Introduction
Cerebral ischemia/reperfusion injury (CIR) is caused
by brain ischemia and further deteriorated by sudden recovery of
the blood supply. Oxidative radical-mediated inflammation and
apoptosis serve a crucial function in CIR-induced neural injury
(1). Therefore, any interventions
aimed at inhibiting inflammation and apoptosis may possess
therapeutic potential for the treatment of IR-induced neural
injury.
MicroRNAs (miRs) are a class of small non-coding and
single-stranded RNAs (~22 nucleotides) first identified in
Caenorhabditis elegans in 1993 (2). miRs exist in virtually all organisms
and are evolutionarily conserved. Their principle function is to
regulate target-gene expression at the post-transcriptional level
(3). To date, >2,000 miRs have
been identified in the human genome, and >60% of protein-coding
genes have been found to be regulated by miRs (4,5). miRs
are known to have pathophysiological relevance in various diseases,
including some that affect the brain (6–8). miR-138
serves a crucial function in various biological processes. The
dysregulation of miR-138 has been demonstrated to be a key factor
in ischemia/perfusion injury of the heart (9) and lungs (10). In a study using zebrafish, it was
found that the disruption of miR-138 function resulted in the
ventricular expansion of gene expression generally restricted to
the atrioventricular valve region, and ultimately resulted in the
disruption of ventricular cardiomyocyte morphology and cardiac
function (11). By contrast, the
overexpression of miR-138 in zebrafish was found to protect the
heart from mycotoxin-induced cardiotoxicity (12). However, the role of miR-138 in the
development of CIR has not been fully investigated.
Lipocalin 2 (Lcn-2), which is also known as
neutrophil gelatinase-associated lipocalin, has multiple functions
including the regulation of cell death/survival, cell
migration/invasion, cell differentiation and iron delivery. The
expression of Lcn-2 is markedly upregulated in injured spinal cord
and brain tissue (13,14). The involvement of Lcn-2 in the
systemic response to IR has been documented in previous studies of
the heart and kidneys (15–17).
The present study was conducted to investigate the
interrelation and interaction of Lcn-2 and miR-138 in a rat model
of CIR and PC12 cells, with the aim of providing an experimental
basis for the development of an effective therapy for CIR.
Materials and methods
Animals
A total of 60 male Sprague-Dawley (SD) rats were
purchased from Weitong Lihua Laboratory Animal Center (SCXK
2006-0009; Beijing, China). All animal procedures were conducted in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (National Institutes of Health,
Bethesda, MA, USA). The Experimental protocols of the present study
were approved by the Animal Care Committee at Hubei University of
Medicine (Shiyan, China).
Preparation of the CIR rat model
SD rats were randomly allocated to normal, model and
sham-operated groups (n=20 per group). Rats in the CIR model group
underwent middle cerebral artery occlusion while anesthetized by an
intraperitoneal injection with 10% chloral hydrate (350 mg/kg;
Shanghai Biomart Technology Co., Ltd., Shanghai, China). An
incision was made along the cervical midline followed by the
separation of the right common carotid artery (CCA), external
carotid artery (ECA) and internal carotid artery (ICA). The CCA and
ECA were ligated with suture thread at the proximal and distal
ends, respectively. Subsequently, the ICA was clamped with a
bulldog clamp at the distal end and an opening was made ~0.5 cm
away from the junction of the ECA and ICA and a rounded nylon
thread (0.3 mm diameter; Takasago Medical Industry, Co., Ltd.,
Tokyo, Japan) was immediately inserted into the opening at a depth
of ~18 mm, thereby inducing cerebral ischemia. The bulldog clamp
was removed and the nylon thread was immobilized along the
incision, followed by the closing the skin incision. After 2 h of
occlusion, cerebral reperfusion was induced by drawing out the
nylon thread by ~10 mm. Rats in the sham-operated group underwent
an identical surgical procedure to that conducted in the model
group, but without ICA occlusion. Rats in the normal group did not
undergo any surgical procedure. All animals were maintained under a
12-h light/dark cycle with ad libitum access to food and
water.
Harvesting of brain specimens
Following 2 h of cerebral ischemia and 22 h of
reperfusion, rats were guillotined while anesthetized with chloral
hydrate, and brain specimens (0.05–0.11 g) were collected and
frozen in liquid nitrogen.
Cell culture
The PC12 cell line was purchased from the Cell Bank
at Wuhan University (Wuhan, China) and cultured in RMPI-1640 medium
with 10% fetal bovine serum, 2 mM L-glutamine and 1%
penicillin-streptomycin solution (Invitrogen; Thermo Fisher
Scientific, Shanghai, China). Cells were incubated at 37°C in a
humidified, 5% CO2/20% O2 atmosphere. For the
induction of hypoxia/reoxygenation (H/R) injury, 6-well plates
containing PC12 cells (5×105 cells/well) were placed
into a humidified airtight container, with continued flow through
of 95% N2/5% CO2 to achieve an
oxygen-deficient environment. The sealed chamber was placed into a
37°C incubator for 6 h, then returned to a 20% O2
atmosphere. The cells used in this study were limited to within 3
passages.
PC12 cell transfection and luciferase
activity assay
PC12 cells at the logarithmic growth phase were
seeded into 6-well plates (5×105 cells/well). On the following day,
the PC12 cells were transfected with miR-138 mimics, miR-138
inhibitors (Guangzhou RiboBio, Co., Ltd., Guangzhou, China) and
pcDNA 3.1-Lcn-2 (Guangzhou Vipotion Biotechnology Co., Ltd.,
Guangzhou, China) using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific) in accordance with the manufacturer's
instructions. The final concentrations of miR-138 mimics, miR-138
inhibitors and pcDNA 3.1-Lcn-2 were 100, 100 and 500 nM
respectively. A mock transfection was performed in a separate
well.
PC12 cells were also seeded into 96-well plates
(1×104 cells/well) for the luciferase-labeled Lcn-2
transfection and luciferase activity assay. Briefly, PC12 cells
were transfected with luciferase-labeled wild-type Lcn-2
(3′UTR-Lcn2-WT; 500 nM; Guangzhou RiboBio, Co., Ltd.) or
luciferase-labeled mutant Lcn-2 (3′UTR-Lcn2-MUT; 500 nM; Guangzhou
RiboBio, Co., Ltd.), with or without miR-138 mimics (100 nM;
Guangzhou Ribobio, Co., Ltd.), respectively, using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). On day 2
following transfection, 1 µl D-luciferin (0.15 mg/ml; Caliper Life
Sciences, Hopkinton, MA, USA) was added to each well and the cells
were incubated for 10 min. Subsequently, bioluminescence was
measured using the IVIS Spectrum System (Caliper Life Sciences).
The relative bioluminescence intensity was an indicator of the
luciferase activity. All experiments in each group were repeated in
triplicate.
Target search of miR-138
The complementary binding status between miR-138 and
the 3′-untranslated region (3′-UTR) of Lcn-2 was obtained using
TargetScan (www.targetscan.org).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) detection of miR-138 and
Lcn-2
Total RNA was extracted from cerebral tissue and
PC12 cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The isolated RNA was treated with DNase I
(Invitrogen; Thermo Fisher Scientific). First strand cDNA was
synthesized using 3 µl RNA and the Super Script III Reverse
Transcriptase kit (Invitrogen; Thermo Fisher Scientific) with
oligonucleotide dTs (0.5 µg/µl). Then, 1 µg cDNA product was used
for the amplification procedure in a 20-µl reaction mixture
containing 10 µl SYBR Green PCR Master mix (Invitrogen; Thermo
Fisher Scientific, Inc.), 7 µl diethylpyrocarbonate-H2O
and 1 µl forward and reverse primers (Table I). The amplification was conducted
using the 7900HT Fast Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The protocol consisted of an
initial denaturation and enzyme activation at 95°C for 10 min,
followed by 35 cycles of 30 sec denaturation at 95°C, an attachment
of primers for 1 min at 60°C and extension at 72°C for 30 sec, and
finally 1 cycle at 72°C for 10 min for final elongation.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and U6 were used
as internal controls for Lcn-2 and miR-138, respectively. Relative
expression levels of Lcn-2 and miR-138 were calculated using the
2−∆∆Cq method (18).
 | Table I.Primer sequences used for miR and mRNA
expression analysis. |
Table I.
Primer sequences used for miR and mRNA
expression analysis.
| Primer | Primer sequence
(5′–3′) |
|---|
| miR-138-RT |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCGGCCTGAT |
| U6-RT |
CGCTTCACGAATTTGCGTGTCAT |
| U6-F |
CTCGCTTCGGCAGCACA |
| U6-R |
AACGCTTCACGAATTTGCGT |
| miR-138-F |
ACACTCCAGCTGGGAGCTGGTGTTG |
| Universal-R | GTGCAGGGTCCGAGGT |
| Lcn-2-F |
CTGATCAGTGTGCCCCTGCAG |
| Lcn-2-R |
GGAGCTTGGAACGAATGTTCTG |
| GAPDH-F |
ACACCCACTCCTCCACCTTT |
| GAPDH-R |
TTACTCCTTGGAGGCCATGT |
Methyl thiazolyl tetrazolium (MTT)
assay
The transfected PC12 cells were cultured in 96-well
plates at a density of 1×104 cells per well. After 6 h of
incubation under hypoxic conditions, 20 µl MTT (5 mg/ml;
Sigma-Aldrich, St. Louis, MO, USA) was added to each well following
0, 24, 48, 72 and 96 h of reoxygenation and incubated for an
additional 4 h. Then, the supernatant was removed and dimethyl
sulfoxide (150 µl/well) was added to dissolve the blue formazan
crystals that had been converted from MTT by live cells. Cell
viability was assessed by measuring the optical density at 570 nm
using a microplate spectrophotometer (Jupiter G19060; Montréal
Biotech, Inc. Dorval, Canada). The absorbance (A) values were
calculated as a percentage of the control untreated wells, and used
to construct a time-course curve. The survival rate of the PC12
cells following each transfection was calculated as a percentage
using the following formula: Growth rate =
(Atransfected-Ablank)/(Acontrol-Ablank)
× 100. Each group was measured in 5 wells on the same plate in
three independent experiments.
Immunoblotting analysis
Immunoblotting analysis was used to detect the Lcn-2
protein expression levels in the cerebral tissues. Protein (50 µg)
from the homogenized sample was mixed at a ratio of 1:4 with
loading buffer (Invitrogen; Thermo Fisher Scientific, Inc.),
separated using 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to a nitrocellulose membrane
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). After blocking the
non-specific binding sites with 5% milk powder for 2 h, the
membrane was incubated overnight at 4°C with rabbit anti-human
Lcn-2 (1:1,000; sc-50351; Santa Cruz Biotechnology, Santa Cruz, CA,
USA) and rabbit anti-human GAPDH (1:1,000; sc-25778; Santa Cruz
Biotechnology) polyclonal antibodies. After washing three times
with Tris-buffered saline supplemented with 0.05% Tween-20 (Abcam,
Shanghai, China), the membrane was incubated with horseradish
peroxidase-conjugated goat-anti-rabbit IgG (1:5,000; cat no.
1706515; Bio-Rad Laboratories, Inc.) for 1.5 h. The
antigen-antibody complex was detected using a standard enhanced
chemiluminescence detection system (Wuhan Boster Bio-Engineering
Ltd., Co., Wuhan, China). The detected protein was quantified using
Gel-pro Analyzer 4.0 software (Media Cybernetics, Inc., Rockville,
MD, USA) and was expressed as the ratio to GAPDH protein.
Statistical analysis
Numerical data are expressed as the mean ± standard
deviation. Statistical differences between the mean for different
groups were assessed using one-way analysis of variance with Prism
version 4.0 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of miR-138 and Lcn-2 in the
cerebral tissue of CIR rats
RT-qPCR was used to detect the expression levels of
miR-138 and Lcn-2, while the protein expression levels of Lcn-2
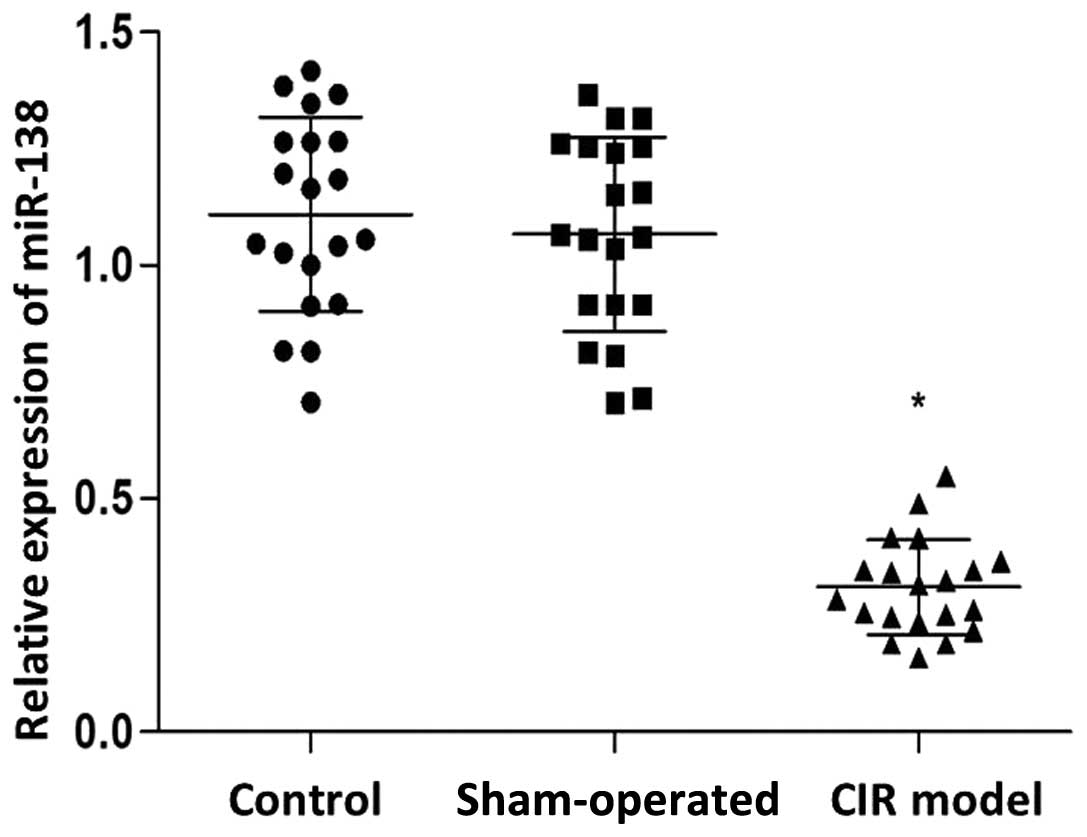
were determined by western blot analysis. As shown in Fig. 1, the expression levels of miR-138 in
the CIR model group was significantly reduced compared with those
in the control and sham-operated groups (P<0.05). By contrast,
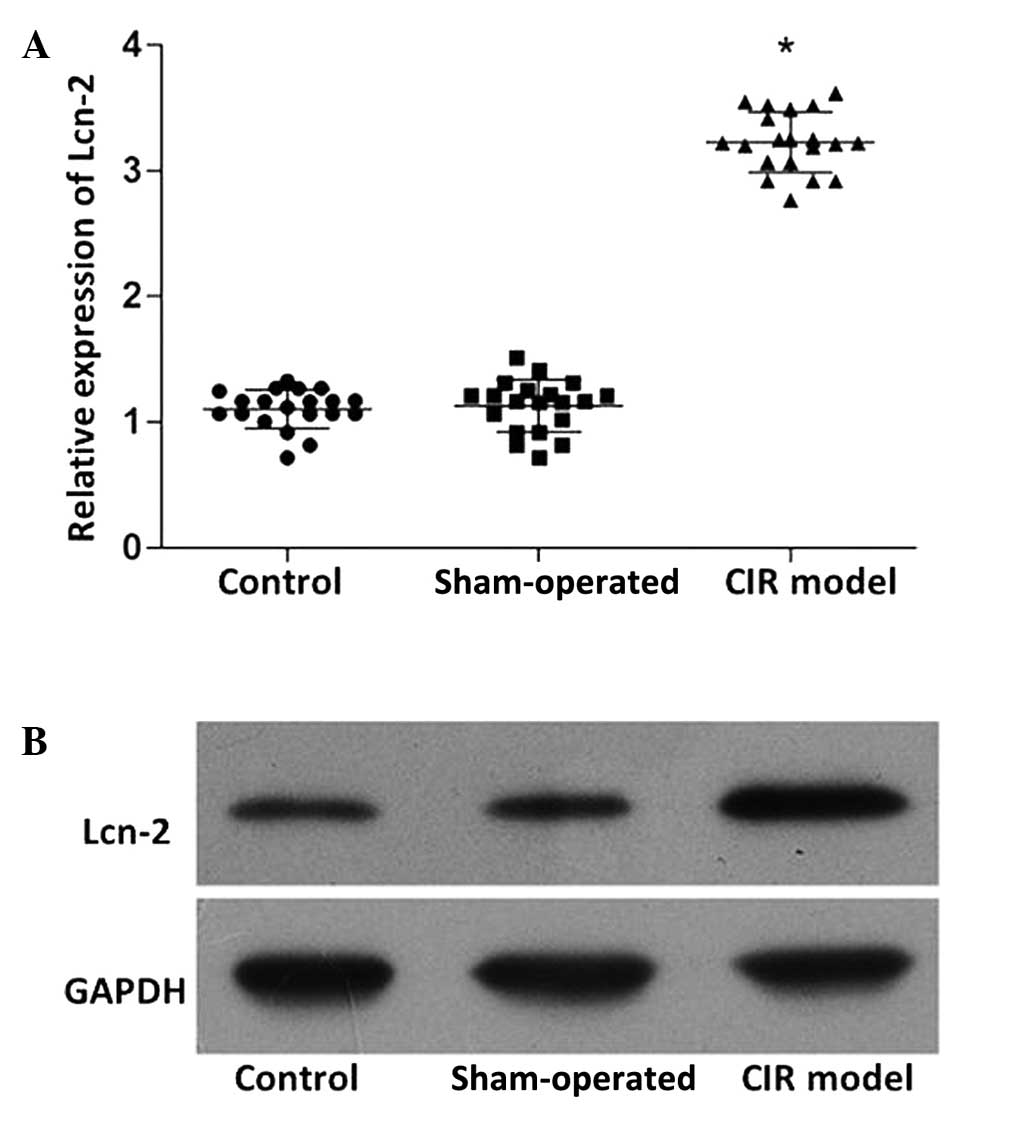
the mRNA (Fig. 2A) and protein
(Fig. 2B) expression levels of Lcn-2
in the CIR model group were markedly increased compared with those
in the control and sham-operated groups (P<0.05). No
statistically significant difference was detected in the miR-138
and Lcn-2 expression levels between the control and sham-operated
groups.
Expression of miR-138 in PC12 cells
and miR-138-induced cell proliferation
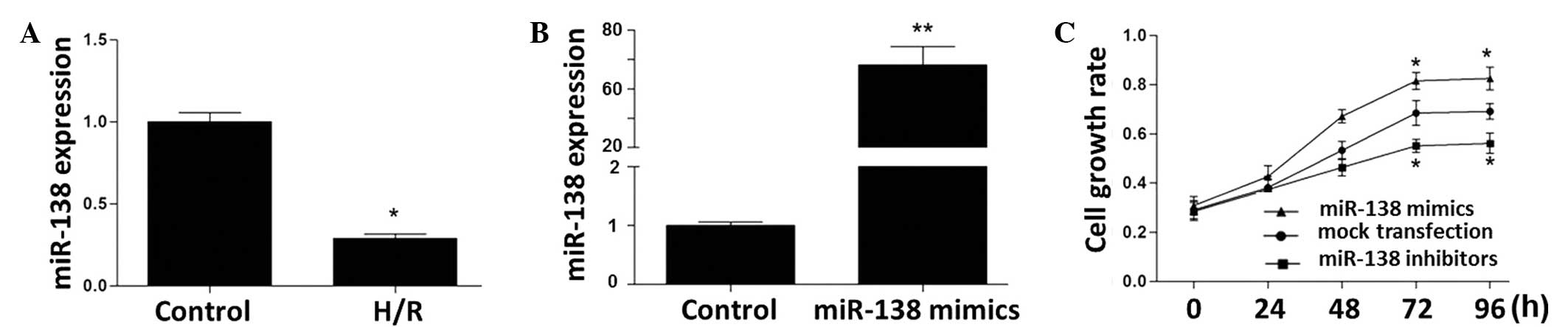
At 24 h of reoxygenation following 6 h of hypoxia,
PC12 cells showed markedly reduced expression levels of miR-138
(Fig. 3A). The expression level of
miR-138 in the miR-138 mimic-transfected PC12 cells was increased
by 68-fold compared with that in the untransfected cells (Fig. 3B). miR-138 expression in the miR-138
inhibitor-transfected PC12 cells was reduced to a non-detectable
level (data not shown). As assessed by MTT assay, the PC12 cell
growth rate was stimulated by transfection with miR-138 mimics and
inhibited by miR-138 inhibitors (Fig.
3C).
Action of miR-138 on Lcn-2
As verified by RT-qPCR and western blot analysis,
the expression levels of Lcn-2 in the PC12 cells was significantly
increased by H/R (Fig. 4A and B). A
TargetScan search indicated that the seed sequence of miR-138 was
base-paired with the 3′-UTR region of Lcn-2 via the nucleotides at
positions 25–31 (Fig. 4C). The
expression of Lcn-2 was inhibited by miR-138 mimics and stimulated
by miR-138 inhibitors (Fig. 4D). As
shown in Fig. 4E, miR-138 inhibited
wild-type Lcn-2 (Lcn-2 WT)-induced luciferase activity without
acting on mutant Lcn-2 (Lcn-2 MUT), functionally demonstrating the
specificity of miR-138.
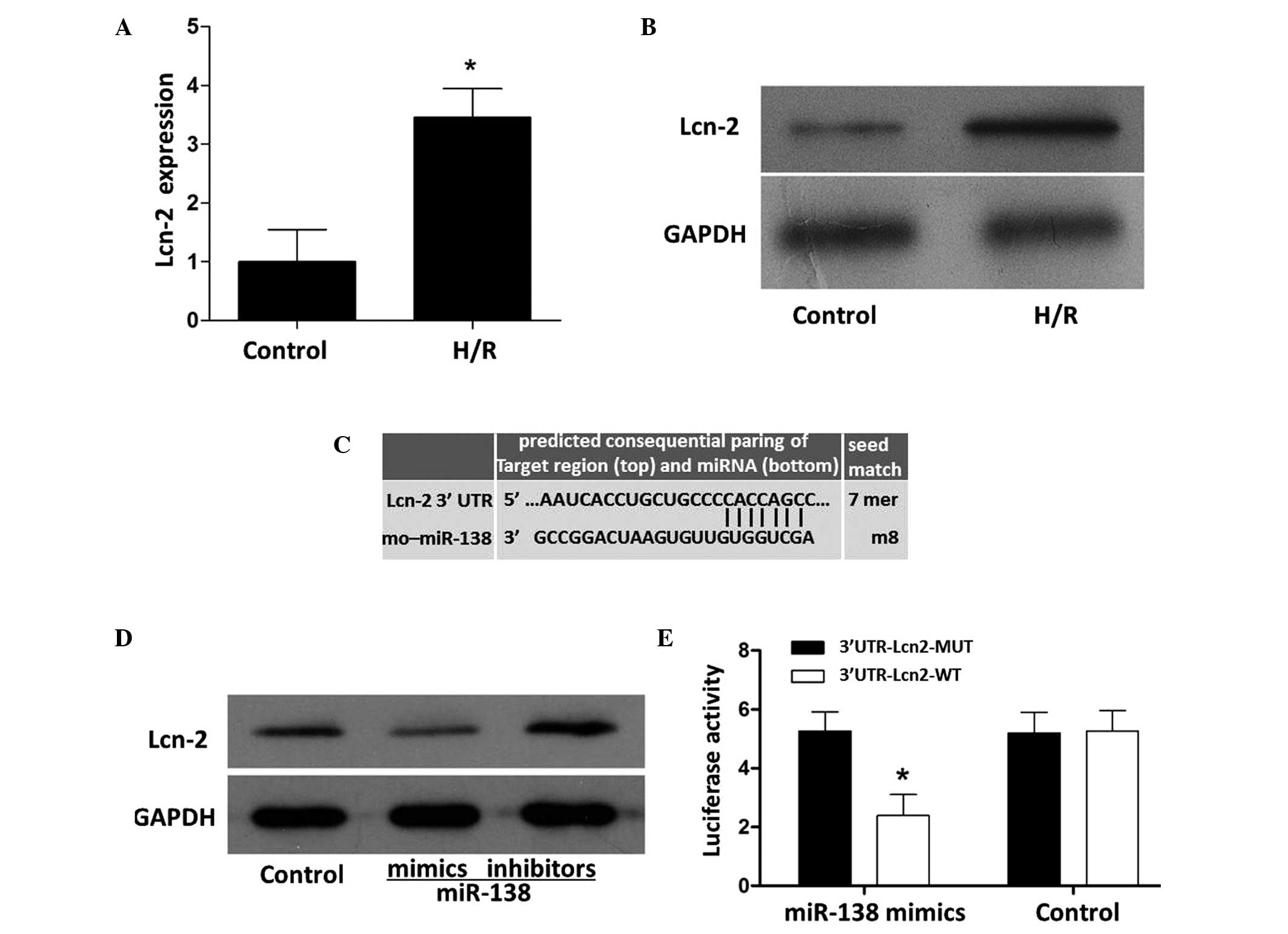 | Figure 4.Effects of miR-138 mimics and
inhibitors on Lcn-2 expression in PC12 cells. The (A) mRNA
(*P<0.05 vs. the control) and (B) protein expression levels of
Lcn-2 were detected by reverse transcription-quantitative
polymerase chain reaction and western blot analysis, respectively.
(C) Seed match status. (D) Compared with the control, the
expression of Lcn-2 was inhibited by miR-138 mimics and enhanced by
miR-138 inhibitors. (E) miR-138 mimics attenuated
3′UTR-Lcn2-WT-induced luciferase activity without affecting
3′UTR-Lcn2-MUT-induced luciferase activity. Numerical data are
expressed as the mean ± standard deviation and analyzed by one-way
analysis of variance (*P<0.05 vs. the 3′UTR-Lcn2-MUT). Lcn-2,
lipcalin-2; H/R, hypoxia/reperfusion; GAPDH, glyceraldehyde
3-phosphate dehydrogenase; UTR, untranslated region; miR, microRNA;
MUT, mutant, WT, wild-type. |
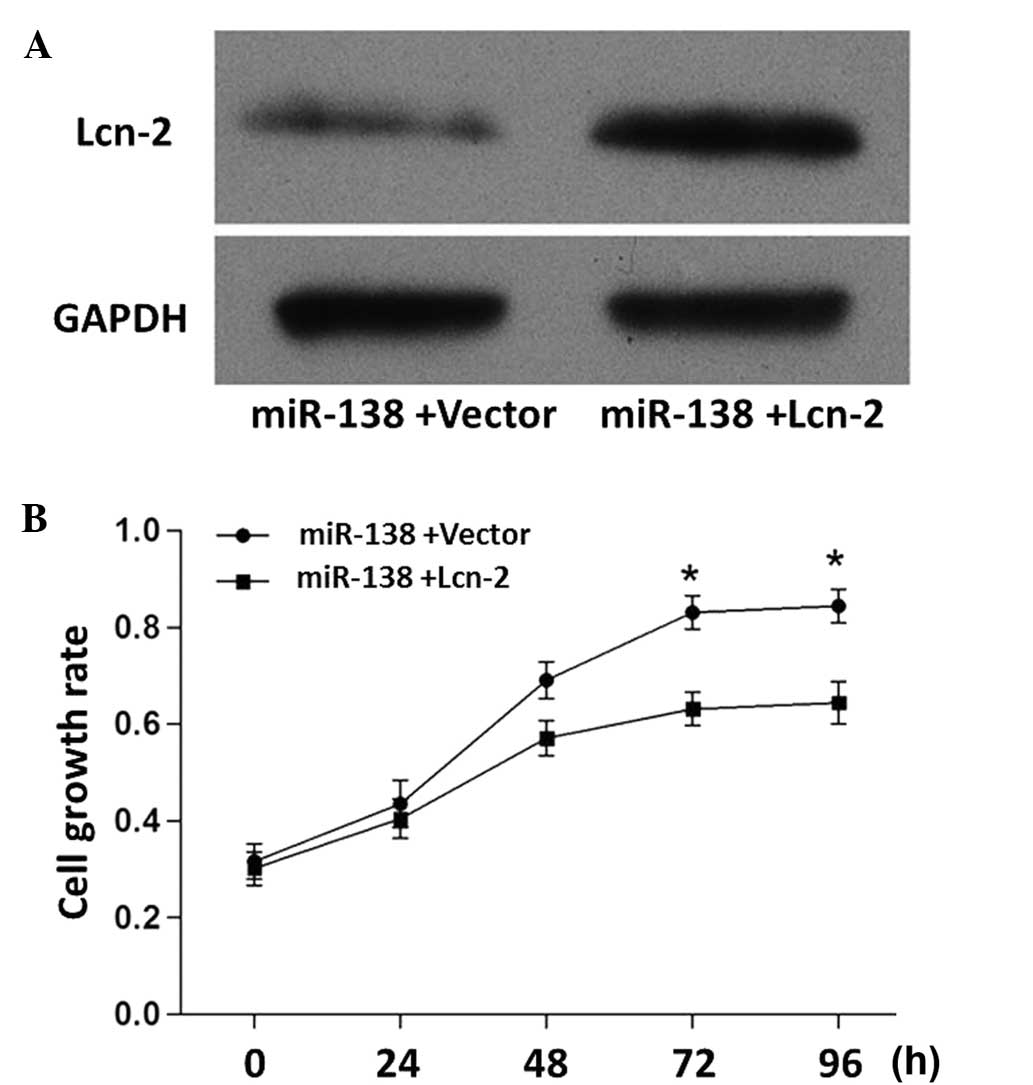
As shown in Fig. 5A,
the miR-138-induced inhibition of Lcn-2 was attenuated when the
cells were co-transfected with miR-138 mimics and Lcn-2 expression
vector. In addition, the miR-138-induced PC12 cell proliferation
was attenuated when the cells were co-transfected with miR-138
mimics and Lcn-2 expression vectors (Fig. 5B). These results further demonstrated
the inhibitory effect of Lcn-2 on PC12 cell growth.
Discussion
Cerebral injury induced by ischemia/reperfusion
(CIR) is notably more severe than damage induced by ischemia alone.
It is speculated that oxidative radical-induced inflammation is
crucially involved during the progression of CIR injury (17). Since miRs were identified as common
regulators of DNA transcription and mRNA translation, numerous miRs
have been associated with oxidative radical-mediated
pathophysiological alterations (19). As a crucial protein in IR-induced
injury, Lcn-2 is included in the list of miR-138 targets (20). In the present CIR rat model, the
expression of miR-138 was significantly reduced, while the Lcn-2
was markedly upregulated. The inverse correlation between miR-138
and Lcn-2 suggests that miR-138 functions as a negative regulator
of Lcn-2 expression. Diverse mechanisms underlie miR activity
(21); for example, miRNAs may
increase the translation of target mRNAs by recruiting protein
complexes to the AU rich elements of the mRNA, or they may switch
the regulation from repression to activation of target gene
translation in conditions of cell cycle arrest (22). As miRs frequently target hundreds of
mRNAs, miR regulatory pathways are complex. In the present study,
in vitro synthesized miR-138-specific mimics and inhibitors
were used in PC12 cells to verify the association between miR-138
and Lcn-2.
CIR was simulated in PC12 cells by H/R treatment in
the current study. The PC12 cell line originates from rat
pheochromocytoma, representing the most widely used system for the
study of regulatory mechanisms of neuronal survival and apoptosis
(23). The expression patterns of
miR-138 and Lcn-2 in PC12 cells were comparable with those observed
in the CIR rat model. The application of miR-138 mimics
downregulated the expression of Lcn-2, while miR-138 inhibitors
upregulated Lcn-2 expression. Accordingly, the cell growth rate was
enhanced by miR-138 mimics and depressed by inhibitors. The
interaction of miRs with the 3′-UTR of protein-coding genes via
RNA-RNA base-pairing is considered to be the primary mechanism
underlying the actions of miRs, which usually leads to a reduction
in protein output, either by mRNA degradation or by translational
repression (21). Furthermore,
previous studies have suggested that miRs may interact with the
5′UTR of protein-coding genes via complementarity and cause the
translational repression or activation of the targeted proteins.
Similarly, miRs could target the coding sequence and thereby
repress gene translation (24–26). The
specificity of miR targeting is determined by the complementary
extent of the ‘seed’ sequence (positions 2–8 from the 5′ end of the
miR) and the ‘seed-match’ sequence (generally in the 3′UTR of the
target mRNA) (27). The base-pairing
between miR-138 and Lcn-2 mRNA is in accordance with that described
above. To verify the direct interaction between miR-138 and Lcn-2,
miR-138 mimics and inhibitors were applied to PC12 cells
transfected with luciferase-labeled wild-type Lcn-2
(3′UTR-Lcn-2-WT) or luciferase-labeled mutant Lcn-2
(3′UTR-Lcn-2-MUT). miR-138 specifically attenuated the luciferase
activity in PC12 cells transfected with 3′UTR-Lcn-2-WT. In
addition, miR-138-induced alterations of Lcn-2 expression and cell
growth rate were neutralized by Lcn-2 overexpression.
In conclusion, the results of the present study
demonstrated that the expression levels of miR-138 were inversely
correlated with the expression levels of Lcn-2 in the CIR rat model
and H/R-treated PC12 cells. The expression of Lcn-2 was inhibited
by miR-138 mimics and enhanced by miR-138 inhibitors, thereby
indicating that miR-138 functions as a negative regulator for Lcn-2
expression. Therefore, the present study provides an experimental
basis for the use of miR-138-based therapy for the treatment of CIR
injury.
Acknowledgements
The present study was supported by the Taihe
Hospital Foundation. The authors would like to thank Mr. James Dai
(LJ Resources Co., Ltd., Vancouver, Canada) for his assistance in
preparing the manuscript and English proofreading.
References
|
1
|
Linkermann A, Hackl MJ, Kunzendorf U,
Walczak H, Krautwald S and Jevnikar AM: Necroptosis in immunity and
ischemia-reperfusion injury. Am J Transplant. 13:2797–2804. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee RC, Feinbaum RL and Ambros V: The
C. elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yates LA, Norbury CJ and Gilbert RJ: The
long and short of microRNA. Cell. 153:516–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Callegari E, Elamin BK, Sabbioni S,
Gramantieri L and Negrini M: Role of microRNAs in hepatocellular
carcinoma: A clinical perspective. Onco Targets Ther. 6:1167–1178.
2013.PubMed/NCBI
|
|
6
|
Smits M, Nilsson J, Mir SE, van der Stoop
PM, Hulleman E, Niers JM, de Witt Hamer PC, Marquez VE, Cloos J,
Krichevsky AM, et al: miR-101 is down-regulated in glioblastoma
resulting in EZH2-induced proliferation, migration and
angiogenesis. Oncotarget. 1:710–720. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malizia AP and Wang DZ: MicroRNAs in
cardiomyocyte development. Wiley Interdiscip Rev Syst Biol Med.
3:183–190. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mosakhani N, Guled M, Lahti L, Borze I,
Forsman M, Pääkkönen V, Ryhänen J and Knuutila S: Unique microRNA
profile in Dupuytren's contracture supports deregulation of
β-catenin pathway. Mod Pathol. 23:1544–1552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He S, Liu P, Jian Z, Li J, Zhu Y, Feng Z
and Xiao Y: miR-138 protects cardiomyocytes from hypoxia-induced
apoptosis via MLK3/JNK/c-jun pathway. Biochem Biophys Res Commun.
441:763–769. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li S, Ran Y, Zhang D, Chen J, Li S and Zhu
D: MicroRNA-138 plays a role in hypoxic pulmonary vascular
remodelling by targeting Mst1. Biochem J. 452:281–291. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morton SU, Scherz PJ, Cordes KR, Ivey KN,
Stainier DY and Srivastava D: microRNA-138 modulates cardiac
patterning during embryonic development. Proc Natl Acad Sci USA.
105:17830–17835. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu TS, Yang JJ, Yu FY and Liu BH:
Cardiotoxicity of mycotoxin citrinin and involvement of
microRNA-138 in zebrafish embryos. Toxicol Sci. 136:402–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rathore KI, Berard JL, Redensek A, Chierzi
S, Lopez-Vales R, Santos M, Akira S and David S: Lipocalin 2 plays
an immunomodulatory role and has detrimental effects after spinal
cord injury. J Neurosci. 31:13412–13419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chia WJ, Dawe GS and Ong WY: Expression
and localization of the iron-siderophore binding protein lipocalin
2 in the normal rat brain and after kainate-induced excitotoxicity.
Neurochem Int. 59:591–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aigner F, Maier HT, Schwelberger HG,
Wallnöfer EA, Amberger A, Obrist P, Berger T, Mak TW, Maglione M,
Margreiter R, et al: Lipocalin-2 regulates the inflammatory
response during ischemia and reperfusion of the transplanted heart.
Am J Transplant. 7:779–788. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Parikh CR, Jani A, Mishra J, Ma Q, Kelly
C, Barasch J, Edelstein CL and Devarajan P: Urine NGAL and IL-18
are predictive biomarkers for delayed graft function following
kidney transplantation. Am J Transplant. 6:1639–1645. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng L, Xing H, Mao X, Li L, Li X and Li
Q: Lipocalin-2 promotes m1 macrophages polarization in a mouse
cardiac ischaemia-reperfusion injury model. Scand J Immunol.
81:31–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Z, Wen L, Martin M, Hsu CY, Fang L,
Lin FM, Lin TY, Geary MJ, Geary GG, Zhao Y, et al: Oxidative stress
activates endothelial innate immunity via sterol regulatory element
binding protein 2 (SREBP2) transactivation of microRNA-92a.
Circulation. 131:805–814. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pontrelli P, Accetturo M and Gesualdo L:
miRNome analysis using real-time PCR. Methods Mol Biol.
1186:201–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ling H, Fabbri M and Calin GA: MicroRNAs
and other non-coding RNAs as targets for anticancer drug
development. Nat Rev Drug Discov. 12:847–865. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vasudevan S, Tong Y and Steitz JA:
Switching from repression to activation: microRNAs can up-regulate
translation. Science. 318:1931–1934. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Posse Chaves EI: Sphingolipids in
apoptosis, survival and regeneration in the nervous system. Biochim
Biophys Acta. 1758:1995–2015. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tay Y, Zhang J, Thomson AM, Lim B and
Rigoutsos I: MicroRNAs to Nanog, Oct4 and Sox2 coding regions
modulate embryonic stem cell differentiation. Nature.
455:1124–1128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lytle JR, Yario TA and Steitz JA: Target
mRNAs are repressed as efficiently by microRNA-binding sites in the
5′ UTR as in the 3′ UTR. Proc Natl Acad Sci USA. 104:9667–9672.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Knoll M, Lodish HF and Sun L: Long
non-coding RNAs as regulators of the endocrine system. Nat Rev
Endocrinol. 11:151–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|