Introduction
A carotid endarterectomy (CEA) is a surgical
procedure that is performed in order to remove deposits of fat,
called plaque, from the carotid arteries in the neck (1). Plaque builds up in large- and
medium-sized arteries as people get older, and this build up is a
vascular disease called atherosclerosis (1). During a CEA, a surgeon removes the
fatty deposits in order to correct the narrowing and allow blood
and oxygen to flow freely to the brain (1). Previous studies have demonstrated that
conducting a CEA in patients with significant carotid stenosis,
including those with or without symptoms, is successful in
preventing stroke in >70% of cases (2,3).
However, the effects of CEA on cognitive function remain unclear
(4,5). Irvine et al (6) reported that cognitive improvements were
detected in 15/22 studies that had previously investigated patients
undergoing CEA. Conversely, other reports have suggested that the
CEA procedure may exert limited effects on cognitive function, and
subtle cognitive dysfunction has been detected in 24–28% of
patients following CEA (7,8), which may have been associated with
alterations in the distribution of cerebral flow, intraoperative
cerebral ischemia and reperfusion injury.
Dexmedetomidine (DEX), which is the
dextro-enantiomer of medetomidine, is a highly selective
α2-adrenoreceptor (α2-AR) agonist. It has been shown to exhibit
various properties, including sedative, analgesic and sympatholytic
activities, and an ability to stabilize hemodynamics. DEX is
considered a useful adjuvant to general anesthesia, since it is
able to decrease the required doses of anesthetics and analgesics,
and promote hemodynamic stability (9). In addition, previous studies have
demonstrated neuroprotective effects for DEX in animal models of
stroke (10,11). Furthermore, α2-ARs have been found to
be closely associated with cognitive function (12), which may be affected by increased
activity in the dorsal noradrenergic bundles, where α2-AR are
located, and in the frontal lobe, where α2-ARs mediate increased
attention (13). Therefore, the
present study hypothesized that DEX treatment may improve cognitive
performance in patients following CEA.
The present study aimed to investigate the effects
of the intravenous administration of DEX, as an adjunct to general
anesthesia, on cognitive function and cerebral injury in patients
post-CEA. In addition, the protein expression levels of the
cerebral ischemia and injury markers S100 calcium-binding protein B
(S100B) and neuron-specific enolase (NSE), and the concentration of
the oxidative stress marker malondialdehyde (MDA), were
determined.
Materials and methods
Ethics statement and patients
The present study was approved by the Bioethics
Committee at the Subei People's Hospital of Jiangsu Province (no.
2011032). The exclusion criteria were as follows: i) The patient
refused general anesthetic or enrollment into the present study,
ii) the patient was undergoing treatment with psychiatric drugs,
iii) the patient had an initial Mini-Mental State Examination
(MMSE) score of <20, iv) the patient was unable to write or
spell, and v) the patient underwent emergency surgery. Patients
included in the present study were diagnosed with a carotid
stenosis of ≥70%, with or without symptoms, and had not previously
undergone carotid surgery. Written informed consent was obtained
from all patients. A total of 50 patients due to undergo elective
CEA were randomized, using computer-generated random numbers, into
two groups: The study group (group D; n=25) and the control group
(group C; n=25). The mean age of the patients in group C was 72±4
years (age range, 67–83 years) and the male/female ratio was 13/11.
The mean age of the patients in group D was 70±3 years (age range,
65–84 years) and the male/female ratio was 16/8. Reasons to abandon
the protocol included the use of anesthetic drugs other than those
indicated in the protocol, the use of an intraoperative shunt,
diagnosis of severe hypotension, administration of a second
anesthetic within the first 24 h, and an inability to perform a
MMSE evaluation.
Anesthetic management
On the evening prior to surgery, patients were
orally administered 25 mg clorazepate (Wanbang Biopharmaceuticals,
Co., Ltd., Xuzhou, China) in order to decrease the preoperative
anxiety of the patients; however, no oral pre-medication was
administered on the day of surgery. Anesthesia was induced with
intravenous midazolam (0.05–0.1 mg/kg; Enhua Pharmaceutical Group,
Co., Ltd., Xuzhou, China), fentanyl (2 µg/kg; Renfu Nuosheng
Pharmaceutical, Co., Ltd., Wuhan, China) and etomidate (0.2–0.3
mg/kg; Enhua Pharmaceutical Group, Co., Ltd.). Following treatment
with rocuronium bromide (1.0 mg/kg; Xincheng Company, Lianyungang,
China), the trachea was intubated using an armored endotracheal
tube (Meinuo Medical Appliances, Co., Ltd., Suzhou, China). In
order to maintain a normal end-tidal CO2
(ETCO2, 30–40 mmHg), the mechanical ventilation
parameters were set as follows: Tidal volume, 8 ml/kg; fraction of
inspired O2 (FiO2), 0.5; and breathing
frequency, 10–14 breaths/min. Anesthesia was maintained with the
intravenous infusion of remifentanil (0.1–0.3 µg/kg/min; Renfu
Nuosheng Pharmaceutical, Co., Ltd.) and propofol (2–6 mg/kg/h); the
infusion rate was adjusted according to the bispectral index. In
addition, patients were intermittently administered rocuronium
bromide (0.1 mg/kg), in order to maintain muscle relaxation.
Group D patients were intravenously administered 0.3
µg/kg DEX 10 min prior to anesthesia, and then at a rate of 0.3
µg/kg/h intraoperatively until 30 min prior to the completion of
surgery. The patients in group C received an equal volume of normal
saline. The mean arterial blood pressure (MAP) was controlled
intraoperatively within ±20% of the baseline value. During clamping
of the carotid artery, MAP values were maintained at +25% of the
baseline by treating the patients with dopamine (2–8 µg/kg/min) or
nitroglycerine (0.1–8.0 µg/kg/min), as appropriate. Following
unclamping of the carotid artery, the surgical field was
infiltrated with 10 ml ropivacaine (5 mg/ml; Hengrui Medicine, Co.,
Ltd., Lianyungang, China) for analgesia. All patients were treated
with tropisetron (4 mg/kg; Jiangsu YANGZIJIANG Pharmaceutical
Group, Co., Ltd. Taizhou, China) intraoperatively to prevent
postoperative nausea and vomiting. The anesthetists were not
blinded to the study protocol; however, the surgeons and post
anesthetic care unit staff were blinded.
Monitoring indicators
Intraoperative monitoring was conducted using the
Philips IntelliVue MP50 patient monitor (Philips Healthcare,
Hamburg, Germany), which incorporates a five-lead
electrocardiogram, and is able to assess invasive arterial blood
pressures, pulse oximetry and ETCO2. The depth of
sedation was measured using the A-2000™ XP bispectral index monitor
(Aspect Medical Systems, Inc., Norwood, MA, USA). MAPs were
recorded at 20 min pre-anesthesia, immediately and at 10 min
following tracheal intubation, at 5, 10 and 15 min following
clamping of the carotid artery, and at 5 and 10 min following
unclamping of the carotid artery. A shunt was inserted and the
patient was excluded from the present study if the carotid artery
stump pressure was <50 mmHg following clamping. One peripheral
venous catheter and a radial arterial line for continuous
monitoring of arterial blood pressure were inserted. A retrograde
internal jugular vein catheter, ipsilateral to the operated
carotid, was inserted into the jugular bulb, in order to obtain
blood samples. Blood samples were drawn from the jugular bulb 20
min prior to anesthesia (t0), 10 min following tracheal intubation
(t1), 15 min following clamping of the carotid artery (t2), 15 min
following unclamping of the carotid artery (t3), and at 6 and 24 h
(t4–5) post-CEA. The blood samples (2 ml) were centrifuged at 2,054
× g for 15 min at room temperature, and the serum was maintained at
−80°C until further analysis.
Quantification of the serum protein
expression levels of S100B and NSE, and the plasma concentration of
MDA
The serum protein expression levels of S100B and NSE
were measured using S100B and NSE enzyme-linked immunosorbent assay
kits (cat nos. MY1168 and MY1163, respectively; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China), according to the
manufacturer's protocol. Plasma MDA levels were determined using
high performance liquid chromatography (HPLC; Shimadzu Corporation,
Kyoto, Japan), as demonstrated in a previous study (14), although with minor alterations.
Briefly, 10 µl blood samples were mixed with 190 µl NaOH (1.3 M),
and were incubated at 60°C for 60 min. Subsequently, 100 µl 35%
HClO4 was added to the cooled mixture, and centrifuged
at 10,000 × g for 10 min at 4°C. The supernatants of the samples
(300 µl) were transferred into 1.5 ml HPLC tubes, after which 50 µl
5 mM, 2,4-dinitrophenylhydrazine solution was added to the mixture
and incubated for 30 min at room temperature. Subsequently, 40-µl
samples were injected into the HPLC instrument. The levels of MDA
are presented as the concentration of MDA in nmol/ml.
MMSE scoring
In order to evaluate cognitive function, all
patients underwent the MMSE 1 day prior to surgery (T0), and at 24,
48 and 72 h (T1–3), and 7 days and 1 month post-CEA (T4–5). MMSE
tests were conducted by a single psychologist blinded to the study
groups. The MMSE scoring system ranges from 0–30, with higher
scores indicating a superior cognitive performance.
Sample size analysis
Power analysis was conducted at 1 and 24 h post-CEA
on MMSE scores from 10 patients in a pilot study, in order to
assess what may be classed as a significantly different MMSE score
between the two groups. The pilot study was designed to achieve a
power of ≥0.80 and two-tailed α of 0.05; with these parameters, the
total required number of participants was 38. Therefore, a total of
50 participants was deemed an adequate sample size in the present
study in order to ensure confidence in the obtained results.
Statistical analysis
Data are presented as the mean ± standard deviation,
and categorical variables are presented as percentages. The normal
distribution of values was examined using the Kolmogorov-Smirnov
test, and comparisons for normally distributed variables were
conducted using an independent t-test between the two groups.
Non-normally distributed variables were analyzed using the
Kruskal-Wallis test. In intragroup analyses, involving comparisons
of the same subjects at different time points, normally distributed
variables were analyzed using the repeated-measures analysis of
variance or the paired t-test, and non-normally distributed
variables were compared using the Friedman test or the Wilcoxon
signed-rank test. The χ2 test was conducted in order to
assess statistical differences in the ratio of variables between
the two groups. All statistical analyses were conducted using SPSS
software, version 19.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patients and clinical parameters
A total of 50 patients were enrolled into the
present study, of which 48 patients fulfilled the inclusion
criteria. Two patients were excluded; 1 patient from group C and 1
patient from group D due to the use of a shunt and excessive
bleeding, respectively. Patient characteristics and clinical data
are presented in Table I. There were
no significant differences between the two groups regarding age,
gender, American Society of Anesthesiologists (ASA) classification,
and existing medications and diseases, including hypertension,
coronary artery disease and diabetes. Perioperative data, including
anesthesia and clamp durations, and the endarterectomy site, were
not significantly different between the two groups. The number of
patients requiring treatment with vasoconstrictors, including
phenylephrine and/or ephedrine, were 11 (45.8%) in group C and 15
(62.5%) in group D; however, this was not significantly different
(P>0.05). Nitroglycerine usage was significantly higher in group
C (62.5%), as compared with group D (33.3%; P~0.04). Three patients
(12.5%) in group C were treated with atropine, as compared with 8
patients (33.3%) in group D; however this difference was not
statistically significant (P=0.08). The dosage of propofol was
significantly lower in group D (542±36 mg), as compared with group
C (572±37 mg; P=0.01). The postoperative duration of
hospitalization was not significantly different between the two
groups (4.57±1.12 days in group C vs. 4.63±1.30 days in group D;
P=0.89).
 | Table I.Patient demographics and other
clinical data. |
Table I.
Patient demographics and other
clinical data.
| Variable | Group C | Group D | P-value |
|---|
| Age (years) | 72±4 | 70±3 | 0.06 |
| Gender |
|
|
|
|
Male | 13 (54.2) | 16 (66.7) | 0.37 |
|
Female | 11 (45.8) | 8 (33.3) |
| BMI
(kg/m2) | 27±3 | 27±4 | 0.72 |
| ASA grade |
|
|
|
| I | 0 (0) | 0 (0) | 0.23 |
| II | 17 (70.8) | 13 (54.2) |
|
III | 7 (29.2) | 11 (45.8) |
| Lateral carotid
stenosis ≥70% | 2 (8.3) | 4 (16.7) | 0.38 |
| Peripheral artery
disease | 2 (8.3) | 3 (12.5) | 0.64 |
| Coronary artery
disease | 8 (33.3) | 9 (37.5) | 0.76 |
| Hypertension | 21 (87.5) | 22 (91.7) | 0.63 |
| Diabetes
mellitus | 8 (33.3) | 5 (20.8) | 0.32 |
| Hyperlipidemia | 13 (54.2) | 17 (70.8) | 0.23 |
| Smoker | 12 (50.0) | 8 (33.3) | 0.24 |
| Antiplatelet
drugs | 16 (66.7) | 14 (58.3) | 0.55 |
| Calcium channel
blockers | 7 (29.2) | 10 (41.7) | 0.37 |
| Statins | 13 (54.2) | 15 (62.5) | 0.56 |
| Usage of
vasoconstrictors | 11 (45.8) | 15 (62.5) | 0.25 |
| Usage of
nitroglycerine | 15 (62.5) | 8
(33.3)a | 0.04 |
| Usage of
atropine | 3 (12.5) | 8 (33.3) | 0.08 |
| Anesthesia duration
(min) | 124±8 | 120±5 | 0.09 |
| Clamp duration
(min) |
31±4 |
28±3 | 0.06 |
| Endarterectomy
site |
|
|
|
|
Left | 15 (62.5) | 13 (54.2) | 0.56 |
|
Right | 9 (37.5) | 11 (45.8) |
|
| Dosage of propofol
(mg) | 572±37 | 542±36a | 0.01 |
| Postoperative
hospitalization (days) | 4.57±1.12 | 4.63±1.30 | 0.89 |
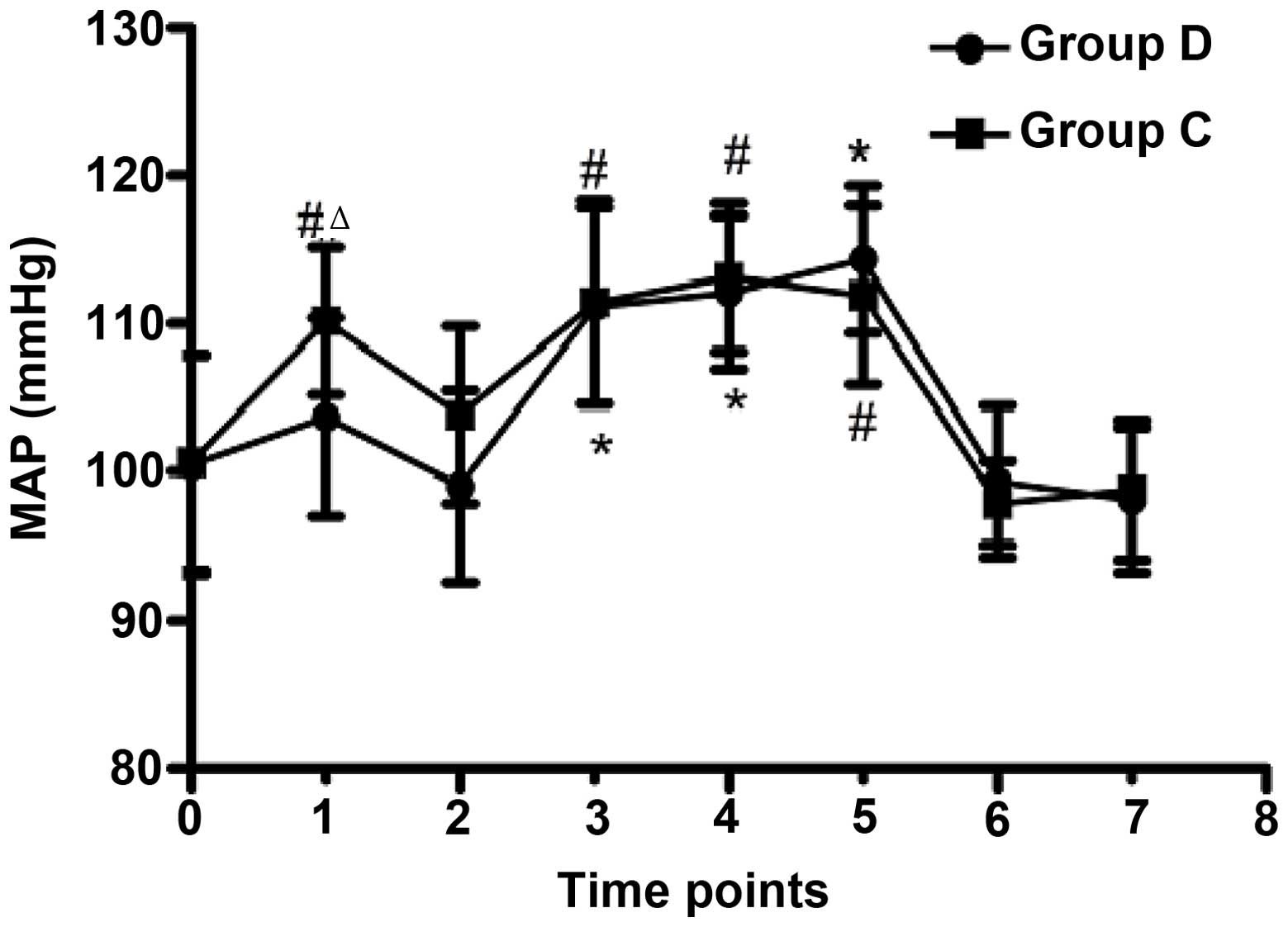
Comparison of MAP
MAP was maintained above the baseline (+25% of
baseline) in both groups during carotid clamping (Fig. 1), and was significantly increased in
group C immediately following tracheal intubation, as compared with
the baseline (P≤0.001) and group D (P=0.001). The ETCO2
was maintained within the target range (30–40 mmHg) in both groups
(Fig. 1).
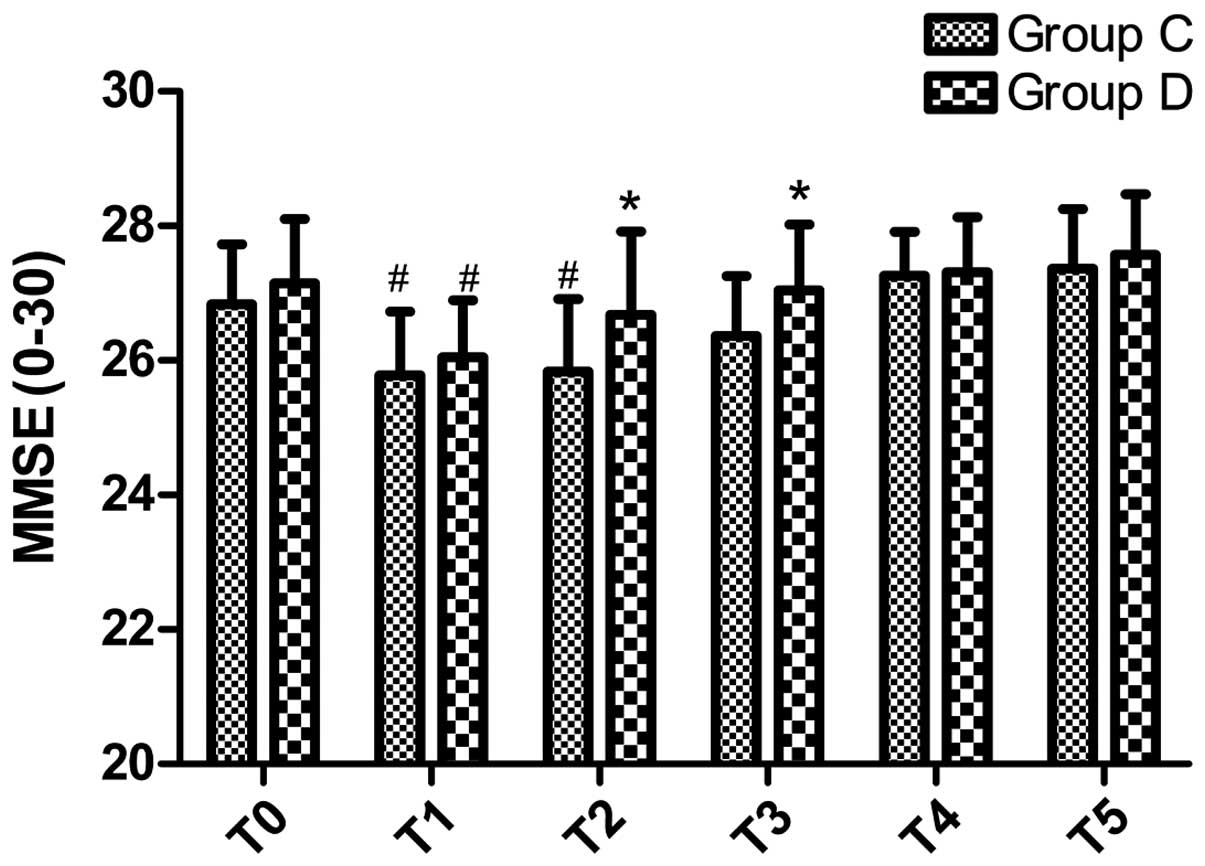
Comparison of MMSE scores
MMSE scores did not differ significantly between the
two groups prior to surgery (P>0.05; Fig. 2). However, MMSE scores were
significantly declined in both groups at T1 (P≤0.001 for both), and
at T2 in group C (P=0.001), as compared with T0. However, MMSE
scores were significantly higher in group D at T2 and T3, as
compared with group C (P=0.025 and P=0.03, respectively).
Conversely, MMSE scores did not differ significantly between the
two groups at T4 and T5, although MMSE scores were markedly
increased at T5 (P>0.05; Fig.
2).
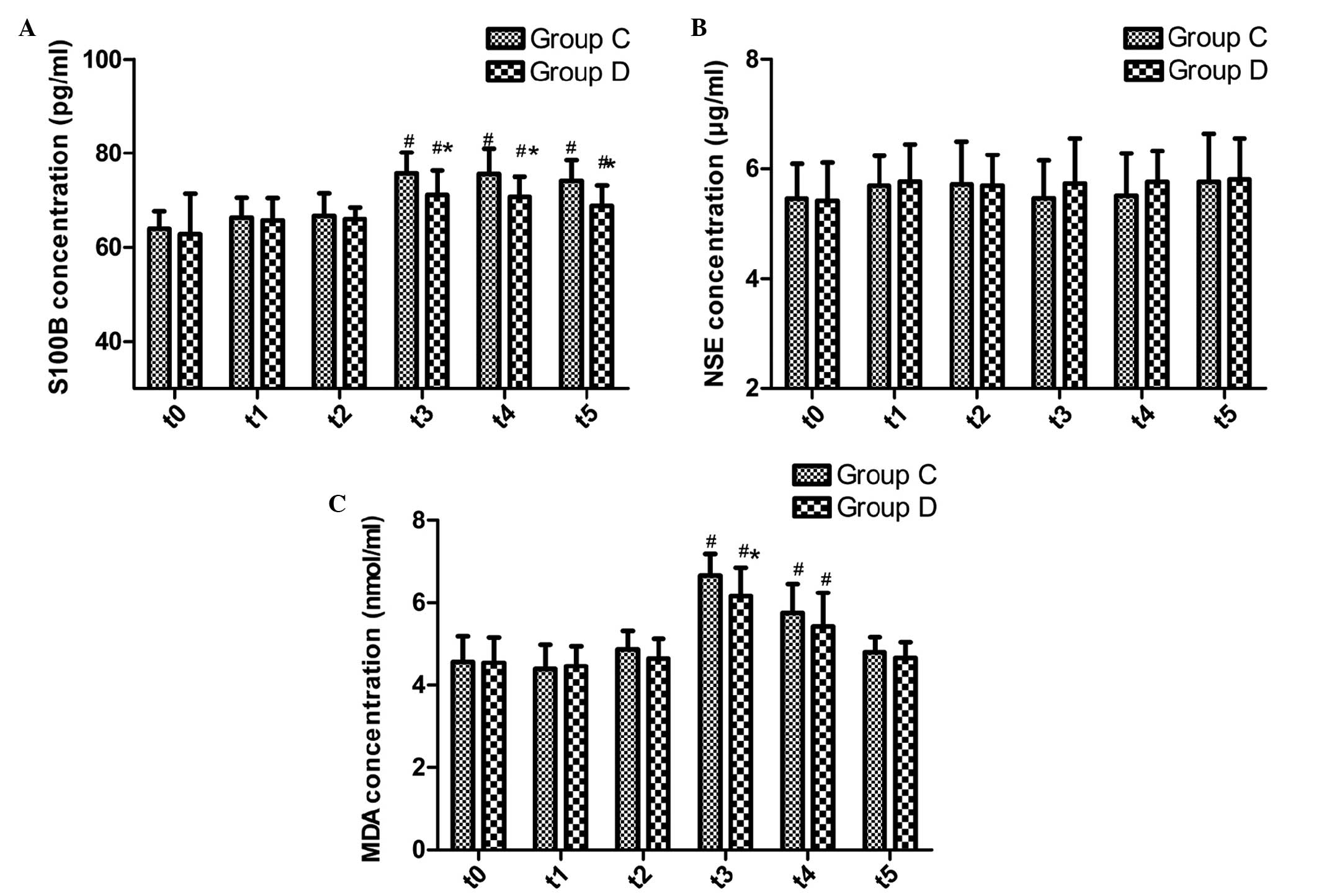
Comparison of serum protein expression
levels of S100B and NSE, and the plasma concentration of MDA
The protein expression levels of S100B and NSE prior
to surgery were not significantly different between the two groups
(P>0.05; Fig. 3A and B). The
serum protein expression levels of S100B were significantly
increased in the two groups at t3–5 (P≤0.001); however, they were
significantly lower in group D at t3–5, as compared with group C
(P=0.005, 0.005 and 0.001, respectively; Fig. 3A). There were no significant
differences in the serum protein expression levels of NSE between
the two groups at any time point (Fig.
3B).
The plasma concentration of MDA increased in the two
groups at t3 and t4 (P≤0.001 for all); however, it was
significantly decreased in group D at t3, as compared with group C
(P=0.017; Fig. 3C).
Discussion
The results of the present study suggested that DEX
infusion during CEA may aid the recovery of postoperative cognitive
function, as demonstrated by elevated MMSE scores and increased
protein expression levels of S100B and NSE in group D at various
time points post-CEA, as compared with group C. In addition, DEX
infusion during CEA was associated with decreased concentrations of
MDA.
In the present study, the number of patients
requiring atropine and vasoconstrictor intervention for the
treatment of bradycardia and severe hypotension, respectively, was
comparable between the two groups. These results suggested that
intravenously administered DEX at the dose used in the present
study may be considered safe for use as an adjuvant to general
anesthetic in patients undergoing CEA. As demonstrated by the
hemodynamic data, the MAP stability was increased following
tracheal intubation in group D, as compared with group C. In
addition, fewer patients were treated with nitroglycerine
intraoperatively in group D, as compared with group C; thus
suggesting that DEX was able to maintain stable circulation.
Previous studies have reported a role for CEA in
stroke prevention in patients with significant carotid stenosis,
including those with and without symptoms (2,3).
However, the effects of CEA on cognitive function remain
controversial, due to mixed reports in the literature.
CEA-associated cognitive dysfunction has previously been detected
in 24–28% of patients following CEA (7,8).
Similarly, in the present study, ~27% of patients exhibited a
reduction in MMSE scores at 48 h post-CEA, as compared with
pre-surgery scores. Previous studies have suggested various
possible mechanisms regarding cognitive impairment post-CEA,
including cerebral ischemia during clamping, emboli from air or the
surgical site, oxidative stress, and postoperative cerebral
hyperperfusion (15,16).
MMSE scores are commonly used as an indication of
cognitive abilities, including memory, attention and language, and
have been demonstrated to be a useful tool for the evaluation of
cognitive dysfunction following CEA (17,18). The
MMSE can be conducted in <10 min by following simple
instructions. Various other neuropsychological scales and tests for
cognitive dysfunction, including the Montreal Cognitive Assessment
and Wechsler Memory Scale, are of limited use due to the complex
training required (19). The present
study applied the MMSE for the evaluation of cognitive dysfunction.
Previous studies demonstrated that a deterioration in MMSE scores
may be associated with clinically identifiable ischemic events,
including subtle stroke and transient ischemic attack (20,21). In
both groups there was a subtle decline in MMSE scores in the early
postoperative period (≤48 h), which may be attributed to asymmetry
in the distribution of cerebral blood flow post-CEA (22,23). In
addition, general anesthesia may have various affects on cognition;
previous studies have suggested that local anesthesia may be
preferable to general anesthesia with regard to the effects on
cognitive function (24,25). In the present study, MMSE scores did
not differ significantly between the two groups at T4 and T5;
however, higher MMSE scores and a lower proportion of patients
exhibiting a decline in MMSE scores were observed in group D at T2
and T3, as compared with group C. These results suggested that DEX
may promote the recovery of cognition following CEA, which is
consistent with theoretical results obtained previously (26).
Previous studies have suggested that adrenergic
receptors, in particular α2-ARs, may have an important role in
promoting cognitive functions, including memory, learning and
selective attention (12,27). Cognition has been shown to be
affected by increased stimulation at the dorsal noradrenergic
bundles located in the locus ceruleus of the mesocephalon, and this
is where α2-ARs are located (13).
Therefore, as a highly selective α2-AR agonist, DEX may enhance
cognitive function following CEA. Previous studies have
demonstrated that DEX may exert organ-protective properties,
including reducing cerebral, cardiac, intestinal and renal injury,
and these effects have been shown to be abolished by atipamezole,
which is an α2-AR antagonist (28–31).
Furthermore, DEX may protect the kidney against I/R injury by
inhibitory effects on injury-induced activation of the Janus
kinase/signal transducer and activator of transcription signaling
pathway (32). Accordingly, DEX may
exert neuroprotective effects on I/R injury during CEA, and further
promote the recovery of cognition in patients.
Reactive oxygen species may have a critical role in
neuronal damage during CEA; the release of oxidative mediators may
be partly facilitated by the injured cerebral endothelium, which
may be further damaged by these mediators, leading to an impairment
of cerebrovascular autoregulation (33). MDA, which is a highly reactive
three-carbon dialdehyde, is a byproduct of polyunsaturated fatty
acid peroxidation. In the present study, the concentration of MDA
was significantly decreased in the jugular bulb of group D patients
following a period of carotid clamping, as compared with group C;
thus suggesting that DEX is able to exert antioxidative activities.
However, the concentration of MDA was not significantly different
between the two groups following carotid declamping, which may be
due to the release of oxidative mediators from the carotid
endothelium following cerebral reperfusion, which may have obscured
the cerebral antioxidative effects of DEX (33). However, the decreased concentration
of MDA in the DEX-treated group following carotid clamping suggest
that DEX may provide an antioxidative benefit in cerebral ischemia,
and this may be the basis of the improved cognitive performance
associated with DEX.
In the present study, improved cognitive function
and the suppression of MDA concentrations were observed to be
associated with decreased protein expression levels of S100B in
group D. S100B is a highly specific biochemical marker of neuronal
damage, and is hypothesized to mediate nuclear factor-κB activation
and the release of proinflammatory cytokines (34). Disrupted astrocytes have been shown
to release massive quantities of S100B following cerebral ischemia,
which may further aggravate cerebral injury. Previous animal
studies have demonstrated an important role for elevated S100B
levels in cognitive decline (35,36). An
increased concentration of S100B may upregulate the expression of
cyclooxygenase-2, which in turn may lead to the deterioration of
spatial memory and learning capabilities via the receptor for
advanced glycation end-products (37,38). The
present study detected increased protein expression levels of S100B
following carotid unclamping, and this was associated with cerebral
injury and a subtle decline in MMSE scores. Furthermore, decreased
protein expression levels of S100B were detected in the DEX-treated
group, as compared with group C, and this appears to be associated
with the neuroprotective effects of DEX. However, the underlying
molecular mechanisms remain unclear.
In the present study, the serum protein expression
levels of NSE were determined as an additional marker of cerebral
injury; the serum protein expression levels of NSE were not
significantly different between the two groups at any time point.
These results may be due to the short sampling time; previous
studies have reported that NSE is a promising marker of cerebral
damage induced by cardiac arrest at later (>24 h) stages only
(39,40). Therefore, significant differences in
the protein expression levels of NSE post-CEA between the groups
may have been detected if the study duration had been
increased.
Various limitations were associated with the present
study, including the small number of patients that were enrolled.
In addition, the cognition evaluation was conducted using a single
test, whereas a combination of various cognitive function tests may
have increased the objectivity of the evaluation. Furthermore, the
post-CEA administration of other drugs was not standardized, which
may have had knock-on effects on cognitive impairment, and the
present study only investigated the effects of a single dose of
DEX, such that the neuroprotective effects of DEX in larger or
lower doses remain unknown. Therefore, future studies incorporating
a larger sample size, and which extend the study duration to later
time points post-CEA, are required.
In conclusion, the results of the present study
suggested that the infusion of DEX during CEA may improve the
recovery of cognitive performance post-CEA. These cognitive
improvements may be due to the antioxidative effects of DEX in the
ischemic cerebral circulation and associated with decreased levels
of S100B. Future studies should investigate the neuroprotective
potential of DEX at various doses, as well as studying the
underlying molecular mechanism.
Acknowledgements
This study was funded by the National Natural
Science Fund (grant no. 81171838), and the Twelfth Five-year Key
Talents Project of Jiangsu Province (grant no. RC2011041).
References
|
1
|
Barnett HJ and Haines SJ: Carotid
endarterectomy for asymptomatic carotid stenosis. N Engl J Med.
317:1468. 1998.
|
|
2
|
North American Symptomatic Carotid
Endarterectomy Trial Collaborators: Beneficial effect of carotid
endarterectomy in symptomatic patients with high-grade carotid
stenosis. N Engl J Med. 325:445–453. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Halliday A, Mansfield A, Marro J, Peto C,
Peto R, Potter J and Thomas D: MRC Asymptomatic Carotid Surgery
Trial (ACST) Collaborative Group: Prevention of disabling and fatal
strokes by successful carotid endarterectomy in patients without
recent neurological symptoms: Randomised controlled trial. Lancet.
363:1491–1502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berman L, Pietrzak RH and Mayes L:
Neurocognitive changes after carotid revascularization: A review of
the current literature. J Psychosom Res. 63:599–612. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Rango P, Caso V, Leys D, Paciaroni M,
Lenti M and Cao P: The role of carotid artery stenting and carotid
endarterectomy in cognitive performance: A systematic review.
Stroke. 39:3116–3127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Irvine CD, Gardner FV, Davies AH and
Lamont PM: Cognitive testing in patients undergoing carotid
endarterectomy. Eur J Vasc Endovasc Surg. 15:195–204. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heyer EJ, Sharma R, Rampersad A, Winfree
CJ, Mack WJ, Solomon RA, Todd GJ, McCormick PC, McMurtry JG, Quest
DO, et al: A controlled prospective study of neuropsychological
dysfunction following carotid endarterectomy. Arch Neurol.
59:217–222. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heyer EJ, DeLaPaz R, Halazun HJ, Rampersad
A, Sciacca R, Zurica J, Benvenisty AI, Quest DO, Todd GJ, Lavine S,
et al: Neuropsychological dysfunction in the absence of structural
evidence for cerebral ischemia after uncomplicated carotid
endarterectomy. Neurosurgery. 58:474–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guler G, Akin A, Tosun Z, Eskitascoglu E,
Mizrak A and Boyaci A: Single-dose dexmedetomidine attenuates
airway and circulatory reflexes during extubation. Acta
Anaesthesiol Scand. 49:1088–1091. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goyagi T, Nishikawa T, Tobe Y and Masaki
Y: The combined neuroprotective effects of lidocaine and
dexmedetomidine after transient forebrain ischemia in rats. Acta
Anaesthesiol Scand. 53:1176–11832009. View Article : Google Scholar
|
|
11
|
Sato K, Kimura T, Nishikawa T, Tobe Y and
Masaki Y: Neuroprotective effects of a combination of
dexmedetomidine and hypothermia after incomplete cerebral ischemia
in rats. Acta Anaesthesiol Scand. 54:377–382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, Yan J, Zhou P, Li J, Gao H, Xia Y
and Wang Q: Neurotransmitter receptors and cognitive dysfunction in
Alzheimer's disease and Parkinson's disease. Prog Neurobiol.
97:1–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Milstein JA, Lehmann O, Theobald DE,
Dalley JW and Robbins TW: Selective depletion of cortical
noradrenaline by anti-dopamine beta-hydroxylase-saporin impairs
attentional function and enhances the effects of guanfacine in the
rat. Psychopharmacology (Berl). 190:51–63. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sani NF, Belani LK, Sin CP, Rahman SN, Das
S, Chi TZ, Makpol S and Yusof YA: Effect of the combination of
gelam honey and ginger on oxidative stress and metabolic profile in
streptozotocin-induced diabetic Sprague-Dawley rats. Biomed Res
Int. 2014:1606952014.PubMed/NCBI
|
|
15
|
Wilson DA, Mocco J, D'Ambrosio AL, Komotar
RJ, Zurica J, Kellner CP, Hahn DK, Connolly ES, Liu X, Imielinska C
and Heyer EJ: Post-carotid endarterectomy neurocognitive decline is
associated with cerebral blood flow asymmetry on post-operative
magnetic resonance perfusion brain scans. Neurol Res. 30:302–306.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wolf O, Heider P, Heinz M, Poppert H,
Sander D, Greil O, Weiss W, Hanke M and Eckstein HH: Microembolic
signals detected by transcranial Doppler sonography during carotid
endarterectomy and correlation with serial diffusion-weighted
imaging. Stroke. 35:e373–e375. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sabbagh M, Cummings J, Christensen D,
Doody R, Farlow M, Liu L, Mackell J and Fain R: Evaluating the
cognitive effects of donepezil 23 mg/d in moderate and severe
Alzheimer's disease: Analysis of effects of baseline features on
treatment response. BMC Geriatr. 13:562013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mukonzo JK, Okwera A, Nakasujja N, Luzze
H, Sebuwufu D, Ogwal-Okeng J, Waako P, Gustafsson LL and Aklillu E:
Influence of efavirenz pharmacokinetics and pharmacogenetics on
neuropsychological disorders in Ugandan HIV-positive patients with
or without tuberculosis: A prospective cohort study. BMC Infect
Dis. 13:2612013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arpaci AH and Bozkırlı F: Comparison of
sedation effectiveness of remifentanil-dexmedetomidine and
remifentanil-midazolam combinations and their effects on
postoperative cognitive functions in cystoscopies: A randomized
clinical trial. J Res Med Sci. 18:107–114. 2013.PubMed/NCBI
|
|
20
|
Arciniegas DB, Kellermeyer GF, Bonifer NM,
Anderson-Salvi KM and Anderson CA: Screening for cognitive decline
following single known stroke using the Mini-Mental State
Examination. Neuropsychiatr Dis Treat. 7:189–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Capoccia L, Sbarigia E, Rizzo A, Mansour W
and Speziale F: Silent stroke and cognitive decline in asymptomatic
carotid stenosis revascularization. Vascular. 20:181–187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gaunt ME, Martin PJ, Smith JL, Rimmer T,
Cherryman G, Ratliff DA, Bell PR and Naylor AR: Clinical relevance
of intraoperative embolization detected by transcranial Doppler
ultrasonography during carotid endarterectomy: A prospective study
of 100 patients. Br J Surg. 81:1435–1439. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kügler CF, Funk H, Vlajic P and Platt D:
The relationship between endothelin-1, event-related P300
potentials, and prognosis in cerebral arteriosclerosis. J Am
Geriatr Soc. 45:427–434. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aleksic M, Huff W, Hoppmann B, Heckenkamp
J, Pukrop R and Brunkwall J: Cognitive function remains unchanged
after endarterectomy of unilateral internal carotid artery stenosis
under local anaesthesia. Eur J Vasc Endovasc Surg. 31:616–621.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weber CF, Friedl H, Hueppe M, Hintereder
G, Schmitz-Rixen T, Zwissler B and Meininger D: Impact of general
versus local anesthesia on early postoperative cognitive
dysfunction following carotid endarterectomy: GALA Study Subgroup
Analysis. World J Surg. 33:1526–1532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen J, Yan J and Han X: Dexmedetomidine
may benefit cognitive function after laparoscopic cholecystectomy
in elderly patients. Exp Ther Med. 5:489–494. 2013.PubMed/NCBI
|
|
27
|
Galeotti N, Bartolini A and Ghelardini C:
Alpha-2 agonist-induced memory impairment is mediated by the
alpha-2A-adrenoceptor subtype. Behav Brain Res. 153:409–417. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sanders RD, Sun P, Patel S, Li M, Maze M
and Ma D: Dexmedetomidine provides cortical neuroprotection: Impact
on anaesthetic-induced neuroapoptosis in the rat developing brain.
Acta Anaesthesiol Scand. 54:710–716. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang XY, Liu ZM, Wen SH, Li YS, Li Y, Yao
X, Huang WQ and Liu KX: Dexmedetomidine administration before, but
not after, ischemia attenuates intestinal injury induced by
intestinal ischemia-reperfusion in rats. Anesthesiology.
116:1035–1046. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoshitomi O, Cho S, Hara T, Shibata I,
Maekawa T, Ureshino H and Sumikawa K: Direct protective effects of
dexmedetomidine against myocardial ischemia-reperfusion injury in
anesthetized pigs. Shock. 38:92–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gu J, Sun P, Zhao H, Watts HR, Sanders RD,
Terrando N, Xia P, Maze M and Ma D: Dexmedetomidine provides
renoprotection against ischemia-reperfusion injury in mice. Crit
Care. 15:R1532011. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Si Y, Bao H, Han L, Shi H, Zhang Y, Xu L,
Liu C, Wang J, Yang X, Vohra A and Ma D: Dexmedetomidine protects
against renal ischemia and reperfusion injury by inhibiting the
JAK/STAT signaling activation. J Transl Med. 11:1412013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saito H, Ogasawara K, Komoribayashi N,
Kobayashi M, Inoue T, Otawara Y and Ogawa A: Concentration of
malondialdehyde-modified low-density lipoprotein in the jugular
bulb during carotid endarterectomy correlates with development of
postoperative cognitive impairment. Neurosurgery. 60:1067–1073.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mori T, Asano T and Town T: Targeting
S100B in cerebral ischemia and in Alzheimer's disease. Cardiovasc
Psychiatry Neurol. 2010:6870672010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leclerc E, Sturchler E and Vetter SW: The
S100B/RAGE axis in Alzheimer's disease. Cardiovasc Psychiatry
Neurol. 2010:5395812010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bell K, Shokrian D, Potenzieri C and
Whitaker-Azmitia PM: Harm avoidance, anxiety, and response to
novelty in the adolescent S-100beta transgenic mouse: Role of
serotonin and relevance to Down syndrome. Neuropsychopharmacology.
28:1810–1816. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sorci G, Bianchi R, Riuzzi F, Tubaro C,
Arcuri C, Giambanco I and Donato R: S100B protein, a
damage-associated molecular pattern protein in the brain and heart,
and beyond. Cardiovasc Psychiatry Neurol. 2010:6564812010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peng M, Wang YL, Wang FF, Chen C and Wang
CY: The cyclooxygenase-2 inhibitor parecoxib inhibits
surgery-induced proinflammatory cytokine expression in the
hippocampus in aged rats. J Surg Res. 178:e1–e8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rosén H, Sunnerhagen KS, Herlitz J,
Blomstrand C and Rosengren L: Serum levels of the brain-derived
proteins S-100 and NSE predict long-term outcome after cardiac
arrest. Resuscitation. 49:183–191. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rech TH, Vieira SR, Nagel F, Brauner JS
and Scalco R: Serum neuron-specific enolase as early predictor of
outcome after in-hospital cardiac arrest: A cohort study. Crit
Care. 10:R1332006. View
Article : Google Scholar : PubMed/NCBI
|