Introduction
Ulcerative colitis (UC) is a chronic, non-specific
colitis of unknown etiology (1,2). Its
etiology and pathogenesis are complex. UC is generally thought to
be caused by the interaction between environmental factors,
heredity, immune system, infection and mentality (3,4).
Assessment of the disease activity and severity of UC has clinical
value in guiding clinical treatment and evaluating prognosis.
Hypoxia inducible factor-1 (HIF-1) (5–7) was
identified by Semenza and Wang in 1992 (8) from the nuclear extract of the Hep3B
cell line in which the protein was found to be bound to the
enhancer of the erythropoietin (EPO) gene. HIF-1 in humans
and other mammals under anoxic conditions forms a heterodimer with
the HIF-1α and HIF-1β subunit (9–11). The
HIF-1α subunit is regulated in an oxygen dependent-manner. HIF-1α
under low oxygen conditions escapes proteasome-mediated degradation
and accumulates in the cell cytoplasm where it binds with HIF-1β,
and subsequently translocates to the nucleus to activate the
transcription of target genes.
The present study aimed to measure HIF-1α in serum
and colonic mucosa of UC patients and UC in remission patients to
examine its regulatory role in the pathogenesis of UC and to assess
its relationship with disease activity and severity, thus providing
a basis in the search of a clinically effective index to evaluate
disease activity and severity.
Materials and methods
Patients
A total of 60 clinically confirmed UC patients who
had visited the affiliated Xiangyang Hospital of Hubei Medical
College (Wuhan, China) during the period January 2010 to September
2013 were included in this study. Of the 60 patients, 47 were
active UC (28 males and 19 females) and 13 were UC in remission (9
males and 4 females). Ten healthy subjects (6 males and 4 females)
were also included in this study and served as controls. The
diagnostic criteria for UC were in accordance with China's IBD
diagnosis consensus opinion in 2007 (12). Disease activity was measured
following the improved Mayo scoring system and disease severity was
graded according to the standards of Truelove and Witts while
endoscopic grading was in line with the standards of Truelove.
Approval for the current study was obtained by the
Institutional Ethics Committee of the affiliated Xiangyang Hospital
of Hubei Medical College.
Analysis of HIF-1α in serum
Blood (4 ml) was drawn in the morning and the serum
portion was extracted. HIF-1α was measured in the serum following
the manufacturer's instructions (Sino-American Biotechnology Co.,
Ltd., Wuhan, China) using the ELISA method. Colonic mucosal tissue
specimens were obtained from UC patients, UC in remission patients
and from controls. The obtained specimens were fixed in 10%
formalin and subsequently embedded in paraffin. The paraffin blocks
were cut into 4 µm sections which were stained with conventional
hematoxylin and eosin (H&E) and HIF-1α specific antibody
through immunohistochemistry. Briefly, the paraffin-embedded
specimens were dewaxed and rehydrated by passing through a graded
series of ethanol to distilled water. The sections were incubated
with 3% hydrogen peroxide for 10 min to block the endogenous
peroxidase activity. The sections were then boiled in sodium
citrate buffer (pH 6.0) to retrieve antigen. The sections were
incubated with goat serum for 15 min at room temperature. HIF-1α
polyclonal rabbit antibody (1:100 dilution, Wuhan Boster
Bio-Engineering Co., Ltd., Wuhan, China) was added and incubated
overnight at 4°C. The following day, the sections were rinsed in
PBS three times and incubated with horseradish-peroxidase
conjugated antibody for 15 min. A DAB color developing substrate
was added and the sections were examined microscopically for color
development for 5–10 min, re-dyed with H&E, mounted and
visualized under the microscope (Olympus BX53, Tokyo, Japan). A
polyclonal anti-goat HIF-1α antibody (1:80) was used with PBS as a
negative control.
Immunohistochemistry
The HIF-1α protein which contained tan fine
particles in the nucleus or cytoplasm was considered positive. Ten
non-overlapping randomly selected fields were analyzed for each
sample at a magnification of ×400 and the images were captured. The
captured images were analyzed using the HPIAS-2000 software
(Techman, Chengdu, China) to scan HIF-1α-positive cells and to
record the percentage and mean values for each sample.
Statistical analysis
The statistical software SPSS 14.0 version (SPSS,
Inc., Chicago, IL, USA) was used to analyze the results. A
normality test and the homogeneity test of variance were used to
evaluate the statistical indicators. The analyzed data were
presented as mean ± standard deviation. The groups were compared by
one-way analysis of variance and a correlation analysis was carried
out using the Pearson linear and Spearman's rank correlation tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
HIF-1α is associated with pathogenesis
of UC
The serum HIF-1α level in UC patients was
(73.21±28.65), which was significantly (P<0.05) higher than that
in UC in remission patients (44.54±14.75) and controls
(42.83±15.49). However, the distribution of serum HIF-1α was more
or less similar between UC in remission patients and the controls
(P>0.05, Table I). The expression
of HIF-1α in colonic mucosa was found to be extremely high in UC
patients (58.05±13.83) in comparison with UC in remission patients
(3.00±2.72) and controls (3.04±2.69). The difference between UC
patients and UC in remission patients and controls was significant
(P<0.05, Table I). A low
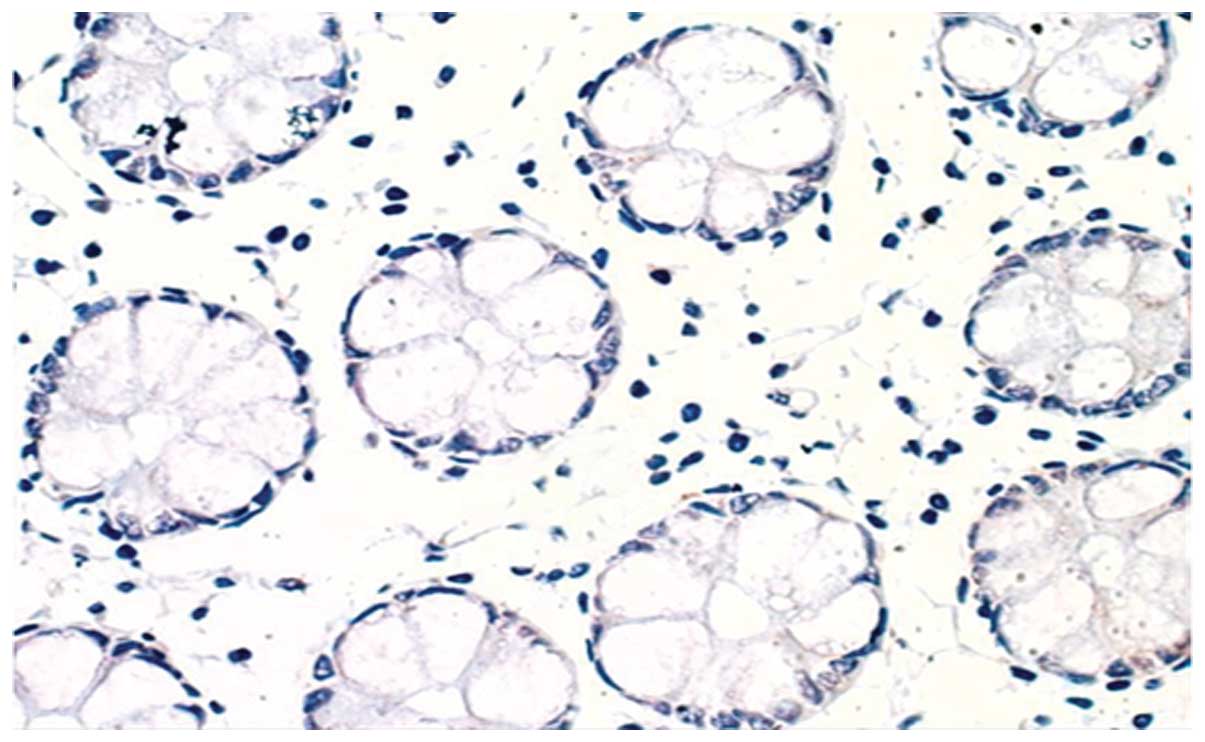
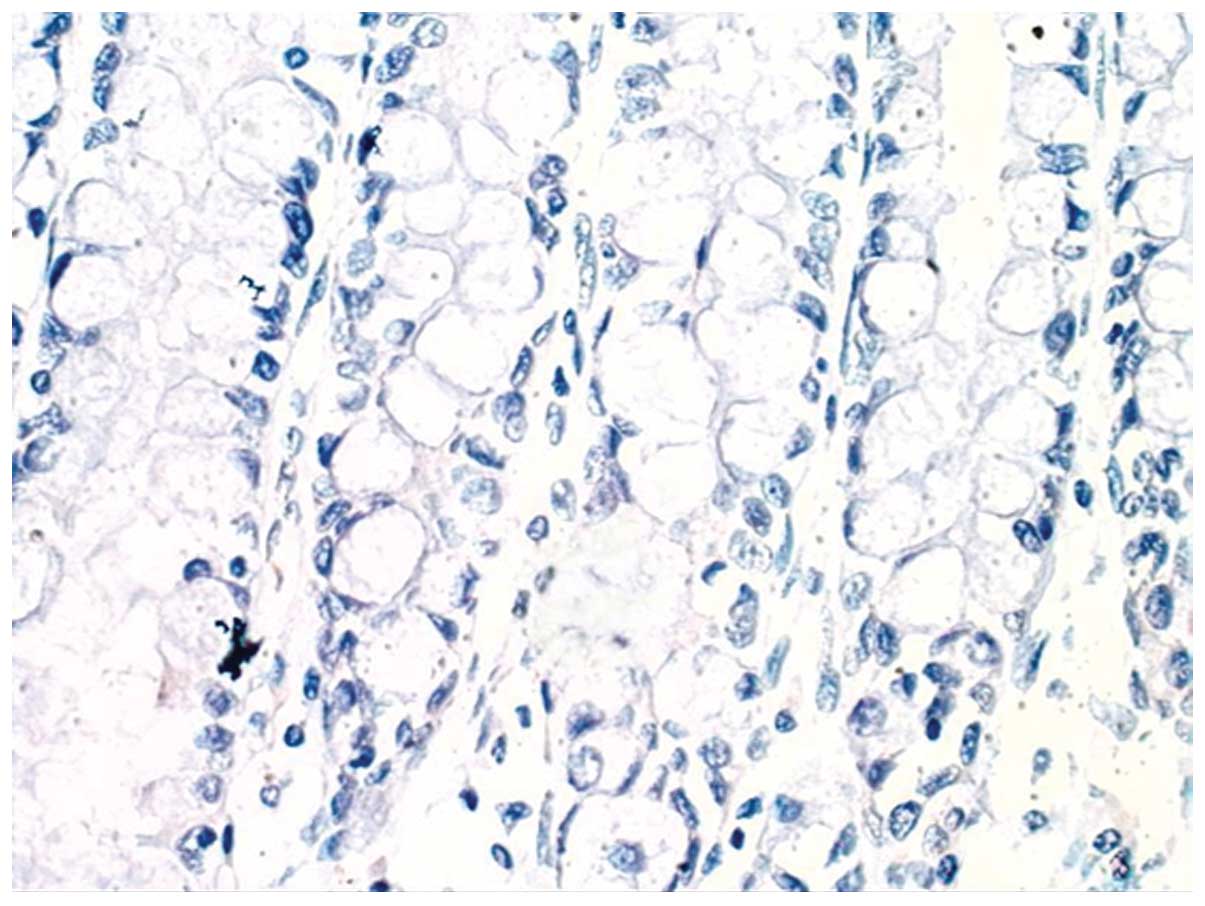
expression of HIF-1α in colonic mucosa and no expression of HIF-1α
in healthy intestinal tissue of UC in remission patients were
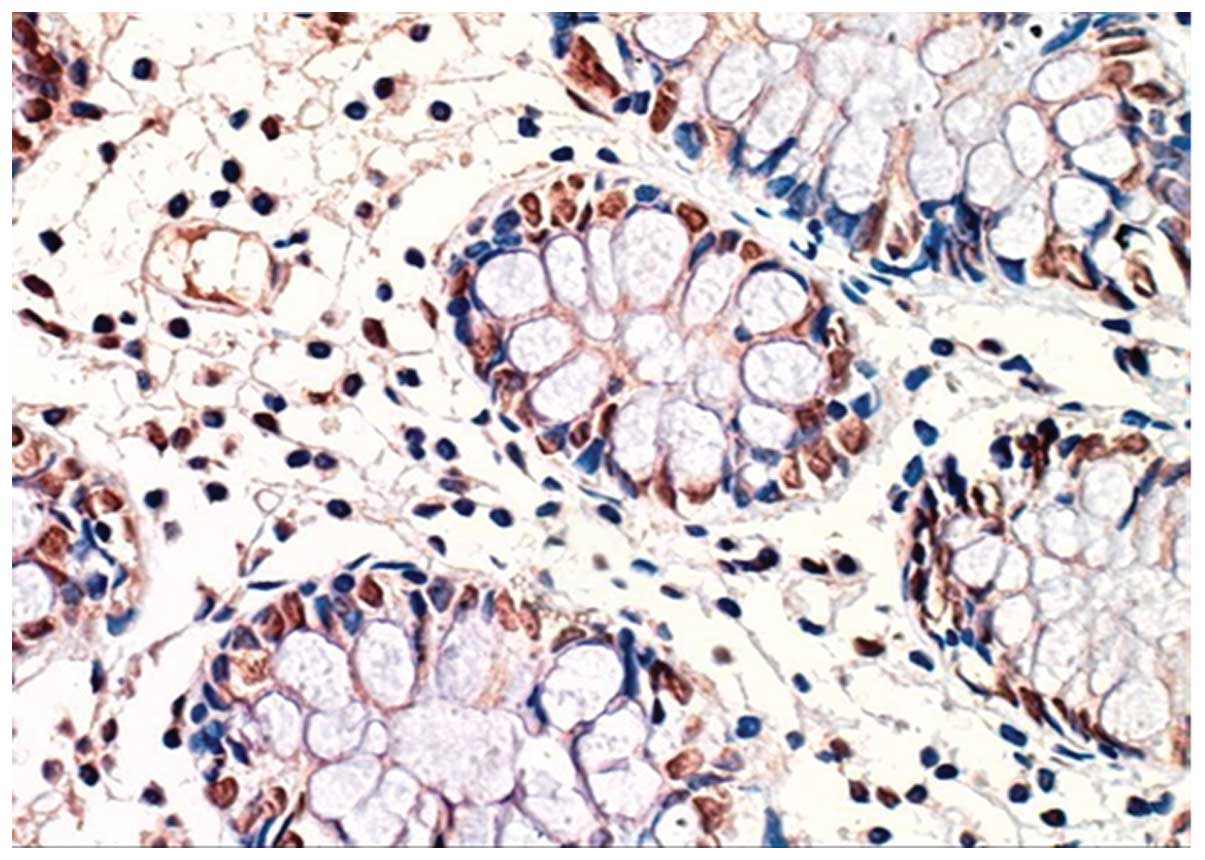
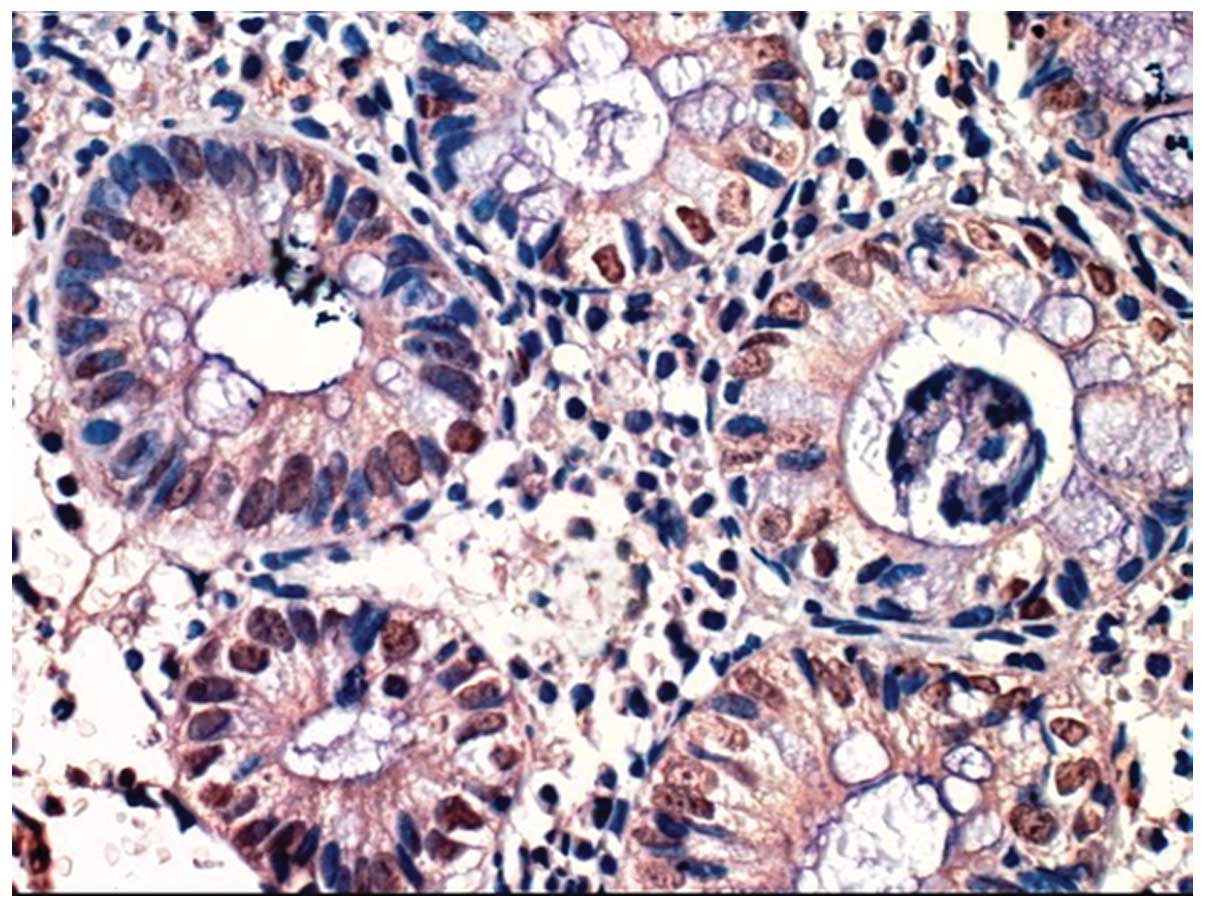
identified (Figs. 1 and 2). However, HIF-1α was expressed was
observed in epithelial cells of the enteric cavity and gland,
endothelial cells of blood vessels, connective tissue cells on
matrices and inflammatory cells of the intestinal tissue (Figs. 3–5).
Thus, HIF-1α expression increased with disease progression.
 | Table I.HIF-1α level in patients and
controls. |
Table I.
HIF-1α level in patients and
controls.
| Subjects | No. | Mayo scoring | Serum HIF-1α
(ng/l) | HIF-1α-positive cells
(%) |
|---|
| Controls | 10 | – | 42.83±15.49 | 3.04±2.69 |
| UC in remission
patients | 13 | 0.54±0.66 | 44.54±14.75 | 3.00±2.72 |
| UC patients | 47 | 6.11±2.50 | 73.21±28.65 | 58.05±13.83 |
Correlation analysis
HIF-1α level in serum and colonic mucosa of UC in
remission patients was not correlated with Mayo scoring (serum
HIF-1α, r=0.139, P>0.05; colonic mucosa HIF-1α, r=0.108,
P>0.05). However, the expression level of HIF-1α in serum and
colonic mucosa was positively associated with Mayo scoring (serum
HIF-1α, r=0.699, P<0.01; colonic mucosa HIF-1α; r=0.743,
P<0.01) in UC patients. Additionally, the expression level of
HIF-1α in serum demonstrated a positive correlation with disease
severity (r=0.696, P<0.01) and endoscopic grading (r=0.674,
P<0.01; Table II) in UC
patients. Similarly, the expression level of HIF-1α in the colonic
mucosa of UC patients showed a significant correlation with disease
severity (r=0.699, P<0.01) and endoscopic grading (r=0.677,
P<0.01, Table III).
 | Table II.Correlation of serum HIF-1α level with
disease severity and endoscopic grading in UC patients. |
Table II.
Correlation of serum HIF-1α level with
disease severity and endoscopic grading in UC patients.
| Parameters | No. of subjects | Serum HIF-1α (mean ±
SD) | Correlation index
(r) | P-value |
|---|
| Disease severity |
|
| 0.696 | 0.0002 |
| Low | 23 |
56.60±17.96 |
|
|
|
Moderate | 16 |
73.61±18.14 |
|
|
|
Severe | 08 |
120.16±15.81 |
|
|
| Endoscopic
grading |
|
| 0.674 | 0.00023 |
| I | 18 |
55.65±19.94 |
|
|
| II | 22 |
72.28±18.99 |
|
|
| III | 7 |
121.29±16.73 |
|
|
 | Table III.Correlation of HIF-1α of colonic
mucosa with disease severity and endoscopic grading in UC
patients. |
Table III.
Correlation of HIF-1α of colonic
mucosa with disease severity and endoscopic grading in UC
patients.
| Parameters | No. of subjects | HIF-1α-positive cells
(%) | Correlation index
(r) | P-value |
|---|
| Disease severity |
|
| 0.699 | 0.00013 |
| Low | 23 |
49.02±10.69 |
|
|
|
Moderate | 16 |
58.75±10.23 |
|
|
| Severe | 08 | 76.87±2.44 |
|
|
| Endoscopic
grading |
|
| 0.677 | 0.00026 |
| I | 18 |
48.04±11.85 |
|
|
| II | 22 | 58.11±9.98 |
|
|
| III | 7 | 77.04±2.58 |
|
|
Discussion
Oxygen plays an essential role in metabolic activity
and cell survival (13). Under
anoxic conditions, several genes in cells are stimulated in order
to respond to hypoxic stress such as CXCR4, KlHEM13 (14–16).
Hypoxia causes metabolic disorders, functional disorders and
various pathological and physiological changes that ultimately
result in disease (17).
In UC, the apoptosis, proliferation of intestinal
epithelial cells, endothelial cells, lymphocytes, accelerated
metabolism and activation of the large number of blood platelets
collectively affect the microcirculation in intestinal mucosa
(18,19). Those processes together constitute a
local hypoxic microenvironment in the intestinal mucosa. Previous
findings have shown different degrees of hypoxia in the intestinal
mucosa of murine models and in patients with inflammatory bowel
disease (20–22). During hypoxia the balance between
oxygen supply and oxygen consumption in cells is damaged, which
enables some hypoxia-inducible factors such as HIF-1α to regulate
different physiological and pathological reactions through
different mechanisms (23). HIF-1α
is associated with diseases due to hypoxia, but is also involved in
ischemia and many types of cancer. It also has a close association
with the restoration and maintenance of intestinal barrier
functions. HIF-1α is able to regulate the expression of numerous
target genes such as vascular endothelial growth factor, EPO,
GAPDH, inducible nitric oxide synthase (iNOS), cyclooxygenase
(COX)-2, insulin-like growth factor 2, ET-1, HIF-1α transferrin and
glycolytic enzymes. In chronic hypoxia, HIF-1α and some of its
target gene products, such as iNOS, COX-2, interleukin (IL)-6 and
IL-8 (24), may also be involved in
the various pathogenic processes in the manifestation of UC such as
the regulation of inflammation, immunity and apoptosis. Findings of
a previous study (25) showed that
COX-2 and iNOS, which are regulated by HIF-1α, were not or were
weakly expressed in normal intestinal mucosa, although their
expression significantly increased in the intestinal mucosa of UC
patients.
The current findings have confirmed that, in UC
patients, serum HIF-1α in colonic mucosa was expressed
significantly higher than that of UC in remission patients and
controls. Thus, hypoxia is present in UC and HIF-1α and plays an
important role in the pathogenesis of UC.
HIF-1α expression is associated with inflammation.
For example, lipopolysaccharide (LPS), the product of bacterial
metabolism, strongly induces HIF-1α expression in macrophage
(26). Thus, it plays as an
important role in LPS-induced inflammation. HIF-1 gene knockout
inhibited the neutrophils and macrophages from eliciting an
inflammatory response under hypoxic conditions, thereby preventing
of inflammation (26). HIF-1α has
been studied in many inflammation-mediated diseases, including
rheumatoid arthritis, chronic bronchitis, chronic obstructive
pulmonary disease, acute pancreatitis, periodontitis and
periodontal disease, chronic hepatitis, chronic nephritis, and
verrucous gastritis (27–31) and it was identified that HIF-1α
participation in eliciting the inflammatory response was
experimentally confirmed. HIF-1α is a core transcription factor in
oxygen homeostasis. Oxygen concentration is not the only factor
that regulates HIF-1α, cytokines secreted during inflammatory
condition have a significant regulatory role in HIF-1α induction,
DNA binding activity and expression of the backward genes (32). For instance, inflammation-associated
cytokines such as IL-1β, IL-6, tumor necrosis factor-α (TNF-α) and
NO have a significant regulatory role in HIF-1α protein
accumulation, DNA binding activity and the expression of backward
factors (32). By contrast, the role
of inflammation-associated cytokines such as IL-1β, IL-6, TNF-α,
and NO in the pathogenesis of UC have been confirmed (33–35).
Under anoxic conditions, a positive feedback loop may be created as
‘inflammation-inflammation medium-HIF-1α-inflammation
medium-inflammation’, in which HIF-1α at least plays a role in the
amplification of inflammation, making the existing inflammation
response amplified and persistent.
In the present study, the HIF-1α expression level in
the serum and colonic mucosa of UC in remission patients was not
correlated with Mayo scoring. However, in the UC patients, the
HIF-1α expression level in serum and colonic mucosa demonstrated an
obvious correlation with Mayo scoring and had a significant
positive correlation with disease severity and endoscopic grading
(36). As the abovementioned
hypotheses have been demonstrated in the current study, HIF-1α may
serve as a good index to assess disease activity and severity of
UC.
In conclusion, the findings of the current study
show that HIF-1α is correlated with UC. However, its complicated
molecular mechanism requires additional investigation, which might
be of great significance in further explaining the pathogenesis of
UC and in pursuing effective, innovative treatments targeting
HIF-1α.
References
|
1
|
Diamanti A, Knafelz D, Panetta F, De
Angelis P, Candusso M, Bracci F, Papadatou B, Francalanci P, Monti
L and Torre G: Thalidomide as rescue therapy for acute severe
ulcerative colitis. Eur Rev Med Pharmacol Sci. 18:1690–1693.
2014.PubMed/NCBI
|
|
2
|
Hu D, Xia SL, Shao XX, Yu LQ, Lin XX, Guo
M, Lin XQ and Jiang Y: Association of ulcerative colitis with
TNF-related apoptosis inducing ligand (TRAIL) gene polymorphisms
and plasma soluble TRAIL levels in Chinese Han population. Eur Rev
Med Pharmacol Sci. 19:467–476. 2015.PubMed/NCBI
|
|
3
|
Dong WG, Liu SP, Yu BP, Wu DF, Luo HS and
Yu JP: Ameliorative effects of sodium ferulate on experimental
colitis and their mechanisms in rats. World J Gastroenterol.
9:2533–2538. 2003.PubMed/NCBI
|
|
4
|
Pica R, Cassieri C, Pronio AM, Zippi M,
Avallone EV, Montesani C, Occhigrossi G and Paoluzi P: Quality of
life in ulcerative colitis patients treated medically versus
patients undergoing surgery. Eur Rev Med Pharmacol Sci. 18:693–698.
2014.PubMed/NCBI
|
|
5
|
Bai R, Zhao AQ, Zhao ZQ, Liu WL and Jian
DM: MicroRNA-195 induced apoptosis in hypoxic chondrocytes by
targeting hypoxia-inducible factor 1 alpha. Eur Rev Med Pharmacol
Sci. 19:545–551. 2015.PubMed/NCBI
|
|
6
|
Wei H, Li F, Fu P and Liu X: Effects of
the silencing of hypoxia-inducible factor-1 alpha on metastasis of
pancreatic cancer. Eur Rev Med Pharmacol Sci. 17:436–446.
2013.PubMed/NCBI
|
|
7
|
Zhang M, Gao X, Bai SJ, Ye XM and Zhang J:
Effect of pioglitazone on expression of hypoxia-inducible factor 1α
and vascular endothelial growth factor in ischemic hindlimb of
diabetic rats. Eur Rev Med Pharmacol Sci. 18:1307–1314.
2014.PubMed/NCBI
|
|
8
|
Semenza GL and Wang GL: A nuclear factor
induced by hypoxia via de novo protein synthesis binds to the human
erythropoietin gene enhancer at a site required for transcriptional
activation. Mol Cell Bio. 12:5447–5454. 1992. View Article : Google Scholar
|
|
9
|
Maxwell PH, Pugh CW and Ratcliffe PJ:
Activation of the HIF pathway in cancer. Curr Opin Genet Dev.
11:293–299. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thornton RD, Lane P, Borghaei Re, et al:
Interleukin 1 induceshypoxia-inducible factor 1 in human gingival
and synovialfibroblasts. Biochem J. 350:307–312. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Albina JE, Mastrofrancesco B, Vessella JA,
et al: HIF-1 expression in healing wounds: HIF-1alpha induction in
primary inflammatory cells by TNF-alpha. Am J Physiol Cell Physiol.
281:1971–1977. 2001.
|
|
12
|
Ouyang Q, Hu PJ, Qian JM, Zheng JJ and Hu
RW: Consensus on the management of inflammatory bowel disease in
China in 2007. J Dig Dis. 8:545–561. 2007.
|
|
13
|
Urgesi R, Zampaletta C, Masini A, Pelecca
G, Pastorelli A, De Lorenzo A and Faggiani R: Spontaneous right
ventricular thrombus in a patient with active ulcerative colitis
and protein C deficiency: a review with a case report. Eur Rev Med
Pharmacol Sci. 14:455–463. 2010.PubMed/NCBI
|
|
14
|
Federico A, Tuccillo C, Grossi E, Abbiati
R, Garbagna N, Romano M, Tiso A, del Blanco CV and Loguercio C: The
effect of a new symbiotic formulation on plasma levels and
peripheral blood mononuclear cell expression of some
pro-inflammatory cytokines in patients with ulcerative colitis: a
pilot study. Eur Rev Med Pharmacol Sci. 13:285–293. 2009.PubMed/NCBI
|
|
15
|
Blanco MI, Becerra M, González-Siso MI and
Cerdán ME: Functional characterization of KlHEM13, a hypoxic gene
of Kluyveromyces lactis. Can J Microbiol. 51:241–249. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Le QT, Denko NC and Giaccia AJ: Hypoxic
gene expression and metastasis. Cancer Metastasis Rev. 23:293–310.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sanges M, Valente G, Rea M, Della Gatta R,
De Franchis G, Sollazzo R and D'Arienzo A: Probiotics in
spondyloarthropathy associated with ulcerative colitis: a pilot
study. Eur Rev Med Pharmacol Sci. 13:233–234. 2009.PubMed/NCBI
|
|
18
|
Schmidt C, Lautenschläger C, Petzold B,
Sakr Y, Marx G and Stallmach A: Confocal laser endomicroscopy
reliably detects sepsis-related and treatment-associated changes in
intestinal mucosal microcirculation. Br J Anaesth. 111:996–1003.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harrois A, Baudry N, Huet O, Kato H, Lohez
M, Ziol M, Duranteau J and Vicaut E: Synergistic deleterious effect
of hypoxemia and hypovolemia on microcirculation in intestinal
villi. Crit Care Med. 41:e376–e384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lehmann Ch, Abdo I, Kern H, Maddison L,
Pavlovic D, Sharawi N, Starkopf J, Hall R, Johnson P, Williams L,
et al: MiDAS (Microcirculation Diagnostics and Applied Studies)
group: Clinical evaluation of the intestinal microcirculation using
sidestream dark field imaging - recommendations of a round table
meeting. Clin Hemorheol Microcirc. 57:137–146. 2014.PubMed/NCBI
|
|
21
|
Yeh YC, Sun WZ, Ko WJ, Chan WS, Fan SZ,
Tsai JC and Lin TY: Dexmedetomidine prevents alterations of
intestinal microcirculation that are induced by surgical stress and
pain in a novel rat model. Anesth Analg. 115:46–53. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Armuzzi A, De Pascalis B, Lupascu A,
Fedeli P, Leo D, Mentella MC, Vincenti F, Melina D, Gasbarrini G,
Pola P, et al: Infliximab in the treatment of steroid-dependent
ulcerative colitis. Eur Rev Med Pharmacol Sci. 8:231–233.
2004.PubMed/NCBI
|
|
23
|
Giatromanolaki A, Sivridis E, Maltezos E,
Papazoglou D, Simopoulos C, Gatter KC, Harris AL and Koukourakis
MI: Hypoxia inducible factor 1alpha and 2alpha overexpression in
inflammatory bowel disease. J Clin Pathol. 56:209–213. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeong HJ, Chung HS, Lee BR, Kim SJ, Yoo
SJ, Hong SH and Kim HM: Expression of proinflammatory cytokines via
HIF-1alpha and NF-kappaB activation on desferrioxamine-stimulated
HMC-1 cells. Biochem Biophys Res Commun. 306:805–811. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu CM, Dong WG, Yu BP, et al: Expression
of hypoxia-inducible factor-1α and inflammation-induced enzyme in
inflammatory bowel disease. J Clinical Gastroente. 17:103–105.
2005.
|
|
26
|
Cramer T, Yamanishi Y, Clausen BE, Förster
I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V,
et al: HIF-1alpha is essential for myeloid cell-mediated
inflammation. Cell. 112:645–657. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu F, Liu H, Xu L, et al:
Hypoxia-inducible factor-1α perpetuates synovial fibroblast
interactions with T cells and B cells in rheumatoid arthritis. Eur
J Immunol. November 25–2015.(Epub ahead of print). doi:
10.1002/eji.201545784. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang X, Liu J, Wan L, et al: Up-regulated
expressions of HIF-1α, VEGF and CD34 promote synovial angiogenesis
in rats with adjuvant arthritis. Xi Bao Yu Fen Zi Mian Yi Xue Za
Zhi. 31:1053–1056. 2015.PubMed/NCBI
|
|
29
|
Lee SH, Lee SH, Kim CH, et al: Increased
expression of vascular endothelial growth factor and hypoxia
inducible factor-1α in lung tissue of patients with chronic
bronchitis. Clin Biochem. 47:552–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tao H, Luo W, Pei H, Zhu S, Zhang M, Chen
B, He J, Zhang M and Zhou R: Expression and significance of
hypoxia-inducible factor-1α in patients withchronic obstructive
pulmonary disease and smokers with normal lung function. Xi Bao Yu
Fen Zi Mian Yi Xue Za Zhi. 30:852–855. 2014.PubMed/NCBI
|
|
31
|
Liu JB, Ding JQ and Wang L:
Immunohistochemical study of dental pulp inflammation of
hypoxia-inducible factor-1α. J Clin Stomatol. 19:77–78. 2003.
|
|
32
|
Hellwig-Bürgel T, Rutkowski K, Metzen E,
Fandrey J and Jelkmann W: Interleukin-1beta and tumor necrosis
factor-alpha stimulate DNA binding of hypoxia-inducible factor-1.
Blood. 94:1561–1567. 1999.PubMed/NCBI
|
|
33
|
Wang BL and Wang XR: Regulation of hypoxia
inducible factor-1α by the inflammatory mediators. Int J Pathol
Clin Med. 90:1673–2588. 2008.
|
|
34
|
Ngo DT, Farb MG, Kikuchi R, Karki S,
Tiwari S, Bigornia SJ, Bates DO, LaValley MP, Hamburg NM, Vita JA,
et al: Antiangiogenic actions of vascular endothelial growth
factor-A165b, an inhibitory isoform of vascular endothelial growth
factor-A, in human obesity. Circulation. 130:1072–1080. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cătană CS, Neagoe Berindan I, Cozma V,
Magdaş C, Tăbăran F and Dumitraşcu DL: Contribution of the
IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease.
World J Gastroenterol. 21:5823–5830. 2015.PubMed/NCBI
|
|
36
|
Avallone EV, Pica R, Cassieri C, Zippi M,
Paoluzi P and Vernia P: Azathioprine treatment in inflammatory
bowel disease patients: type and time of onset of side effects. Eur
Rev Med Pharmacol Sci. 18:165–170. 2014.PubMed/NCBI
|