Introduction
The central retinal artery (CRA), as branch of the
ophthalmic artery and the internal carotid artery, can be regarded
as an extracranial part of the cerebrovascular system. Since it is
possible to examine the CRA ophthalmoscopically, assessment of CRA
pressure may be of assistance in obtaining information regarding
the impediment of intracranial blood circulation in patients with
stenosis of the internal carotid artery or common carotid artery
(1–6). The previously developed technique of
contact lens-associated ophthalmodynamometry allows the
non-invasive estimation of the diastolic CRA pressure (diastCRAP)
(7–10). The ophthalmodynamometer consists of a
corneal contact lens which facilitates visualization of the central
retinal artery using slit-lamp based ophthalmoscopy. This technique
allows pressure to be exerted on the eye to increase the
intraocular pressure up to the point when the central retinal
artery begins to pulsate, which facilitates measurement of the
pressure exerted on the eye at the point when the retinal artery
begins pulsating (7–10). Previous studies have demonstrated
that patients who have undergone internal carotid artery dissection
exhibited abnormally low diastCRAP values, as determined by
ophthalmodynamometry (10–12). Notably, these
ophthalmodynamometry-assessed diastCRAP values correlated with
diastolic arterial blood pressure measurements which were
conventionally performed on the upper arm. Furthermore, patients
with ischemic ophthalmopathy exhibited abnormally low diastCRAP
values following ophthalmodynamometry. Carotid artery stenosis is
one of the most common causes of cerebral stroke, which remains one
of the most common causes of increased disability adjusted life
years (13).
Larger studies investigating the association between
the presence and degree of carotid artery stenosis (CAS) and
ophthalmodynamometry-determined diastCRAP remain scarce. Therefore,
the aim of the present study was to examine whether diastCRAP is
associated with the presence and degree of CAS and whether
diastCRAP alters when patients undergo surgical treatment for
CAS.
Materials and methods
Patient enrolment
This prospective clinical observational study
included patients of a study group who suffered from a CAS or
carotid artery occlusion and who were consecutively admitted to the
Department of Neurosurgery at Xuanwu Hospital, Capital Medical
University (Beijing, China) between January 2014 and April 2014.
The protocol of the present study was approved by the Medical
Ethics Committee of Xuanwu Hospital (BCT01994187) and all
participants provided written informed consent. Exclusion criteria
were signs of iris neovascularization, neovascular glaucoma or any
other type of glaucoma. The study additionally included individuals
of a control group of patients with cataract or other ocular
disorders such as refractive error problems without association to
the retina, optic nerve or cerebrovascular system. The examiners
were not masked with respect to study group or control group
status; however, they were masked with respect to the degree of CAS
within the surgical study group or within the non-surgical study
group.
Ophthalmological examination
All study participants underwent a routine
ophthalmological examination including measurement of
best-corrected visual acuity, tonometry and slit lamp-assisted
biomorphometry of the anterior ocular segment and ophthalmoscopy.
At the first day of admission to the hospital and at the first day
after surgery, ophthalmodynamometry was additionally performed for
measurement of diastCRAP. The patients received 1 drop of 0.5%
tropicamide (Santen Pharmaceutical Co., Ltd., Osaka, Japan) to
induce medical mydriasis. Approximately 10 min later, the corneal
surface was topically anesthetized by the instillation of 1 drop of
0.5% proparacaine (Santen Pharmaceutical Co., Ltd.). An
ophthalmodynamometer (Meditron GmbH, Völklingen, Germany) was
placed onto the anesthetized corneal surface. The
ophthalmodynamometer consisted of a conventional Goldmann contact
lens that additionally included a pressure sensor at its margin
where the contact lens was held during the ophthalmoscopic
examination. Using a slit lamp, the optic nerve head was observed
though the Goldmann contact lens. The pressure applied onto the
globe of the eye through the contact lens was slightly and
continuously increased until the CRA started to show pulsations.
The pressure asserted onto the eye through the contact lens at this
time point was noted. The pressure was provided in force units. On
the basis of calculations conducted by Morgan et al, the
force unit of the device was the equivalent of 0.89 mmHg of
intraocular pressure (14). All
measurements were repeated nine times. The mean of the 10
measurements was taken for further statistical analysis. The value
was designated the ophthalmodynamometric value (ODM-value). The
intraocular pressure measured at the baseline of the examination
was added to the ODM-value and the sum of the two values was the
diastCRAP.
The ophthalmodynamometric method has previously been
described in detail (10,11,14–16). The
reproducibility of the technique has been evaluated in previous
studies, in which the coefficient of variation for the
re-determination of the ODM-value was 9.1±4.2% (14,15). The
systolic CRA pressure, which would have been defined as the
pressure asserted onto the globe of the eye at the time point when
the CRA stopped to pulsate without showing any sign of further
perfusion, was not measured since it would have been necessary to
induce complete CRA occlusion by the contact lens-induced pressure
on the globe. Although the retinal ischemic tolerance time may be
≥90 min according to studies by Hayreh and Jonas (17), the risk of inducing damage to the
retina and optic nerve was considered to be too high for such
testing to be conducted.
Statistical analysis
Statistical analysis was performed using a
commercially available statistical software package (SPSS for
Windows, version 21.0; IBM SPSS, Armonk, NY, USA). In a first step
of the analysis, the means ± standard deviations were calculated.
In a second step, the Student's t-test for paired samples was used
to evaluate the significance of measurements between the two eyes
of the same individual, and measurements of the same eyes examined
at baseline and after surgery. Applying the Students t-test for
unpaired samples, the diastCRAP in the study group was compared
with the diastCRAP in the control group. Multivariate analysis was
performed with the degree of CAS as the dependent variable and the
independent variables were the parameters that were determined to
be significantly associated with the degree of CAS in the
univariate analysis. Results are presented with 95% confidence
intervals (CIs). All P-values were based on two-sided tests and
were considered statistically significant when the values were
<0.05.
Results
Patient characteristics
The study group included 95 patients (51 of whom
were men) with a mean age of 62.6±11.3 years (median, 64 years;
range, 34–81 years). The mean systolic blood pressure was 140±22
mmHg (median, 140 mmHg), and the mean diastolic blood pressure was
81±10 mmHg (median, 80 mmHg). The mean blood glucose concentration
was 5.76±1.73 mmol/l, the mean concentration of high-density
lipoproteins was 1.22±0.35 mmol/l, of low-density lipoproteins was
2.04±0.73 mmol/l, and of triglycerides was 1.45±0.66 mmol/l.
Forty-eight (51%) study participants indicated they were smokers.
The control group consisted of 64 subjects with a mean age of
62.4±15.0 years (median, 62.3 years; range, 34.0 to 95.2 years).
The study group and control group did not differ significantly in
age (P=0.92).
CAS location and degree
The CAS was located in the internal carotid artery
in 32 (34%) patients, at the bifurcation in 54 (57%) patients, in
the common carotid artery in 6 (6%) patients, or was combined with
a stenosis of the extracranial artery in 3 (3%) patients. The
degree of the CAS ranged from 32 to 100% (mean, 70.1±19.6%). Out of
the 95 patients, 50 patients (mean age, 63.9±8.6 years) of a
surgical study subgroup had a CAS >75% (mean, 86.2±7.9%) on one
side which then underwent surgery. Thirty-two patients underwent
carotid endarterectomy and 18 individuals received a carotid artery
stent implant. The patients of the surgical study group had
experienced neurologic symptoms such as transient ischemic attacks
that had eventually led to the indication for carotid artery
surgery, as also described in previous studies (18,19).
Following surgery, the residual stenosis on the surgical side was
22.3±3.8% (range, 5–37%). On the contralateral side not undergoing
surgery, the degree of CAS ranged from 0 to 99% (mean,
56.7±9.9%).
diastCRAP values and their association
with CAS degree
In the control group without any CAS, the diastCRAP
value was significantly associated with the brachial diastolic
blood pressure [P<0.001; standardized coefficient β, 0.54;
regression coefficient B, 0.995 (95% CI: 0.57 to 1.42)]. Using the
following regression equation from the control group: diastCRAP =
0.995 × diastolic brachial blood pressure + 8.85 mmHg), an expected
diastCRAP for the whole study population was calculated. The
difference between the measured diastCRAP and the calculated
diastCRAP was then calculated. In the control group, diastCRAP was
not found to be significantly associated with age, neither in
univariate analysis (P=0.50) nor in multivariate analysis after
adjusting for diastolic blood pressure (P=0.14).
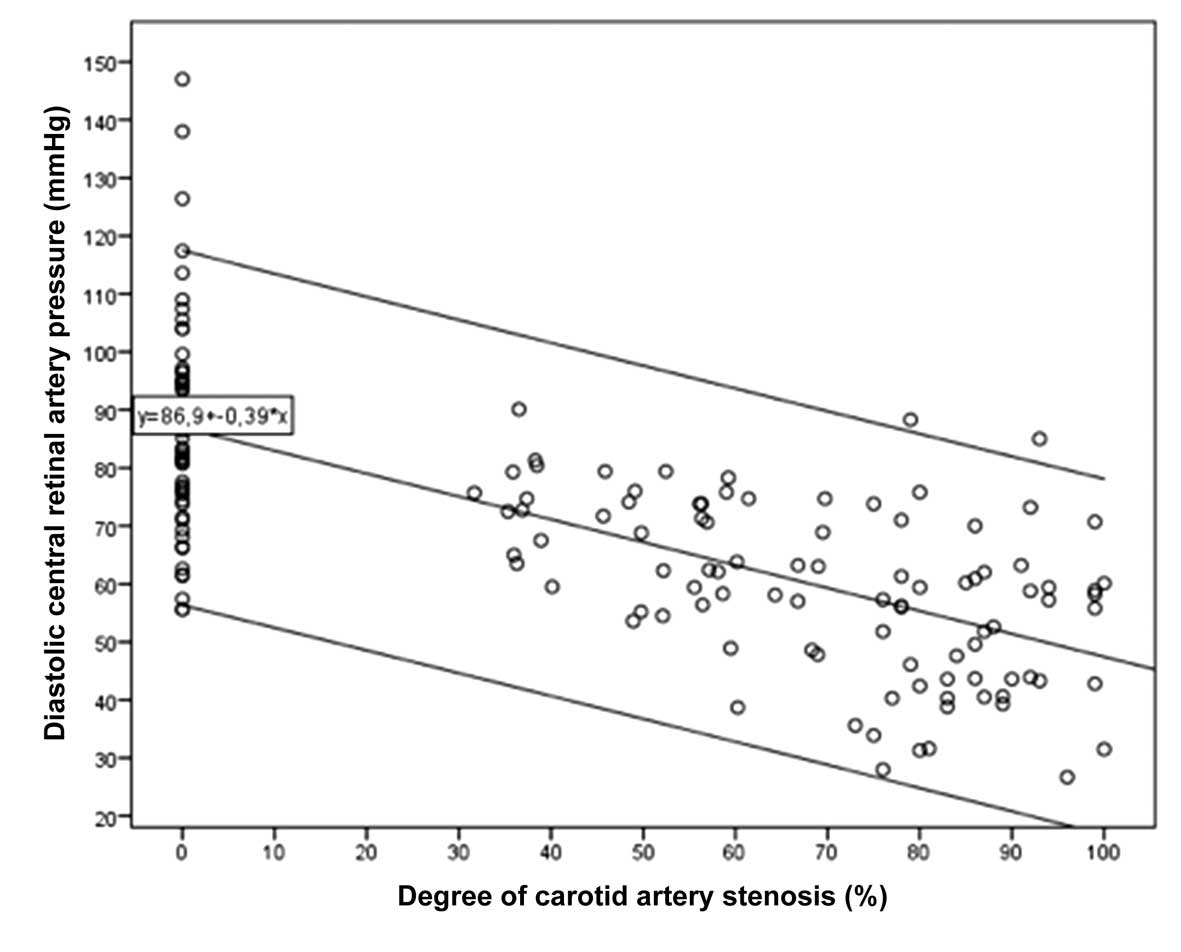
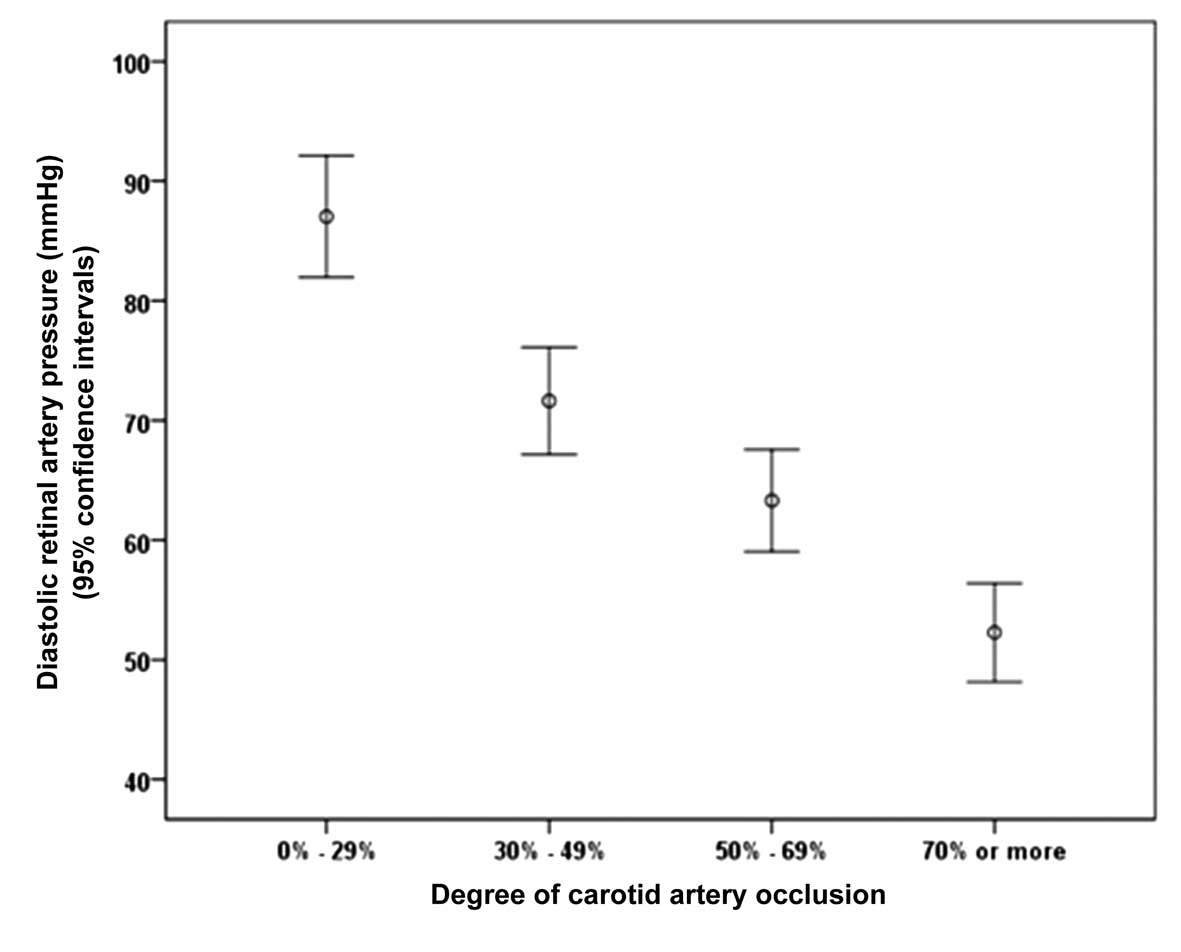
Including all study participants in the statistical
analysis revealed that the diastCRAP was significantly associated
with the degree of CAS [P<0.001; β, −0.69; B, −0.40 (95% CI:
−0.46 to −0.33); Figs. 1 and
2]. If the difference between the
measured diastCRAP and expected diastCRAP was taken, the
correlation became stronger [P<0.001; β, 0.74; B, 1.25 (95% CI:
1.07 to 1.44)]. Correspondingly, a multivariate analysis with the
degree of CAS as dependent variable and measured diastCRAP and
diastolic brachial blood pressure as independent variables showed
that the degree of CAS was significantly (correlation coefficient
overall: r=0.75) associated with a higher brachial diastolic blood
pressure [P<0.001; β, 0.27; B, 1.09 (95% CI: 0.66 to 1.53)] and
lower diastCRAP [P<0.001; β, −0.73; B, −1.28 (95% CI: −1.47 to
−1.09)].
diastCRAP values prior to and
following CAS surgery
Within the surgical study group at the baseline of
the study, the diastCRAP was significantly lower at the surgical
side than at the contralateral side (48.1±13.2 vs. 54.3±14.0 mmHg;
P=0.02). At the surgical side, the diastCRAP value increased
significantly (P<0.001) after surgery from 48.1±13.2 to 66.9±9.9
mmHg, while at the contralateral side, the diastCRAP did not change
significantly [54.3±14.0 mmHg (median, 51.9 mmHg; range, 30.7–88.6
mmHg) vs. 54.3±14.0 mmHg (median, 53.7 mmHg; range: 21.7–77.4 mmHg;
P=0.86]. Subsequently, the diastCRAP value following surgery was
significantly higher at the surgical side than at the contralateral
side (P<0.001).
In the surgical study group at baseline, the
diastCRAP value at the surgical side was not found to be
significantly associated with brachial diastolic blood pressure
(P=0.22). Similarly, diastCRAP at the contralateral side was not
determined to be significantly associated with brachial diastolic
blood pressure (P=0.88). Following surgery, diastCRAP at the
surgical side was significantly associated with brachial diastolic
blood pressure [P=0.001; β, 0.44; B, 0.55 (95% CI: 0.23,
0.87)].
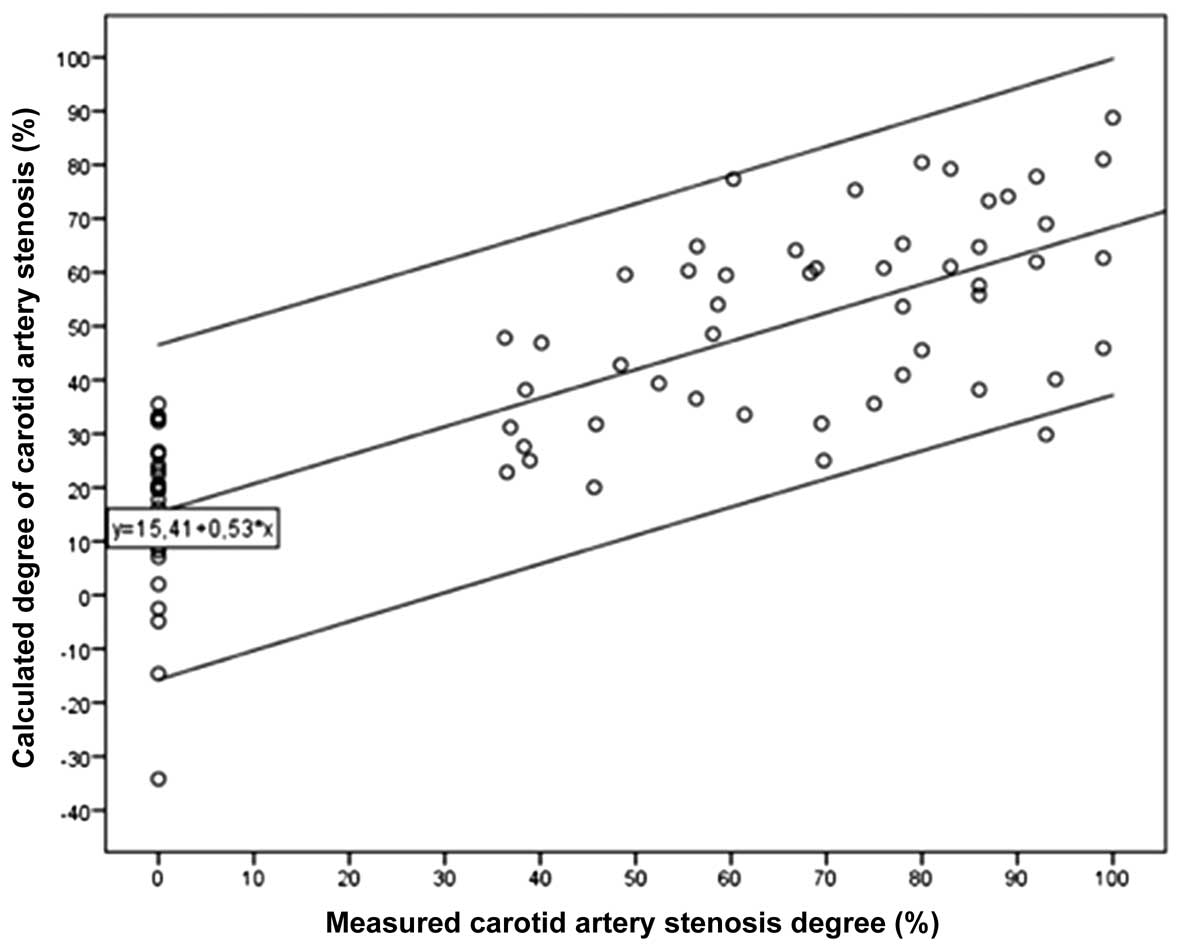
diastCRAP values in the prediction of
CAS degree
The whole study population was randomly divided into
two subgroups. These were a ‘formula subgroup’, comprising 80
individuals whose data were used to calculate the association of
the degree of CAS with diastCRAP and brachial diastolic blood
pressure, and a ‘test subgroup’ of 79 individuals in which the
formula was tested for its ability to predict the measured degree
of CAS. In the formula subgroup, the formula for the degree of CAS
was as follows: CAS degree (%) = 0.95 × brachial diastolic blood
pressure (mmHg) − 1.19 × diastCRAP (mmHg) + 49.54. In the test
subgroup, the calculated CAS value was compared with the measured
CAS degree and it was observed that all but one of the points were
located within the 95% CI of the regression line (Fig. 3).
Discussion
In this observational study on normal individuals
and patients with moderate to severe CAS, diastCRAP as measured by
ophthalmodynamometry was found to be highly significantly and
linearly correlated with the degree of CAS. Correspondingly,
diastCRAP was significantly lower at the side with the more severe
CAS in patients with bilateral CAS, and diastCRAP increased
significantly following successful surgery, resulting in a
reduction in the degree of CAS. Subsequent to successful surgery
for CAS, diastCRAP became correlated with diastolic brachial blood
pressure, in contrast to the preoperative situation. In view of the
strong and linear association between diastCRAP and the degree of
CAS, the results suggest that the diastCRAP may be a surrogate for
the status of the carotid artery and potentially for the
cerebrovascular status in general. Thus, it may be inferred that
ophthalmodynamometry could be a useful tool in the diagnosis and
follow-up of CAS.
The result of the present study that
ophthalmodynamometric measurements of the diastCRAP were correlated
with the degree of CAS is concordant with findings obtained in
studies performed several decades ago and which used
ophthalmodynamometric devices others than the modern Goldmann lens
associated ophthalmodynamometer (2–6,20–23). In
contrast to the old devices, which provide inaccurate pressure
measurements and have difficulties in a precise ophthalmoscopic
examination of the optic nerve head, the Goldmann contact lens as
applied in the present study provided good optical conditions for
the ophthalmoscopic examination of the optic nerve head. The
technique is an established technique of contact lens-associated
ophthalmodynamometry, which has previously been applied in studies
that have examined the central retinal venous pressure in diseases
such as central and branch retinal vein occlusion, endocrine
orbitopathy, glaucoma and increased brain pressure and that have
examined the diastCRAP in diseases such as ischemic ophthalmopathy
and dissection of the internal carotid artery (8,11,12,14,15,24–29).
The central retinal vessels at the optic nerve head usually have a
diameter of 120–150 µm, so that pulse-synchronous vessel wall
movements will occur in the range of ~20 µm. This value is close to
the lateral image resolution of ophthalmoscopy; thus, the modern
ophthalmodynamometer with best possible optical conditions for
clinical ophthalmoscopy will lead to more precise measurements as
compared with the measurements obtained using older
ophthalmodynamometer models.
The findings of the present study on the group of
patients with severe CAS concur with previous reports on small
numbers of patients with a complete occlusion of the carotid artery
or a carotid artery dissection (10,12). The
results are also congruent with the findings of a reduced retinal
blood velocity in patients with CAS and an improvement in blood
flow velocity following carotid endarterectomy or carotid artery
stenting (30–33).
In addition to the results obtained in the previous
investigations, the present study findings showed a correlation
between the degree of severe CAS and the reduction in diastCRAP in
intra-individual inter-eye, inter-individual intra-individual
follow-up comparisons. The results imply that the new Goldmann
lens-associated ophthalmodynamometry may be a useful additional
tool in the assessment of the patients with CAS. Furthermore, the
results may prompt an ophthalmologist to suspect CAS if during a
routinely performed Goldmann contact lens examination of the ocular
fundus, a slight pressure on the contact lens induces a pulsation
of the CRA.
The current study has potential imitations. First,
as a hospital-based investigation, the study by principle carries
the risk of bias due to the referral bias of patients admitted to
the hospital. Secondly, the patients of the study population only
showed severe CAS, and patients with minor degrees of CAS were not
examined. Therefore, it was not possible to explore the association
between diastCRAP and minor degrees of CAS; thus, it was not
possible to determine whether the correlation between diastCRAP and
CAS was linear or curvilinear. One may assume that for minor
degrees of CAS without functional consequences for the
cerebrovascular system, the degree of CAS would not be correlated
with diastCRAP. If that is the case, future studies may investigate
the suitability of ophthalmodynamometry for use in assessment of
the functional impediment of cerebrovascular blood perfusion in
patients with CAS of varying degrees.
In conclusion, diastCRAP was highly significantly
and linearly correlated with the degree of CAS in intra-individual
inter-eye, inter-individual and intra-individual follow-up
comparisons. The strong and linear association between diastCRAP
and the degree of CAS suggests that diastCRAP should be further
explored as a surrogate for cerebrovascular status.
References
|
1
|
Hedges TR, Weinstein JD, Kassell NF and
Langfitt TW: Correlation of ophthalmodynamometry with ophthalmic
artery pressure in the rhesus monkey. Am J Ophthalmol.
60:1098–1101. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wunsh SE: Ophthalmodynamometry. N Engl J
Med. 281:4461969. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galin MA, Baras I and Dodick JM:
Semiautomated suction ophthalmodynamometry. Am J Ophthalmol.
68:237–240. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bettelheim H: The clinical significance of
ophthalmodynamometry and ophthalmodynamography. Klin Monatsbl
Augenheilkd. 155:769–791. 1969.(In German). PubMed/NCBI
|
|
5
|
Van der Werff TJ: The pressure measured in
ophthalmodynamometry. Arch Ophthalmol. 87:290–292. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krieglstein GK and Silva FA: Comparative
measurements of the ophthalmic arterial pressure using the Mikuni
dynamometer and the Stepanik-arteriotonograph. Albrecht Von Graefes
Arch Klin Exp Ophthalmol. 212:77–91. 1979.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Entenmann B, Robert YC, Pirani P,
Kanngiesser H and Dekker PW: Contact lens tonometry - application
in humans. Invest Ophthalmol Vis Sci. 38:2447–2451. 1997.PubMed/NCBI
|
|
8
|
Morgan WH, Yu DY, Cooper RL, Alder VA,
Cringle SJ and Constable IJ: Retinal artery and vein pressures in
the dog and their relationship to aortic, intraocular and
cerebrospinal fluid pressures. Microvasc Res. 53:211–221. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Firsching R, Schütze M, Motschmann M,
Behrens-Baumann W and Meyer-Schwickerath R: Non-invasive
measurement of intracranial pressure. Lancet. 351:523–524. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jonas JB: Retinal arterial collapse
pressure in eyes with retinal arterial occlusive diseases. Br J
Ophthalmol. 88:5892004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jonas JB and Niessen A:
Ophthalmodynamometric diagnosis of unilateral ischemic
ophthalmopathy. Am J Ophthalmol. 134:911–912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jonas JB and Hennerici M:
Ophthalmodynamometry for diagnosis of dissection of internal
carotid artery. Graefe Arch Clin Exp Ophthalmol. 244:129–130. 2006.
View Article : Google Scholar
|
|
13
|
Murray CJ, Barber RM, Foreman KJ, Ozgoren
Abbasoglu A, Abd-Allah F, Abera SF, Aboyans V, Abraham JP, Abubakar
I, Abu-Raddad LJ, et al: GBD 2013 DALYs and HALE Collaborators:
Global, regional, and national disability-adjusted life years
(DALYs) for 306 diseases and injuries and healthy life expectancy
(HALE) for 188 countries, 1990–2013: Quantifying the
epidemiological transition. Lancet. 386:2145–2191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morgan WH, Cringle SJ, Kang MH, Pandav S,
Balaratnasingam C, Ezekial D and Yu DY: Optimizing the calibration
and interpretation of dynamic ocular force measurements. Graefes
Arch Clin Exp Ophthalmol. 248:401–407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jonas JB: Reproducibility of
ophthalmodynamometric measurements of the central retinal artery
and vein collapse pressure. Br J Ophthalmol. 87:577–579. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zaret CR, Sacks JG and Holm PW: Suction
ophthalmodynamometry in the diagnosis of carotid stenosis.
Ophthalmology. 86:1510–1512. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hayreh SS and Jonas JB: Optic disk and
retinal nerve fiber layer damage following transient central
retinal artery occlusion: An experimental study in rhesus monkeys.
Am J Ophthalmol. 129:786–795. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
North American Symptomatic Carotid
Endarterectomy Trial Collaborators: Beneficial effect of carotid
endarterectomy in symptomatic patients with high-grade carotid
stenosis. N Engl J Med. 325:445–453. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
European Carotid Surgery Trialists'
Collaborative Group: MRC European Carotid Surgery Trial: Interim
results for symptomatic patients with severe (70-99%) or with mild
(0-29%) carotid stenosis. Lancet. 337:1235–1243. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sanborn GE, Miller NR, McGuire M and Kumar
AJ: Clinical-angiographic correlation of ophthalmodynamometry in
patients with suspected carotid artery disease: A prospective
study. Stroke. 12:770–774. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mullie MA and Kirkham TH:
Ophthalmodynamometry revisited. Can J Ophthalmol. 18:165–168.
1983.PubMed/NCBI
|
|
22
|
Weinberger J: Clinical applications of
noninvasive carotid artery testing. J Am Coll Cardiol. 5:137–148.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barańska-Gieruszczak M,
Laskowska-Studniarska W, Myga W and Ryglewicz D: Diagnostic value
of ultrasonography and ophthalmodynamometry in the diagnosis of
stenosis and occlusion of the internal carotid artery. Neurol
Neurochir Pol. 21:281–285. 1987.(In Polish). PubMed/NCBI
|
|
24
|
Morgan WH, Hazelton ML, Balaratnasingamm
C, Chan H, House PH, Barry CJ, Cringle SJ and Yu DY: The
association between retinal vein ophthalmodynamometric force change
and optic disc excavation. Br J Ophthalmol. 93:594–596. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morgan WH, Yu DY, Alder VA, Cringle SJ and
Constable IJ: Relation between pressure determined by
ophthalmodynamometry and aortic pressure in the dog. Br J
Ophthalmol. 82:821–825. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jonas JB and Harder B:
Ophthalmodynamometric estimation of cerebrospinal fluid pressure in
pseudotumor cerebri. Br J Ophthalmol. 87:361–362. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jonas JB: Central retinal artery and vein
pressure in patients with chronic open-angle glaucoma. Br J
Ophthalmol. 87:949–951. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jonas JB: Ophthalmodynamometric
measurement of orbital tissue pressure in thyroid-associated
orbitopathy. Acta Ophthalmol Scand. 82:2392003. View Article : Google Scholar
|
|
29
|
Jonas JB and Harder B:
Ophthalmodynamometric differences between ischemic versus
non-ischemic retinal vein occlusion. Am J Ophthamol. 143:112–116.
2007. View Article : Google Scholar
|
|
30
|
Ishikawa K, Kimura I, Shinoda K, Eshita T,
Kitamura S, Inoue M and Mashima Y: In situ confirmation of retinal
blood flow improvement after carotid endarterectomy in a patient
with ocular ischemic syndrome. Am J Ophthalmol. 134:295–297. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cohn EJ Jr, Sandager GP, Benjamin ME,
Lilly MP, Hanna DJ and Flinn WR: Assessment of ocular perfusion
after carotid endarterectomy with color-flow duplex scanning. J
Vasc Surg. 29:665–671. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Costa VP, Kuzniec S, Molnar LJ, Cerri GG,
Puech-Leão P and Carvalho CA: The effects of carotid endarterectomy
on the retrobulbar circulation of patients with severe occlusive
carotid artery disease. An investigation by color Doppler imaging.
Ophthalmology. 106:306–310. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wong YM, Clark JB, Faris IB, Styles CB and
Kiss JA: The effects of carotid endarterectomy on ocular
haemodynamics. Eye (Lond). 12:367–373. 1998. View Article : Google Scholar : PubMed/NCBI
|