Introduction
Vertebrobasilar ischemia (VBI) vertigo is an
otolaryngological disease that is common in elderly patients. The
cerebellum and brain stem rely on blood flow from the
vertebrobasilar arteries; however, when lesions develop in the
vertebrobasilar arteries, poor blood supply to the brain leads to
dizziness, referred to as central vestibular vertigo (1). Lesions within the vestibular nuclei,
brain stem, cerebellum and temporal lobe are the predominant cause
of central vestibular vertigo, which presents with a continuous and
progressive set of symptoms (2). VBI
vertigo accounts for >20% of defective cerebrovascular disease
(3), and is a common cause of
vertigo in senile patients, ~5% of which develop complete cerebral
apoplexy (4). Clinical
manifestations, including dizziness, nausea and vomiting, often
occur, and resistance to treatment is characteristic of VBI vertigo
(5). The primary symptom in 50–80%
of patients with VBI is dizziness (6) with tinnitus and hearing loss. VBI has
multiple risk factors, including increased platelet aggregation
rate, high blood pressure, high blood viscosity, hyperlipidemia,
cervical spondylosis, bradycardia and low blood sugar (1). During an acute episode, patients suffer
from dizziness combined with nausea, vomiting, instability and
difficulty of movement, which should be treated rapidly. Generally
comprehensive measures, which aim to reduce artery spasm, improve
brain microcirculation, improve brain tissue oxygen supply and
restore the brain function, as well as intramuscular or intravenous
injection or reducing oral drugs are considered to be the primary
measures for the treatment of VBI vertigo.
Kudzu root, which is known as ‘Ge gen’ in
traditional Chinese medicine, is the dried root of Pueraria
lobata (Willd.) Ohwi; a perennial, leguminous vine that is
native to southeast Asia. For >2,000 years, kudzu root has been
used as a herbal medicine in the treatment of fever, acute
dysentery, diarrhoea, diabetes and cardiovascular disease,
containing isoflavonoids and triterpenoids as its predominant
active constituents (7). Puerarin is
a flavonoid glycoside, and is the major bioactive ingredient and
the most abundant secondary metabolite present in the root of P.
lobata (Willd.) Ohwi. Puerarin is available in numerous foods,
is used in alternative medicine (8)
and is able to expand the cerebrovascular and coronary arteries
(9). This has been hypothesized to
produce numerous downstream effects, including reducing myocardial
oxygen consumption, inhibiting platelet aggregation, improving
erythrocyte deformability, eliminating oxygen free radicals and
improving the function of blood rheology, thereby ameliorating the
insufficient vertebrobasilar blood supply (10). Puerarin is increasingly employed in
treatment of cardiovascular and cerebrovascular diseases, diabetes
with its associated complications (11), Parkinson's and Alzheimer's disease
(12), osteonecrosis (13), cancer and endometriosis (14). The effectiveness of puerarin in these
diverse medicinal contexts may be due to its numerous suggested
pharmacological functions, which potentially include vasodilative
(15), cardioprotective (16), neuroprotective (17), antioxidative (18), anticancer (19) and anti-inflammatory effects, in
addition to pain alleviation, increased osteogenesis (20), reduced alcohol intake and decreased
resistance to insulin (8).
Previous studies have increasingly reported that
combining puerarin with betahistine or with other, conventional
drugs may enhance its effects in relieving dizziness and other side
effects in patients with VBI (21–29).
Furthermore, the treatment of insufficient blood supply within the
vertebrobasilar arteries using a single drug has its limitations,
since different treatments should be adopted according to the cause
of insufficient blood supply, which can be complex. For instance,
platelet aggregation inhibitors, vascular expansion agents,
cerebral vasodilator and albumin light quantum therapy can be used
for atherosclerosis, while cervical traction can be used for
cervical hyperostosis. Systematic consideration of all appropriate
evidence on use of a particular factor, such as by meta-analysis,
is therefore required (30). This
may be used to identify relevant studies, extract relevant data,
appraise study methods and statistically evaluate the associated
studies (31,32). To the best of our knowledge, the
majority of the studies regarding puerarin and betahistine combined
treatment of VBI vertigo has not been rigorously investigated.
Therefore, an evaluation of the efficacy and safety of this
combination of traditional Chinese medicine and modern treatment
for VBI vertigo is required. In the present study, a systematic
evaluation of randomized controlled trials (RCTs) of puerarin and
betahistine combinatorial treatment of VBI vertigo was conducted in
order to assess its overall effectiveness and safety and to provide
the basis for subsequent clinical studies regarding its
application.
Materials and methods
Search strategy
Electronic databases were searched, including
entries from the following periods: Chinese National Knowledge
Infrastructure (CNKI; www.cnki.net/), January 1987 to August 2014; Wanfang
Med Online Database (WMOD; www.wanfangdata.com.cn/), April 1998 to August 2014;
Chinese VIP Information (VIP; www.cqvip.com/), February 1989 to August 2014; PubMed
(www.pubmed.cn/), November 1967 to August 2014;
the Cochrane Central Register of Controlled Trials (www.cochranelibrary.com/), March 1999 to August
2014; and the Chinese Biomedical Literature Database (CBM;
www.sinomed.ac.cn/), October 1978 to August 2014.
The search terms used were ‘puerarin’, ‘betahistine’, ‘betahistine
hydrochloride)’ and ‘vertebrobasilar ischemia vertigo’. No language
restriction was applied.
Study selection and eligibility
criteria
The titles and abstracts of potentially relevant
references were identified through the literature search, checked
to determine their suitability with regard to the meta-analysis
inclusion criteria and independently reviewed by two reviewers.
Discrepancies regarding whether to include a study were resolved by
consensus with an additional investigator. The present study only
considered RCTs using puerarin and betahistine treatment of VBI
vertigo, irrespective of language, whether the RCT was from a
periodical or magazine, publishing year and database.
Inclusion criteria were as follows: i) Studies were
RCTs, regardless of whether these were blinded studies; ii) sample
sizes ≥20 participants; iii) based on strict diagnostic criteria of
VBI vertigo, according to the guidelines of the World Health
Organization (33), but regardless
of age and gender; iv) patients in the intervention group were
co-administered an injection of puerarin (such as Chia Tai-Tianqing
Pharmaceutical Group Co., Ltd., Jiangsu, China) and betahistine
(such as Yabao Pharmaceutical Group Co., Ltd., Beijing, China),
administered via multiple routes. The control groups were
administered a routine treatment, such as betahistine, compound
Danshen injection (which consists of extracts of Salvia
miltiorrhiza and Dalbergiae odoriferae), sibelium or low
molecular weight dextran, regardless of manufacturer, preparation
form, dose or duration of treatment.
Selected outcomes
The primary outcome measure was clinical efficacy,
which was expressed as follows: Cured, when signs and symptoms
disappear entirely, and transcranial Doppler (TCD) indicates that
the vertebral/basilar artery blood supply returns to normal;
excellent, when symptoms, such as dizziness, are significantly
reduced and patients can have a regular life, while TCD displays
that the vertebral/basilar artery blood supply is improved by
>60%; valid or effective, in cases when the symptoms are
slightly reduced slightly, and TCD displays improvement of >40%
in the vertebral/basilar artery blood supply; and invalid, in cases
when the symptoms are not evidently relieved, and TCD displays
improvement of <40% in the vertebral/basilar artery blood
supply. The secondary outcome measure was the increase in average
blood flow velocity of the vertebrobasilar arteries (the basilar
artery and the left and right vertebral arteries). The final
outcome considered was the incidence of adverse reactions
(including diarrhea, nausea and polypnea) of the patients in the
two groups.
Data extraction and management
Data from each review were extracted by two
independent authors in accordance with the aforementioned criteria,
using a self-developed data extraction form in Microsoft Excel 2010
(Microsoft Corporation, Redmond, WA, USA), and the data were
validated by a third author. Disagreements were resolved by
discussion between the two reviewers, and by the opinion of a third
reviewer if necessary. Details noted from the studies included
study design, treatment duration, the age of participants, the
number of participants and assessments of the aforementioned
outcomes.
Trial quality assessment
In order to reduce subjective bias, the two authors
independently appraised the methodological quality of each review
in accordance with the Cochrane handbook (34) and quality assessment of the RCTs was
conducted using Jadad's validated score (35). Quality assessment included evaluation
of randomization, blinding of assessors to the treatment groups,
allocation concealment regardless of the results of the allocation
and intentional analysis. Each of these criteria was worth one
point; a study scoring >3 points was considered to be
high-quality, while <3 points indicated a low-quality study.
Statistical analysis
Statistical analyses were conducted using RevMan
version 5.0.18 (The Nordic Cochrane Centre, The Cochrane
Collaboration, Copenhagen, Denmark) software. An examination of
odds ratio (OR) was used to analyze efficacy of the
numerical/quantitative data, the interval size is expressed using
95% confidence interval (CI) and the heterogeneity of the results
was assessed using a χ2 test.
The studies were initially tested for clinical
heterogeneity. A fixed-effects model was used upon detection of
statistical homogeneity (indicated by a P-value of >0.1 or an
I2 value <50%); however, a random effect model would
be used upon detection of statistical heterogeneity (indicated by a
P-value of <0.1 or an I2 value >50%) (36) or when no clinical heterogeneity or no
statistically significant difference existed in the trials. If
there was clinical and methodological heterogeneity,
meta-regression, subgroup analysis and sensitivity analysis were
conducted (37). In the presence of
clear heterogeneity, only a descriptive analysis of the data was
made. Binary classification variables were expressed using OR,
continuous variables were expressed using the weighted mean
differences or standardized mean differences and effect size was
expressed by the 95% CI. P<0.05 was considered to indicate a
statistically significant difference. Publication bias was assessed
by a funnel plot (38).
Results
Study selection
Description of studies
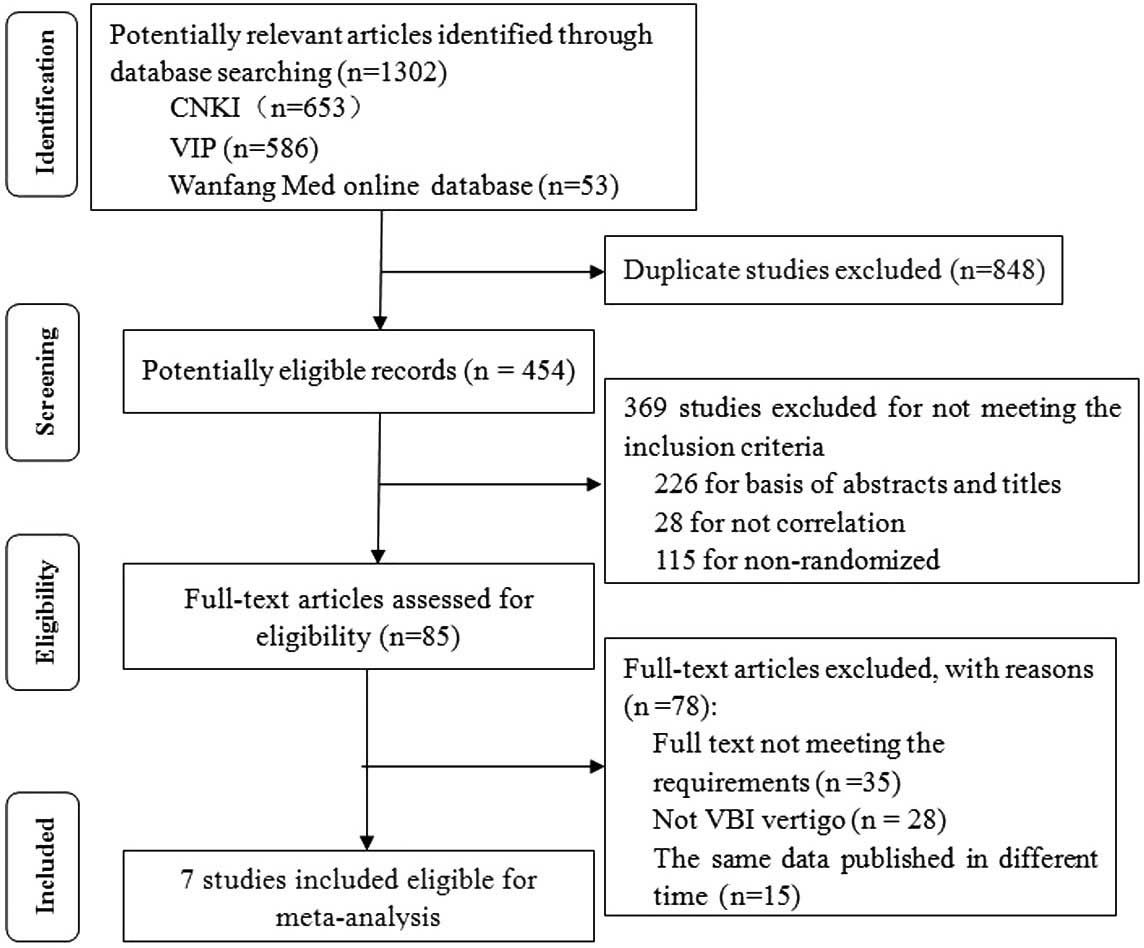
Initial searches yielded 1,302 studies (653 from
CNKI, 586 from VIP, 53 from Wanfang Med Online Database) in
accordance with the aforementioned search strategy. Following
removal of 848 duplicate studies, the abstracts of 454 studies were
evaluated. Subsequent to the evaluation of the abstract and title
of each study, 369 studies were excluded as they did not meet the
inclusion criteria. The full text of each of the remaining 85
trials was read, and 35 trials were excluded in accordance with the
study criteria; 28 of these trials did not examine VBI vertigo and
15 trials included repetition of data, leaving 7 trials remaining.
The results of the present meta-analysis are therefore based on
information from 7 RCTs (21–27),
which included a total of 664 patients. These RCTs were all
conducted in China and published in Chinese. Fig. 1 presents a flow chart of the trial
selection process.
Characteristics of trials included
The characteristics of the included 7 RCTs are
summarized in Table I. Data
regarding patient age, gender, duration of treatment, the number of
participants and adverse reactions are provided for all trials. The
average age of the participants ranged from 53.0–64.32 years, the
duration of treatment ranged from 7–14 days and the number of
patients in the studies ranged from 23–69, with a total of 664
patients (333 patients in treatment groups) across the 7 RCTs. The
available data revealed that studies predominantly included male
patients. Drugs for the intervention group included puerarin
injection in addition to betahistine hydrochloride tablets
(21,24), betahistine mesylate (22,23,26,27),
which was administered orally or through intravenous drip, or
betahistine injection (25).
Conventional drugs administered to the control groups included
Compound Danshen injection with low molecular weight dextran
(21,22,25,26),
Compound Danshen injection only (23), sibelium capsule with low molecular
weight dextran (24) or betahistine
mesylate only (27). Publication
year of the 7 RCTs ranged between 2007–2013, and trials published
in 2010–2013 represented 4/7 of these trials (57.14%). With regard
to the evaluated outcomes, the reviews assessed clinical symptoms
and signs (4/7, 57.14%), vertigo symptoms and signs (3/7, 42.86%)
and alterations in hemorheology (2/7, 28.57%). Adverse reactions
were not reported in 3 trials (21,23,27).
 | Table I.Characteristics of the included
studies. |
Table I.
Characteristics of the included
studies.
| Author, year | Group: no. patients
(male/female) | Average age
(years) | Intervention
group | Control group | Duration
(days) | Outcome
measures | Adverse
reactions | Ref. |
|---|
| Sun and Dang, | 69
(22/47) | 59 | PI + BHT | CDI + LMD | 7 | A | Not mentioned | (21) |
| 2007 | C: 69 (28/41) | 59 |
|
|
|
|
|
|
| Yue et
al., | T: 46 (26/20) | 56±7 | PI + BM | CDI + LMD | 7 | A | Dizziness,
nausea | (22) |
| 2013 | C: 45 (26/19) | 56±7 |
|
|
|
|
|
|
| Yang, | T: 23 (13/10) | 57±8 | PI + BM | CDI | 14 | A,B | Not mentioned | (23) |
| 2010 | C: 23 (14/9) | 57±9 |
|
|
|
|
|
|
| Wang, | T: 30 (21/9) | 55 | PI + BHT | SC + LMD | 14 | A,B | Skin itching | (24) |
| 2011 | C: 30 (20/10) | 53 |
|
|
|
|
|
|
| Xie, | T: 79 (45/34) | 64±10 | PI + BI | CDI + LMD | 14 | A,B | Facial flush, heart
palpitations, dry mouth, abdominal discomfort, nausea, fever | (25) |
| 2008 | C: 79 (47/32) | 64±8 |
|
|
|
|
|
|
| Guo, | T: 43 (24/19) | 56±7 | PI + BM | CDI + LMD | 7 | A,B | Diarrhea,
nausea | (26) |
| 2012 | C: 44 (25/19) | 56±7 |
|
|
|
|
|
|
| Lei, | T: 43 (14/29) | 57 | PI + BM | BM | 7 | A | Not mentioned | (27) |
| 2008 | C: 40 (12/28) | 58 |
|
|
|
|
|
|
Methodological quality of included trials
In the 7 studies included, 1 trial (26) mentioned that the intervention and
control groups were generated using a random digits table; however,
the remaining articles did not mention a randomization method.
Allocation concealment and intentional analysis were not mentioned
in any of the studies included in the present meta-analysis.
Primary outcomes
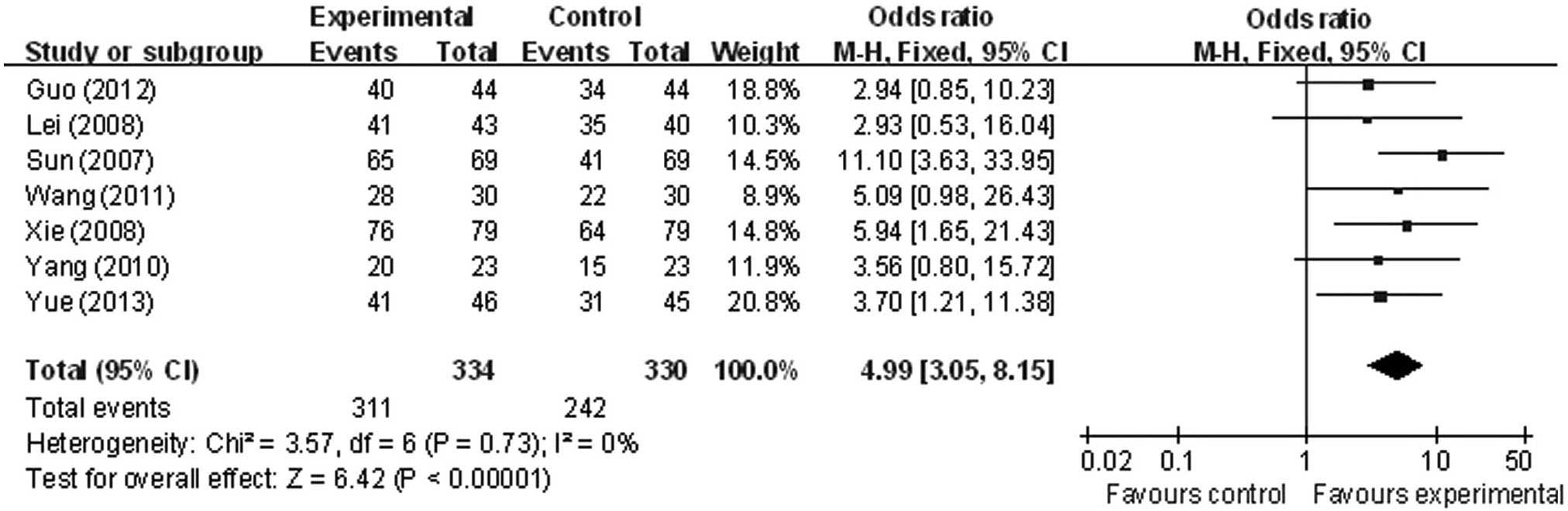
Evaluation of therapeutic efficacy
Clinical efficacy (determined as cured, excellent,
effective or invalid) was the primary factor studied, and the total
effective rate was reported in all 7 studies (21–27).
Analysis revealed statistical homogeneity of the total effective
rate within the present studies (χ2=3.57; P=0.73;
I2=0%), leading to adoption of the fixed-effect model
for meta-analysis. However, a statistically significant difference
was identified in total efficacy rate between the intervention and
control groups (OR, 4.99; 95% CI, 3.05 to 8.15) (Fig. 2).
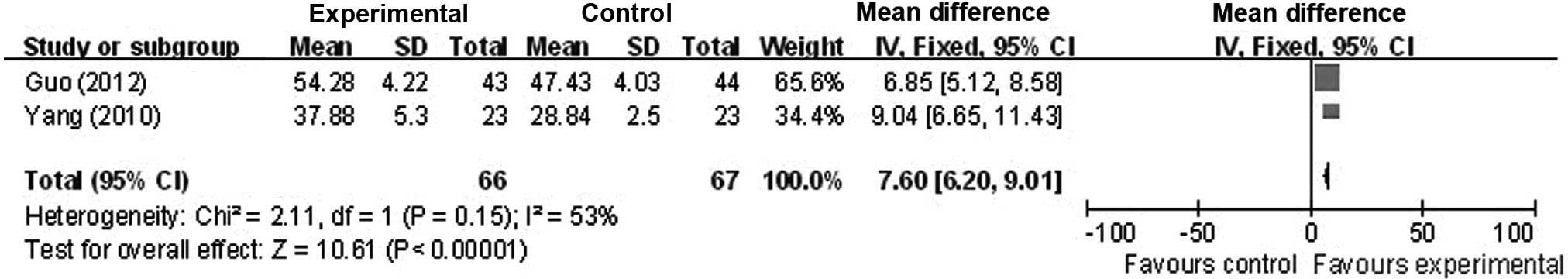
Assessment of the average blood flow velocity of
the vertebrobasilar arteries
Inevitably, studies brought together in a systematic
review will differ; any kind of variability among studies in a
systematic review may be termed heterogeneity (39). Increase in the average blood flow
velocity of the vertebrobasilar arteries (the basilar artery and
left and right vertebral arteries) of patients with VBI vertigo was
reported in 2 studies (23,26). A χ2 test was performed to
determine whether there were significant differences in homogeneity
amongst the studies; this indicated no significant homogeneity in
the change observed in blood flow velocity of the vertebrobasilar
arteries (χ2=2.11; P=0.15; I2=53%). A
meta-analysis was therefore performed using a fixed-effects model.
Statistical analyses revealed a significant increase in blood flow
velocity of vertebrobasilar arteries between the control and
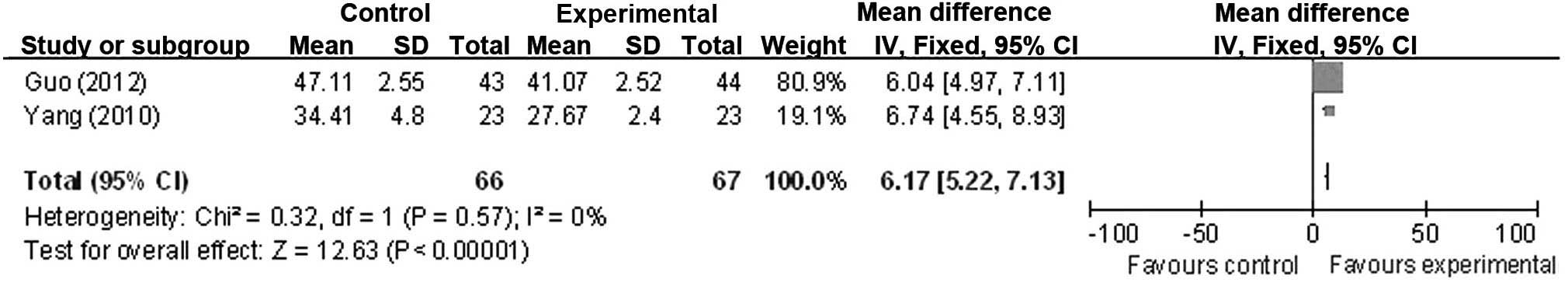
intervention groups (OR, 7.60; 95% CI, 6.20 to 9.01) (Fig. 3). There was no significant
heterogeneity of the increase observed in the average blood flow
velocity of the left vertebral artery amongst the studies
(χ2=0.32; P=0.57; I2=0%), leading to
application of the fixed-effect model. Statistical results revealed
a statistically significant increase in the average blood flow
velocity of the left vertebral artery (OR, 6.17; 95% CI, 5.22 to
7.13) (Fig. 4). However, significant
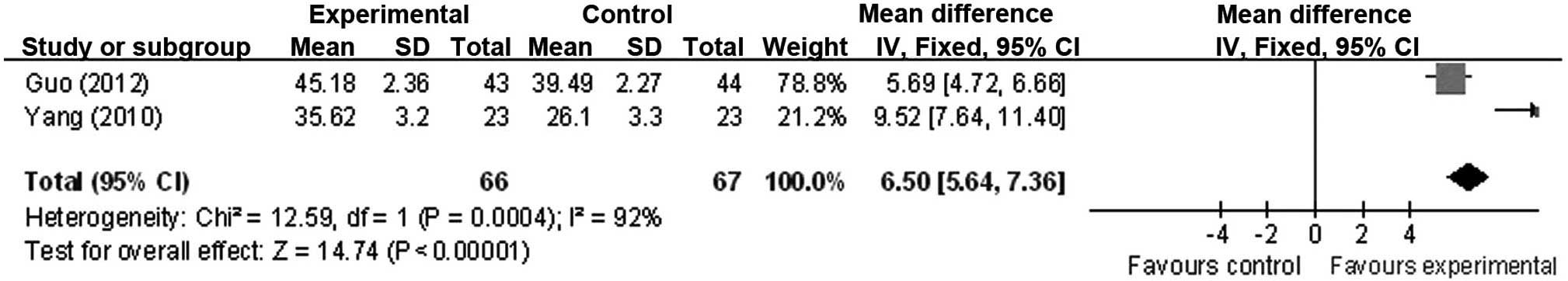
heterogeneity was reported in the change to blood flow velocity of
the right vertebral artery amongst the studies
(χ2=12.59; P=0.0004; I2=92%). In order to
identify the cause of the heterogeneity, a sensitivity analysis and
an additional subgroup analysis were performed; however, they were
unable to indicate the etiology of the heterogeneity, meaning that
descriptive analysis was conducted (Fig.
5).
A total of 2 studies (23,26)
reported that, following treatment, the average blood flow velocity
of the right vertebral artery in the experimental group was
35.62±3.20 and 45.18±2.36 cm/s; the corresponding control values
were 26.10±3.30 and 39.49±2.27 cm/s, respectively. The difference
between the groups in the two trials was statistically significant
(P<0.05).
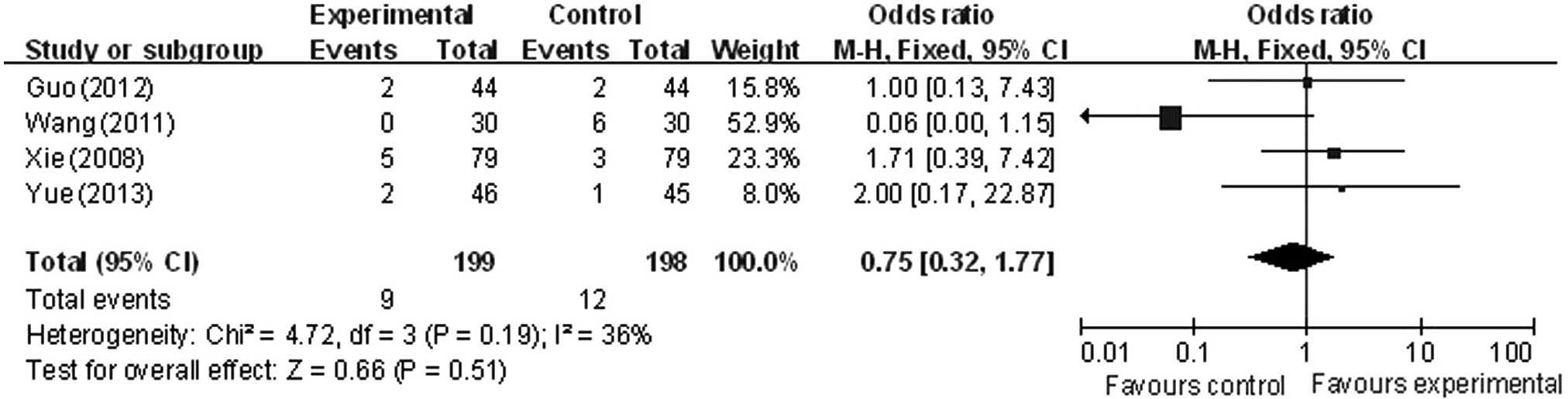
Adverse effects
Adverse reactions of the experimental and control
groups included 396 cases (199 cases reported within the
experimental groups and 198 cases in the control groups), reported
across 4 trials (22,24–26).
Analysis demonstrated that the difference in heterogeneity amongst
the trials was not significant (χ2=4.72; P=0.19;
I2=36%), and the fixed-effect model was used. There was
a statistically significant difference in adverse events between
the intervention and control groups (OR, 0.75; 95% CI, 0.32 to
1.77), suggesting a lower incidence of adverse reactions in the
experimental than the control group in the treatment of VBI vertigo
(Fig. 6).
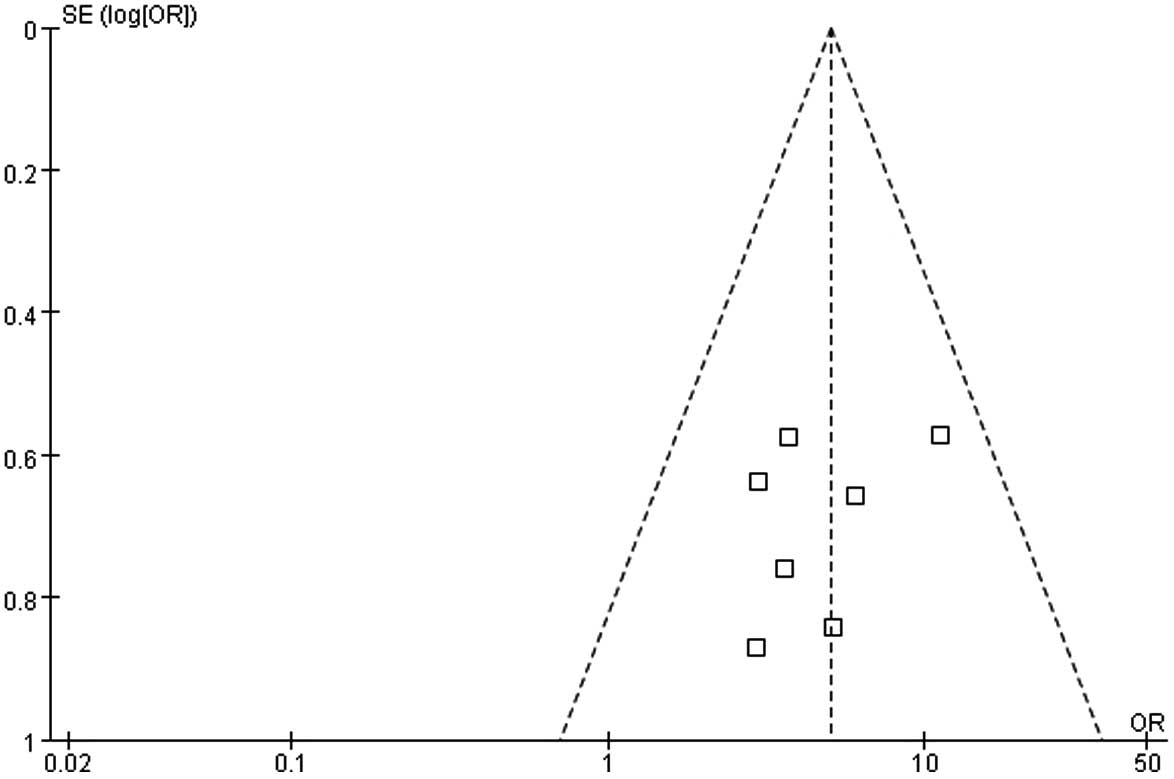
Publication bias
A funnel plot was used to detect publication bias
(Fig. 7). All 7 studies included in
the present meta-analysis were within 95% CI, and the plot is an
inverted funnel shape, with the results predominantly within the
middle-lower region of the funnel plot. However, the distribution
was slightly asymmetric, which suggests a possible publication
bias; however, the publication bias was lower.
Discussion
The present meta-analysis examined 7 RCTs, including
a total of 664 participants, and reviewed clinical studies of
puerarin combined with betahistine in the treatment of VBI vertigo,
evaluating the safety and efficacy of this co-treatment. The
majority of the trials reported that this combination treatment was
effective in treating VBI vertigo, evaluated based on the total
efficacy rate, the improvements of vertigo symptoms, increased
average blood flow velocity of the basilar artery or fewer adverse
reactions. The overall methodological quality of the 7 trials was
‘moderate’ or ‘low.’ However, to the best of our knowledge a number
of the trials included in the present study had not previously been
included in meta-analyses, which should enhance the validity of the
present evaluation of the effects of puerarin co-treatment with
betahistine.
VBI vertigo, caused by ischemia within the
vestibular system (40), is a common
disease in elderly patients, who are likely to be more vulnerable
to its associated risks. Furthermore, VBI vertigo markedly affects
the quality of life and health of patients (10). Previous clinical studies have
reported that puerarin may improve blood circulation in the neck
(28), inhibit platelet aggregation
(41), improve the deformability of
red blood cells (42) and expand the
coronary artery and cerebrovascular system, thereby promoting blood
microcirculation (29). Puerarin is
therefore widely used in clinical prevention and treatment of a
variety of ischemic cardiovascular and cerebrovascular diseases
(43).
The results of the present meta-analysis suggested
that puerarin and betahistine co-treatment produced positive
results in patients with VBI vertigo compared with the control
groups, providing support for its clinical application. The present
results demonstrated that the total efficacy rate was higher in the
experimental groups compared with the control groups. Improvements
of the vertigo symptoms and an increased average blood flow
velocity of the basilar artery were reported in 2 trials (23,26).
However, the small sample size (seven RCTs) and three relatively
low-quality studies (2 points by Jadad's validated score) may have
limited the accuracy of the statistical analyses, which in turn may
have reduced the validity of the present study. The current study
demonstrated that puerarin combinatorial treatment with betahistine
increased the average blood flow velocity of the basilar artery of
the patients with VBI. However, due to the prevalence of limiting
factors, such as short treatment duration, lack of detail regarding
follow-up investigation and small sample sizes, the results of the
present study require additional investigation.
A total of 4 studies (22,24–26)
reported adverse reactions in the intervention and control groups,
which primarily included nausea and abdominal discomfort; however,
these occurred at lower incidence in the intervention group. Due to
the small sample size and quality issues with the studies, this
analysis should be repeated on a greater number of high-quality
RCTs.
The present analysis has a number of potential
limitations, warranting conservative interpretation. Firstly, the
quality of the RCTs included was generally low. The included RCTs
claimed randomization, but provided limited description of the
methodology underlying this, which may lead to selection bias.
Secondly, the majority of the included systematic reviews used the
improvement of vertigo and other clinical symptoms as the primary
outcomes. However, these studies predominantly lacked long-term
follow-up indicators, such as progress and recurrence of vertigo
symptoms, which are crucial for determining the overall effect of
treatment. Thirdly, all previous RCTs claimed that the puerarin
co-treatment with betahistine produced an improvement in vertigo
symptoms compared with use of conventional drugs alone. In
addition, no negative data was identified, despite extensive
searches of unpublished material, which may affect the validity of
conclusions in the reviews and lead to publication bias.
Furthermore, the safety of integrative medicine using conventional
western medicine alongside tradition medicine is unclear (44). References to adverse reactions were
limited in numerous RCTs, and no in-depth analysis of their causes
was performed, which may affect reliability. Finally, only 7
studies were included in the present meta-analysis, which results
in a limitation in the accuracy of statistical analysis. Future
analyses should include a larger number of studies.
All RCTs evaluated in the present study were
published in mainland China, in Chinese, which are therefore not
accessible by the international research community. However, 4
trials (22,24–26) were
of high methodological quality, based on high Jadad's validated
scores (35), which was determined
by trial quality assessment. As the quality of numerous trials
included in the present meta-analysis was low, additional
high-quality trials are required to thoroughly evaluate the effects
of puerarin and betahistine co-treatment of VBI vertigo. Therefore,
trials must follow basic guidelines established for reporting
clinical trials, such as those in the CONSORT statement, and
treatment should be rigorously monitored and reported in future
clinical trials (45–49).
The present meta-analysis provides additional
evidence to suggest that puerarin combined with betahistine may
reduce vertigo and improve the average blood velocity of the
basilar artery, with few adverse side effects, in patients with VBI
vertigo. However, the evidence presented in the current study
should be interpreted cautiously due to the low quality of a number
of the included trials. A greater number of rigorously-designed,
large sample, multi-center RCTs are required to fully evaluate the
effectiveness of puerarin combined with betahistine in VBI vertigo
treatment.
Acknowledgements
The present study was financially supported by the
National Natural Science Foundation of China (grant no. 81473365)
and the program of Innovative Research Team of Beijing University
of Chinese Medicine (grant no. 2011-CXTD-13).
References
|
1
|
Li JQ, Zhang QJ and Li B: Research
progress of diagnosis and treatment of vertebral basilar artery
blood supply deficiency. Zhong Wai Yi Xue Yan Jiu. 30:154–156.
2011.(In Chinese).
|
|
2
|
Chang WL: 130 case analysis of diagnosis
and treatment of vertebral-basilar artery blood supply
insufficiency. Shaanxi Yi Xue Za Zhi. 39:1039–1040. 2010.(In
Chinese).
|
|
3
|
Li L, Yu LX and Sui P: Curative effect
observation of buflomedil hydrochloride combined with shuxuening in
the treatment of posterior circulation ischemic vertigo. Zhongguo
Shi Yong Shen Jing Ji Bing Za Zhi. 13:9–11. 2010.(In Chinese).
|
|
4
|
Wang FX, Jiang ZM, Huang ZW, Han F, Jin YL
and Qiao XL: EAPA turn neck test and research of prognosis of
vertebral-basilar artery blood supply insufficiency. Zhong Feng Yu
Shen Jing Ji Bing Za Zhi. 1:3761994.(In Chinese).
|
|
5
|
Wang XJ: 50 cases of curative effect
observation of puerarin combined with sibelium in the treatment of
vertebrobasilar ischemia vertigo. Baiqiuen Jun Yi Xue Yuan Za Zhi.
7:301–302. 2009.(In Chinese).
|
|
6
|
Kang YQ and Li ZY: Clinical analysis of
vertebrobasilar ischemia. Di Si Jun Yi Da Xue Jilin Jun Yi Xue Yuan
Xue Bao. 22:84–87. 2000.(In Chinese).
|
|
7
|
Wong KH, Li GQ, Li KM, Razmovski-Naumovski
V and Chan K: Kudzu root: Traditional uses and potential medicinal
benefits in diabetes and cardiovascular diseases. J Ethnopharmacol.
134:584–607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou YX, Zhang H and Peng C: Puerarin: A
review of pharmacological effects. Phytother Res. 28:961–975. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi CY, Zhao LX and Guan HY: 102 cases of
puerarin for treatment of vertebral basilar artery insufficiency
vertigo. Shaanxi Zhong Yi. 12:1324–1325. 2005.(In Chinese).
|
|
10
|
Yao LY: Curative effect observation of
alprostadil combined with shuxuening in the treatment of posterior
circulation ischemic vertigo. Zhongguo Shi Yong Shen Jing Ji Bing
Za Zhi. 15:56–57. 2012.(In Chinese).
|
|
11
|
Hsu FL, Liu IM, Kuo DH, Chen WC, Su HC and
Cheng JT: Antihyperglycemic effect of puerarin in
streptozotocin-induced diabetic rats. J Nat Prod. 66:788–792. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng QH, Zhang YM and Lou FL: Mechanisms
of inflammation in AD rats induced by αβ and the intervention
effect of acetyl puerarin and chitosan phosphatidylcholine.
Shandong University. 2013.(In Chinese).
|
|
13
|
Wang XX, Zhang YL and Wu J: The effects of
puerariae radix on bone mass and bone microarchitecture in
ovariectomy mice. Zhongguo Guzhi Shusong Zazhi. 14:349–354.
2008.(In Chinese).
|
|
14
|
Miyazawa M, Sakano K, Nakamura S and
Kosaka H: Antimutagenic activity of isoflavone from Pueraria
lobata. J Agric Food Chem. 49:336–341. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun XH, Ding JP, Li H, Pan N, Gan L, Yang
XL and Xu HB: Activation of large-conductance calcium-activated
potassium channels by puerarin: The underlying mechanism of
puerarin-mediated vasodilation. J Pharmacol Exp Ther. 323:391–397.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan LL, Sun LH, Li J, Yue XH, Yu HX and
Wang SY: The protective effect of puerarin against myocardial
reperfusion injury. Study on cardiac function. Chin Med J (Engl).
105:11–17. 1992.PubMed/NCBI
|
|
17
|
Xu X, Zhang S, Zhang L, Yan W and Zheng X:
The Neuroprotection of puerarin against cerebral ischemia is
associated with the prevention of apoptosis in rats. Planta Med.
71:585–591. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guerra MC, Speroni E, Broccoli M, Cangini
M, Pasini P, Minghett A, Crespi-Perellino N, Mirasoli M,
Cantelli-Forti G and Paolini M: Comparison between Chinese medical
herb Pueraria lobata crude extract and its main isoflavone
puerarin antioxidant properties and effects on rat liver
CYP-catalysed drug metabolism. Life Sci. 67:2997–3006. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu Z and Li W: Induction of apoptosis by
puerarin in colon cancer HT-29 cells. Cancer Lett. 238:53–60. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Zeng X, Zhang L and Zheng X:
Stimulatory effect of puerarin on bone formation through activation
of PI3K/Akt pathway in rat calvaria osteoblasts. Planta Med.
73:341–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun XJ and Dang XR: Curative effect
observation of betahistine combined with puerarin in the treatment
of vertebrobasilar ischemia vertigo. Di 4 Jun Yi Da Xue Xue Bao.
28:22772007.(In Chinese).
|
|
22
|
Yue YM, Qiao B and Zhang YH: 46 cases
analysis of curative effect of puerarin combined with betahistine
in the treatment of vertebrobasilar ischemia vertigo. Zhongguo Yi
Xue Chuang Xin. 10:53–54. 2013.(In Chinese).
|
|
23
|
Yang MG: Curative effect observation of
betahistine combined with puerarin in the treatment of
vertebrobasilar ischemia vertigo. Hebei Yi Yao. 32:3170–3171.
2010.(In Chinese).
|
|
24
|
Wang L: Curative effect observation of
betahistine combined with puerarin in the treatment of
vertebrobasilar ischemia vertigo. Dang Dai Yi Xue. 17:1092011.(In
Chinese).
|
|
25
|
Xie SB: Curative effect observation of
betahistine combined with puerarin in the treatment of
vertebrobasilar ischemia vertigo. Shi Yong Yi Xue Za Zhi.
24:2323–2324. 2008.(In Chinese).
|
|
26
|
Guo R: Curative effect of puerarin
combined with betahistine in the treatment of 43 cases of
vertebrobasilar ischemia vertigo. Zhongguo Shi Yong Shen Jing Ji
Bing Za Zhi. 15:61–62. 2012.(In Chinese).
|
|
27
|
Lei LF: Curative effect of puerarin
combined with betahistine in the treatment of 43 cases of
vertebrobasilar ischemia vertigo. Zhongguo Zhong Yi Ji Zheng.
17:1143–1144. 2008.(In Chinese).
|
|
28
|
Qiao D and Ma YZ: Curative effect
observation of puerarin injection in the treatment of
vertebrobasilar ischemia vertigo. Shijie Zhong Xi Yi Jie He Za Zhi.
3:1632008.(In Chinese).
|
|
29
|
Zhang JR: 60 cases of curative effect
observation of puerarin combined with lidocaine in the treatment of
vertebrobasilar ischemia vertigo. Xian Dai Zhong Xi Yi Jie He Za
Zhi. 14:6012005.(In Chinese).
|
|
30
|
Altman DG and Riley RD: Primer: An
evidence-based approach to prognostic markers. Nat Clin Pract
Oncol. 2:466–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Trivella M, Pezzella F, Pastorino U,
Harris AL and Altman DG: Prognosis In Lung Cancer (PILC)
Collaborative Study Group: Microvessel density as a prognostic
factor in non-small-cell lung carcinoma: A meta-analysis of
individual patient data. Lancet Oncol. 8:488–499. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheung F, Feng Y, Wang N, Yuen MF, Tong Y
and Wong VT: Effectiveness of Chinese herbal medicine in treating
liver fibrosis: A systematic review and meta-analysis of randomized
controlled trials. Chin Med. 7:52012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
American Heart Association:
Recommendations on stroke prevention, diagnosis, and therapy.
Report of the WHO Task Force on Stroke and other Cerebrovascular
Disorders. Stroke. 20:1407–1431. 1989.PubMed/NCBI
|
|
34
|
Green S: Cochrane Handbook for Systematic
Reviews of Interventions. The Cochrane Collaboration. 2011.
|
|
35
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qin F, Liu JY and Yuan JH:
Chaihu-Shugan-San, an oriental herbal preparation, for the
treatment of chronic gastritis: A meta-analysis of randomized
controlled trials. J Ethnopharmacol. 146:433–439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Petitti DB: Approaches to heterogeneity in
meta-analysis. Stat Med. 20:3625–3633. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sutton AJ, Duval SJ, Tweedie RL, Abrams KR
and Jones DR: Empirical assessment of effect of publication bias on
meta-analyses. BMJ. 320:1574–1577. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions. Version 4.2.6. The
Cochrane Collaboration. 2006.
|
|
40
|
Du YQ and Wang XJ: Ozagrel sodium combined
with buflomedil treat vertebrobasilar ischemia vertigo. Dang Dai Yi
Xue. 15:1332009.(In Chinese).
|
|
41
|
Xiao HX: Pharmacological research status
of Pueraria lobata. Shizhen Guo Yi GuoYao. 12:11412000.(In
Chinese).
|
|
42
|
Liao ML, Yu J and Zhou M: New progress of
Puerarin in clinical application. Xian Dai Zhong Xi Yi Jie He Za
Zhi. 12:7762003.(In Chinese).
|
|
43
|
Lei BX, Hou XL and Wang JP: Effect of
anti-cerebral ischemia and hypoxia of kudzu. Zhongguo Yiyuan Yao
Xue Za Zhi. 20:152000.(In Chinese).
|
|
44
|
Yang X, Xiong X, Yang G and Wang J:
Chinese patent medicine Xuefu Zhuyu capsule for the treatment of
unstable angina pectoris: A systematic review of randomized
controlled trials. Complement Ther Med. 22:391–399. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Turner L, Shamseer L, Altman DG, Schulz KF
and Moher D: Does use of the CONSORT Statement impact the
completeness of reporting of randomised controlled trials published
in medical journals? A Cochrane review. 1:602012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Turner L, Shamseer L, Altman DG, Weeks L,
Peters J, Kober T, Dias S, Schulz KF, Plint AC and Moher D:
Consolidated standards of reporting trials (CONSORT) and the
completeness of reporting of randomised controlled trials (RCTs)
published in medical journals. Cochrane Database Syst Rev.
11:MR0000302012.PubMed/NCBI
|
|
47
|
Xiong X, Yang X, Liu W, Feng B, Ma J, Du
X, Wang P, Chu F, Li J and Wang J: Banxia baizhu tianma decoction
for essential hypertension: A systematic review of randomized
controlled trials. Evid Based Complement Alternat Med.
2012:2714622012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xiong X, Yang X, Feng B, Liu W, Duan L,
Gao A, Li H, Ma J, Du X, Li N, et al: Zhen gan xi feng decoction, a
traditional Chinese herbal formula, for the treatment of essential
hypertension: A systematic review of randomized controlled trials.
Evid Based Complement Alternat Med. 2013:9823802013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Smith SM, Chang RD, Pereira A, Shah N,
Gilron I, Katz NP, Lin AH, McDermott MP, Rappaport BA, Rowbotham
MC, et al: Adherence to CONSORT harms-reporting recommendations in
publications of recent analgesic clinical trials: An ACTTION
systematic review. Pain. 153:2415–2421. 2012. View Article : Google Scholar : PubMed/NCBI
|