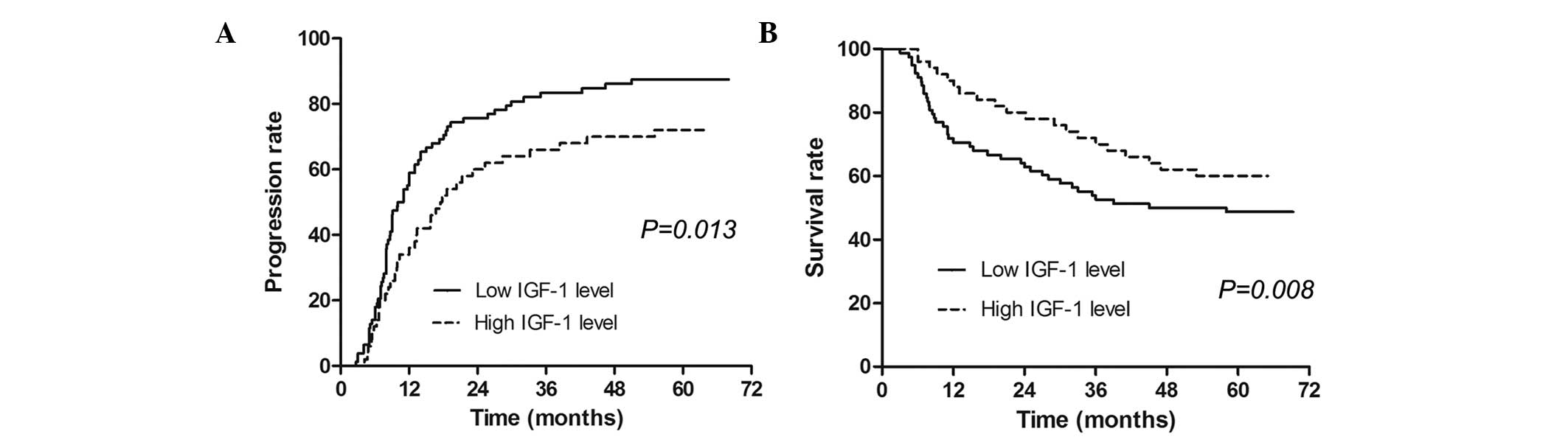
Association of IGF-1 levels with TTP
and OS
The median serum IGF-1 value (57.3 ng/ml) was used
as a cut-off in the univariate model and a low IGF-1 level
(<57.3 ng/ml) was calculated to be associated with shorter TTP
and poorer OS. In the multivariate analysis, IGF-1 was found to be
an independent predictive factor for TTP and OS. The IGF-1 level
was a stronger predictive tool when compared with VEGF, with a
lower P-value (Table III). The
median time to progression in patients with high IGF-1 levels was
significantly longer than that in patients with low levels
(Fig. 1A). The median survival time
in patients with high IGF-1 levels was also significantly longer
than that in patients with low IGF-1 levels (Fig. 1B).
Discussion
The prognosis of patients with HCC is poor, with a
5-year survival rate of 10% (16).
Although the BCLC staging system and Cancer of the Liver Italian
Program (CLIP) score are widely used for the guidance of treatment
decisions and to stratify patients with HCC for clinical trials,
patients within the same HCC stage in these HCC staging systems are
notably heterogeneous (2).
Biomarkers are expected to better predict patient survival and
provide improved prognostic stratification. AFP has served as a
diagnostic and prognostic marker for HCC for many years (17). However, persistent AFP elevation may
be observed in some cirrhotic patients due to hepatocyte
regeneration. Therefore, other markers, such as VEGF, hepatocyte
growth factor (HGF) and transforming growth factor β1 have been
assessed as potentially improved diagnostic and prognostic
predictors for HCC. However, absolute positive and negative markers
for HCC remain deficient, and even those with very high sensitivity
and specificity are not universally useful diagnostically (16). IGF-1 is a 7.6 kDa single chain
molecule with ~50% identity to the sequences of the A- and B-chains
of human insulin (18); it is
produced primarily by the liver. IGF signaling plays an important
role in growth promotion in various tissues and organs, acting via
autocrine, paracrine and endocrine mechanisms. Previous studies
have shown that high baseline levels of IGF-1 in the serum are
associated with an increased risk of cancer of the prostate, breast
cancer, colon/rectum and lung (5–7,19). A number of studies have observed an
association between IGF-1 and HCC. Kaseb et al found that
lower plasma IGF-1 and higher plasma VEGF levels correlated
significantly with advanced clinicopathological parameters and poor
OS, and that when IGF-1 and VEGF were integrated into HCC staging,
the prognostic stratification of patients was significantly
enhanced (2). A recent study has
also demonstrated that in patients with HCC, significant
associations exist between IGF-1 expression and liver cirrhosis and
survival (12). Similarly, Cho et
al found that serum IGF-1 levels correlated with OS in patients
with HCC (20). However, another
study evaluating the clinical utility of serum protein and mRNA
levels of IGF-1 in HCC found that neither serum protein nor mRNA
levels of IGF-1 are prognostic for the outcome of HCC patients
(16). This finding warrants further
investigation because it is based on a small sample size. In the
current study, it was found that serum IGF-1 level was
significantly associated with hepatitis infection status,
Child-Pugh score, bilirubin, tumor size and nodularity, vascular
invasion and BCLC stage. This is consistent with previous reports
(2,12), indicating that serum IGF-1 may be
complementary to other clinical relevant prognostic indicators in
patients with HCC, including Child-Pugh score, tumor parameters and
BCLC variables. Furthermore, a low IGF-1 level predicted shorter
TTP and OS in patients treated with TACE. As serum IGF-1 is
measured by a simple noninvasive approach, it may be widely used in
clinical practice to aid the prognostic stratification of patients
with HCC in clinical trials, which should help to guide treatment
decisions and improve HCC outcomes.
Reduced circulating levels of IGF-1 in patients with
hepatic cirrhosis or HCC have been attributed to liver damage as
hepatocytes are the primary source of IGF-1 (21). The observation that IGF-1 synthesis
is attenuated in chronic hepatitis C and reflects the severity of
liver fibrosis appears to support this hypothesis (22). However, in the present study, the
status of hepatitis infection but not the state of clinical liver
cirrhosis was found to correlate with the serum IGF-1 levels. This
may be due to the comparable liver functions of the patients
enrolled in this study. Although the majority of the patients in
the present study had Child-Pugh class A liver function, the levels
of IGF-1 served to predict progression and mortality, regardless of
the remnant liver function. Therefore, the serum levels of IGF-1
may be considered in addition to other parameters, such as
Child-Pugh class or BCLC stage, to aid the assessment of hepatic
function and prognostically evaluate patients with HCC (20).
In conclusion, the present study shows that the
baseline serum IGF-1 level correlated with clinical factors of
patients with HCC and was an independent predictive factor for TTP
and OS. The results suggest that serum IGF-1 may serve as a novel
factor for use in determining the prognosis of patients with HCC
undergoing TACE.
Acknowledgements
The authors thank International Science Editing for
language editing work on this manuscript.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaseb AO, Morris JS, Hassan MM, Siddiqui
AM, Lin E, Xiao L, Abdalla EK, Vauthey JN, Aloia TA, Krishnan S and
Abbruzzese JL: Clinical and prognostic implications of plasma
insulin-like growth factor-1 and vascular endothelial growth factor
in patients with hepatocellular carcinoma. J Clin Oncol.
29:3892–3899. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ziol M, Sutton A, Calderaro J, Barget N,
Aout M, Leroy V, Blanc JF, Sturm N, Bioulac-Sage P, Nahon P, et al:
ESM-1 expression in stromal cells is predictive of recurrence after
radiofrequency ablation in early hepatocellular carcinoma. J
Hepatol. 59:1264–1270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maki RG: Small is beautiful: Insulin-like
growth factors and their role in growth development and cancer. J
Clin Oncol. 28:4895–4995. 2010. View Article : Google Scholar
|
|
5
|
Chan JM, Stampfer MJ, Giovannucci E, Gann
PH, Ma J, Wilkinson P, Hennekens CH and Pollak M: Plasma
insulin-like growth factor-I and prostate cancer risk, A
prospective study. Science. 279:563–566. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hankinson SE, Willett WC, Colditz GA,
Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE and Pollak M:
Circulating concentrations of insulin-like growth factor-I and risk
of breast cancer. Lancet. 351:1393–1396. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma J, Pollak MN, Giovannucci E, Chan JM,
Tao Y, Hennekens CH and Stampfer MJ: Prospective study of
colorectal cancer risk in men and plasma levels of insulin-like
growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer
Inst. 91:620–625. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elsammak MY, Amin GM, Khalil GM, Ragab WS
and Abaza MM: Possible contribution of serum activin A and IGF-1 in
the development of hepatocellular carcinoma in Egyptian patients
suffering from combined hepatitis C virus infection and hepatic
schistosomiasis. Clin Biochem. 39:623–629. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
García-Galiano D, Sánchez-Garrido MA,
Espejo I, Montero JL, Costán G, Marchal T, Membrives A,
Gallardo-Valverde JM, Muñoz-Castañeda JR, Arévalo E, et al: IL-6
and IGF-1 are independent prognostic factors of liver steatosis and
non-alcoholic steatohepatitis in morbidly obese patients. Obes
Surg. 17:493–503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo SM, Tan WM, Deng WX, Zhuang SM and Luo
JW: Expression of albumin, IGF-1, IGFBP-3 in tumor tissues and
adjacent non-tumor tissues of hepatocellular carcinoma patients
with cirrhosis. World J Gastroenterol. 11:4272–4276. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su WW, Lee KT, Yeh YT, Soon MS, Wang CL,
Yu ML and Wang SN: Association of circulating insulin-like growth
factor 1 with hepatocellular carcinoma, One cross-sectional
correlation study. J Clin Lab Anal. 24:195–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chun YS, Huang M, Rink L and Von Mehren M:
Expression levels of insulin-like growth factors and receptors in
hepatocellular carcinoma A retrospective study. World J Surg Oncol.
12:2312014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shao YY, Huang CC, Lin SD, Hsu CH and
Cheng AL: Serum insulin-like growth factor-1 levels predict
outcomes of patients with advanced hepatocellular carcinoma
receiving antiangiogenic therapy. Clin Cancer Res. 18:3992–3997.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bruix J and Sherman M: American
association for the study of liver diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
American Association for the Study of
Liver Diseases: Management of Hepatocellular Carcinoma: An Update.
http://www.aasld.org/publications/practice-guidelines-0Accessed.
September 16–2014
|
|
16
|
Karabulut S, Duranyildiz D, Tas F, Gezer
U, Akyüz F, Serilmez M, Ozgür E, Yasasever CT, Vatansever S and
Aykan NF: Clinical significance of serum circulating insulin-like
growth factor-1 (IGF-1) mRNA in hepatocellular carcinoma. Tumour
Biol. 35:2729–2739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malaguarnera G, Giordano M, Paladina I,
Berretta M, Cappellani A and Malaguarnera M: Serum markers of
hepatocellular carcinoma. Dig Dis Sci. 55:2744–2755. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Mellow: JS andB axter RC: Growth
hormone-dependent insulin-like growth factor (IGF) binding protein
both inhibits and potentiates IGF-I-stimulated DNA synthesis in
human skin fibroblasts. Biochem Biophys Res Commun. 156:151–156.
1988.
|
|
19
|
Yu H, Spitz MR, Mistry J, Gu J, Hong WK
and Wu X: Plasma levels of insulin-like growth factor-I and lung
cancer risk, A case-control analysis. J Natl Cancer Inst.
91:151–156. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cho E, Kim HC, Lee JH, Yoo JJ, Choi WM,
Cho YY, Lee MJ, Lee YB, Yu SJ, Kim YJ, et al: Serum insulin-like
growth factor-1 predicts disease progression and survival in
patients with hepatocellular carcinoma who undergo transarterial
chemoembolization. PLoS One. 9:e908262014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mazziotti G, Sorvillo F, Morisco F,
Carbone A, Rotondi M, Stornaiuolo G, Precone DF, Cioffi M, Gaeta
GB, Caporaso N and Carella C: Serum insulin-like growth factor I
evaluation as a useful tool for predicting the risk of developing
hepatocellular carcinoma in patients with hepatitis C virus-related
cirrhosis, A prospective study. Cancer. 95:2539–2545. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lorenzo-Zúñiga V, Bartolí R, Masnou H,
Montoliu S, Morillas RM and Planas R: Serum concentrations of
insulin-like growth factor-I (igf-I) as a marker of liver fibrosis
in patients with chronic hepatitis C. Dig Dis Sci. 52:3245–3250.
2007. View Article : Google Scholar : PubMed/NCBI
|