Introduction
The prevalence of diabetic retinopathy (DR) in
patients with established diabetes and newly diagnosed diabetes is
27.9 and 10.5%, respectively (1),
and the incidence of DR is expected to increase substantially
(2). DR is the leading cause of
blindness in patients of working age (3), and one of the early manifestations of
DR is persistent apoptosis of vascular and neural cells in the
retinal tissue (4,5). Other consequences of DR include the
breakdown of the blood retinal barrier, retinal edema,
neovascularization and detachment and loss of vision (5). Although the pathogenesis of DR is
complicated and has yet to be fully elucidated,
hyperglycemia-induced oxidative stress, an imbalance in the
production of reactive oxygen species (ROS) and ROS-induced damage
have been demonstrated to serve a crucial function in the
pathogenesis of DR (6).
Grape seed proanthocyanidin extract (GSPE), which is
a potent antioxidant derived from grape seeds, provides a
concentrated source of polyphenols (7). Previous studies have demonstrated that
GSPE has an important role in antioxidation, anti-inflammation,
radical scavenging and antitumor activity (7,8); and
that the physiological benefits of GSPE are closely associated with
its antioxidative and free radical scavenging properties.
Furthermore, GSPE has been demonstrated to have a protective effect
in DR by reducing the production of advanced glycation end products
(9).
Nuclear factor erythroid 2-related factor 2 (Nrf2)
is a transcription factor involved in the Nrf2-antioxidant response
element signaling pathway, which protects against oxidative stress;
therefore, Nrf2 is crucially involved in the attenuation of
inflammation-associated pathogenesis in numerous diseases (10). Previous studies have demonstrated
that Nrf2 may have a protective role in the retina (11–13).
Furthermore, it has been demonstrated that Nrf2 has a
cytoprotective role for neurons and vasculature in the diabetic
retina (12,14).
Scapagnini et al (15) found that modulation of the Nrf2
pathway was achievable using food polyphenols, which has since
become a nutritional neuroprotective therapeutic strategy. To
further understand the role of GSPE in the protection of DR and the
mechanism of Nrf2 in the pathogenesis of DR, the present study
investigated whether GSPE was capable of modulating the expression
levels of Nrf2 and the downstream molecule, heme oxygenase (HO)-1,
in the retina. Furthermore, whether GSPE administration could
improve the structure and morphology of diabetic retinas was
examined. The authors of the present study hypothesized that GSPE
had a protective role in DR by modulating the Nrf2 pathway.
Materials and methods
Experimental design
A total of 30 Wistar rats, aged 8–10 weeks and
weighing 230–250 g, were purchased from the Animal Center of
Shandong University (Shandong, China; license number, SCXX20050015)
and divided into three equal groups (10 rats/group): The untreated
(control); untreated diabetic (DM); and diabetic treated with GSPE
(DM + GSPE) groups. Animal care and handling in the present study
was approved by the Ethics Committee of Shandong University.
Diabetes was induced in the DM and DM ± GSPE rats
following 18 h of fasting by a single intraperitoneal injection
with 65 mg/kg streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO,
USA) dissolved in 0.1 M citrate buffer (pH 4.5). The control rats
were administered a single intraperitoneal injection of isometric
citrate buffer. The rats were maintained at 25±1°C in a
temperature-controlled room with a 12-h light/dark cycle and ad
libitum access to food and water. Tail venous blood samples
were harvested at 72 h after STZ treatment in order to measure
blood glucose levels using a glucose monitoring system (cat. no.
1620368; Roche Diagnostics, Indianapolis, IN, USA). A total of 20
rats with serum glucose levels >300 mg/dl were included in the
experiment. Following the induction of diabetes, 250 mg/kg GSPE
(Tianjin Jianfeng Natural Product R&D, Co., Ltd., Tianjin,
China) was administered per day in normal saline solution via oral
gavage for 8 weeks.
Upon completion of the experiment, fasted rats were
anesthetized with 80 mg/kg ketamine (Sigma-Aldrich), sacrificed by
cervical dislocation, and their eyes were immediately removed. The
right eyes were fixed in 4% paraformaldehyde (Sigma-Aldrich) for
morphological analysis and apoptosis rate measurement; whereas the
left eyes were harvested and stored at −80°C for the evaluation of
Nrf2 expression levels and determination of redox status.
Retinal morphology analysis
Retinal samples were cut into 4-µm sections, placed
onto glass slides, deparaffinized in xylene (Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China) and serially treated with 100,
96 and 70% ethanol. Subsequently, the slides were stained with
hematoxylin and eosin (HE; Sangon Biotech Co., Ltd., Shanghai,
China) and observed at ×100–400 magnification under a light
microscope (BX53F; Olympus Corporation, Tokyo, Japan).
Morphological analyses were performed by two independent
pathologists in a blinded manner.
Cytoplasmic and nuclear
extraction
Using a nuclear extraction kit (cat. no. P0028;
Beyotime Institute of Biotechnology, Beijing, China), each fresh
isolated retina was homogenized in 200 µl ice-cold cytoplasmic
extraction buffer for 15 min and centrifuged at 15,000 × g for 10
min at 4°C, according to the manufacturer's protocol. The
supernatant containing the cytoplasmic protein fraction was used to
determine the activity levels of superoxide dismutase (SOD) and
glutathione peroxidase (GSH-Px), the quantity of methane
dicarboxylic aldehyde (MDA) and the expression levels of HO-1. The
remaining nuclear pellet was resuspended in 50 µl ice-cold nuclear
extraction buffer for 10 min and centrifuged at 15,000 × g for 10
min at 4°C. The supernatant containing the nuclear fraction was
used for the quantification of Nrf2 in the nucleus. The
Bicinchoninic Acid Assay kit (cat. no. P0012; Beyotime Institute of
Biotechnology) was used to quantify the protein concentrations in
the cytoplasmic and nuclear extracts.
Estimation of redox status in
retinas
SOD and GSH-Px activity levels and MDA content were
estimated using the Total Superoxide Dismutase Assay kit with WST-8
(S0101), the Lipid Peroxidation MDA Assay kit (S0131) and the Total
Glutathione Peroxidase Assay kit (S0058), respectively (all from
Nanjing Jiancheng Bioengineering Institute, Nanjing, China),
according to the manufacturer's protocols. Briefly, T-SOD activity
was assessed based on the xanthine-xanthine oxidase system. GSH-Px
activity was measured according the speed of enzymatic reaction,
whereas MDA levels were determined by the thiobarbituric acid
method.
Western blot analysis
Nuclear extracts were used to detect the expression
levels of Nrf2, whereas cytoplasmic extracts were used to analyze
HO-1 levels. A Bicinchoninic Acid Assay kit was used to determine
the protein concentration in the supernatant, and the samples were
subsequently stored at −80°C. Immediately prior to electrophoresis,
loading buffer (Sangon Biotech Co., Ltd.) was added to the samples
and heated at 95°C for 4 min. Subsequently, 40 µg protein was added
to each gel well and separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (Sangon Biotech Co.,
Ltd.). Separated proteins were electroblotted onto a 0.45-µm
polyvinylidene fluoride (PVDF) membrane (Roche Diagnostics) using
transfer buffer (Beyotime Institute of Biotechnology). Nonspecific
binding was blocked by incubating the membranes in 5% fat-free milk
for 1 h. PVDF membranes were incubated overnight at 4°C with rabbit
anti-rat Nrf2 polyclonal antibody (cat. no. ab31163),
rabbit-anti-rat Lamin B polyclonal antibody (cat. no. ab13248),
mouse anti-rat HO-1 monoclonal antibody (cat. no. ab16048) and
mouse anti-rat β-actin monoclonal antibody (cat. no. ab8226; all
1:1,000; all Abcam, Cambridge, UK). Subsequently, the membranes
were washed three times for 10 min each with Tris-buffered saline
supplemented with Tween-20 (Sangon Biotech Co., Ltd.), prior to
incubation with goat anti-rabbit secondary antibody (1:2,000; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) at 25°C for 2 h.
Chemiluminescence was detected using a Kodak Image Station 2000 MM
(Kodak, Rochester, NY, USA). Grayscale analysis was performed using
Scion Image analysis software 4.03 (Scion Corporation, Frederick,
MD, USA).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) assay
TUNEL staining of the retinal sections on the glass
slides was performed using a one-step TUNEL Apoptosis Assay kit
(cat. no. C1089; Beyotime Institute of Biotechnology), according to
the manufacturer's protocol. In order to stain the nucleus,
4′6′-diamino-2-phenylindole dihydrochloride was added for 10 min at
room temperature. Following staining, slides were observed at a
550-nm excitation wavelength under an Olympus BX53F microscope. The
cells with red fluorescence were defined as apoptotic.
Immunohistochemistry
Immunofluorescence techniques were performed to
investigate the expression levels of Nrf2. Briefly, sections were
blocked with 10% normal goat serum and 0.1 M phosphate-buffered
saline (both Sangon Biotech Co., Ltd.) prior to incubation with
rabbit anti-Nrf2 antibody (Abcam, Cambridge, UK) at 4°C overnight.
SP-9000 SP link Detection kits (cat. no. SP-9000-D; ZSGB-BIO,
Beijing, China) were used according to the manufacturer's protocol.
Slides were counterstained with hematoxylin for detection by light
microscopy (BX53F; Olympus Corporation).
Statistical analysis
All data are expressed as the mean ± standard
deviation (n≥6/group). Comparisons were performed using one-way
analysis of variance for the different groups followed by Dunnett's
post-hoc test for all pair comparisons using SPSS software, version
11.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
General characteristics
Despite consuming an increased quantity of food and
water, compared with the control rats the DM rats gradually lost
weight (242.41±14.63 vs. 301.62±11.69 g; P<0.001) by eight weeks
after the induction of diabetes. Furthermore, the average overnight
8-h fasting serum glucose level of the DM rats was 451.2±18.74
mg/ml, which was significantly higher compared with the control
group (94.53±9.03 mg/ml; P<0.001). No significant differences in
glucose levels were detected between the DM + GSPE and DM groups
(447.25±24.49 vs. 451.2±18.74 mg/dl; P=0.968) (Table I).
 | Table I.Body weight and blood glucose values
in three groups. |
Table I.
Body weight and blood glucose values
in three groups.
| Characteristic | Control | DM | DM + GSPE |
|---|
| Body weight (g) |
|
|
|
| 0
weeks | 243.52±6.30 | 241.61±4.76 | 239.24±6.36 |
| 8
weeks |
301.62±11.69 |
242.41±14.63a |
251.85±12.14 |
| Blood glucose
(mg/dl) |
94.53±9.03 |
451.2±18.74a |
447.25±24.49 |
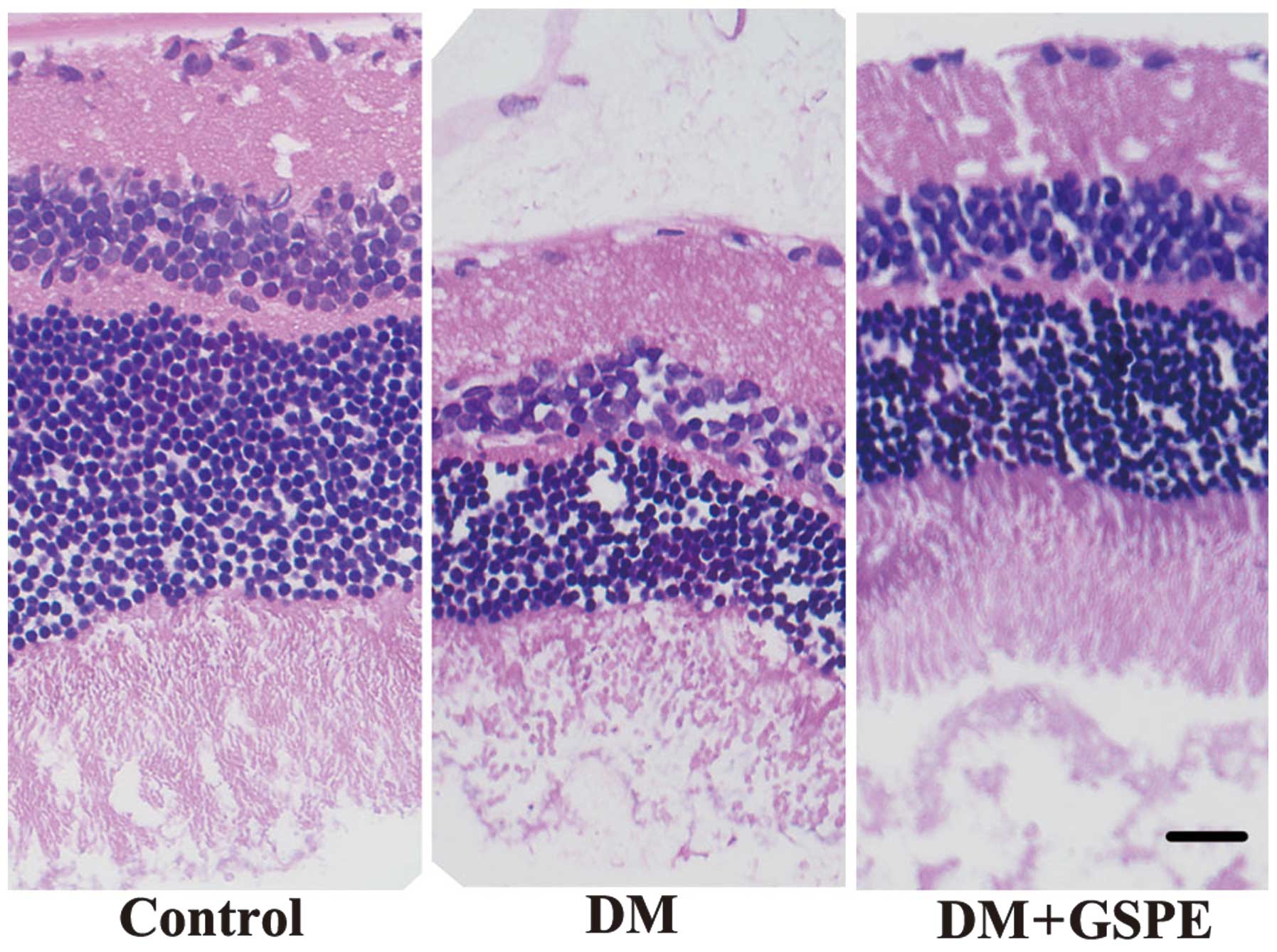
Retinal morphology
Following HE staining, the retinas of the control
group were highly organized with intact layers; whereas
disorganized retinas with impaired layers were detected in the DM
group. Retinal cells in the DM group were irregularly and loosely
arranged and the nerve fiber and ganglion cell layers were
narrower, as compared with the control and DM + GSPE groups. These
results suggest that GSPE is able to attenuate the disorganization
and impairment of the retinal layers associated with DM (Fig. 1).
GSPE attenuates oxidative stress in
diabetic retina
Table II presents
the significant reductions in SOD (n=8; P=0.003) and GSH-Px (n=8;
P=0.003) activity levels in the diabetic retina homogenates, as
compared with the controls. Following GSPE administration, SOD
(n=8; P=0.011) and GSH-Px (n=8; P=0.001) activity levels
significantly increased in the DM + GSPE group, as compared with
the DM group. Furthermore, MDA levels were significantly increased
in the diabetic retina, as compared with the control group (n=8;
P=0.002). MDA levels significantly decreased in the DM + GSPE
group, as compared with the DM group (n=8; P=0.013) (Table II).
 | Table II.Levels of the oxidative stress markers
GSH-Px, SOD and MDA in the three groups (n=8 per group). |
Table II.
Levels of the oxidative stress markers
GSH-Px, SOD and MDA in the three groups (n=8 per group).
| Marker | Control | DM | DM + GSPE |
|---|
| GSH-Px (U/mg) | 18.42±3.38 |
12.12±2.47a |
18.03±2.69b |
| SOD (U/mg) | 16.63±3.27 |
10.80±1.54a |
14.44±2.42b |
| MDA (nmol/mg) |
7.09±2.03 |
16.86±3.97a |
11.24±1.74b |
GSPE activates the Nrf2 pathway
Retinal Nrf2 expression levels were increased in the
DM + GSPE group, as compared with the DM group (Fig. 2A). Nuclear Nrf2 expression levels
were subsequently assessed by western blot analysis. The results
demonstrated that Nrf2 protein expression levels in the nucleus
were significantly increased in the retinas of the DM + GSPE group,
as compared with the untreated DM group (n=6; P=0.038) (Fig. 2B). Furthermore, the expression levels
of HO-1, which is the target gene of the Nrf2 pathway (14), were significantly elevated in the DM
+ GSPE group, as compared with the untreated DM group (n=6;
P=0.043) (Fig. 2C).
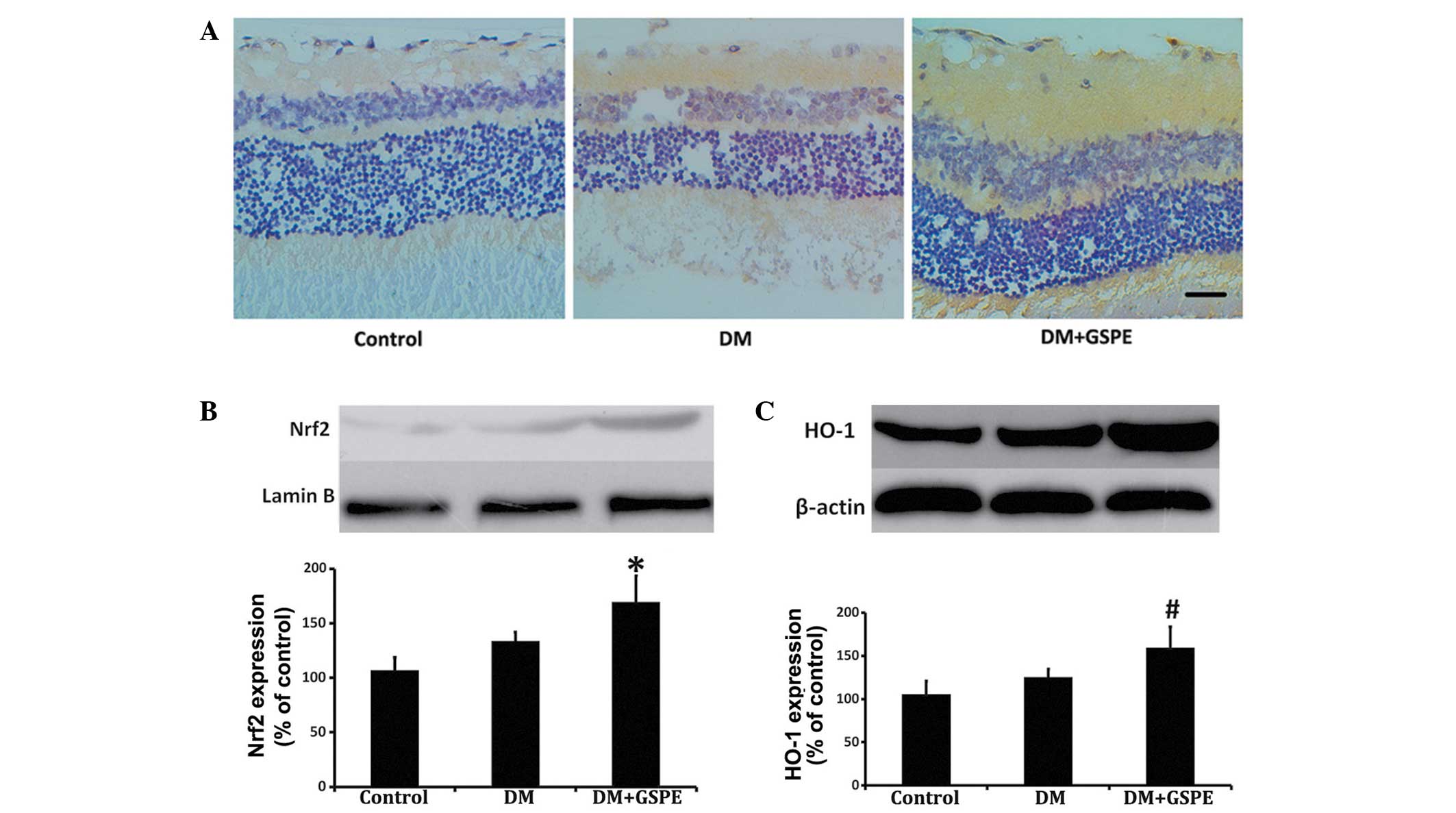 | Figure 2.Expression levels of Nrf2 and HO-1 in
the control, DM and DM + GSPE groups. (A) Immunohistochemical
analysis demonstrated an increase in Nrf2 expression levels in the
retinas of rats in the DM + GSPE group. Nrf2 was predominantly
expressed in the nerve fibers, ganglion cells and inner plexiform
layer of the retina (scale bar, 20 µm; stain, hematoxylin). (B)
Western blot analysis showing increased nuclear Nrf2 expression
levels in the DM + GSPE group, as compared with the DM group. (C)
Western blot analysis showed increased cytoplasmic HO-1 expression
levels in the DM + GSPE group, as compared with the DM group.
*P=0.038 vs. the DM group; #P=0.043 vs. the DM group
(n=6). DM, diabetes mellitus; GSPE, grape seed proanthocyanidin
extract; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1,
heme oxygenase-1. |
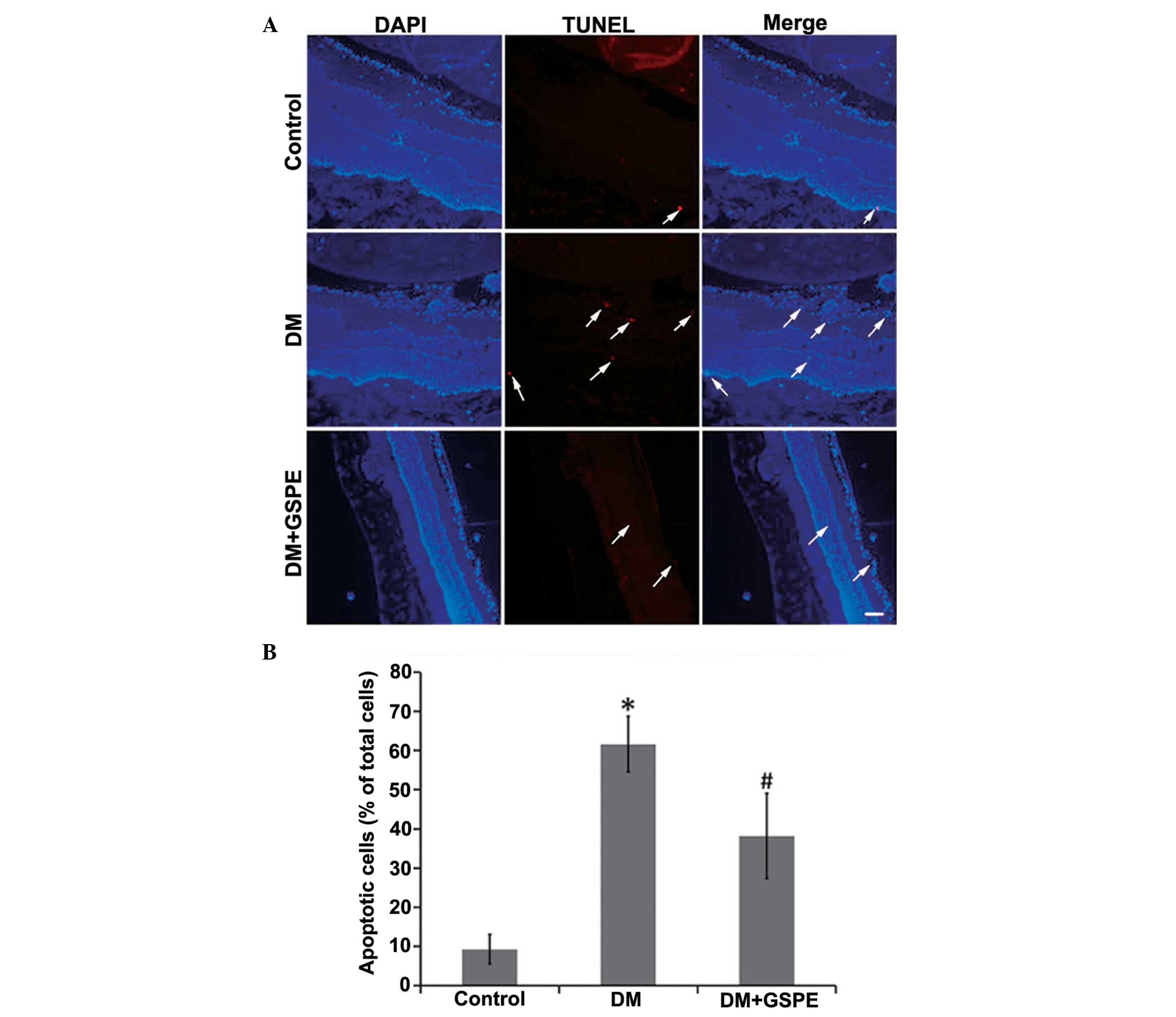
GSPE decreases cell apoptosis
The results of TUNEL staining demonstrated that the
rate of apoptosis in retinal cells in the DM group was
significantly increased, as compared with the control group (n=5;
P<0.001). Apoptotic cells were predominantly detected in the
nerve fiber, ganglion cell and inner plexiform layers of the
retina. Treatment with GSPE significantly decreased the number of
apoptotic cells (n=5; P=0.014) (Fig.
3).
Discussion
DR remains the leading cause of blindness in adults
of working age worldwide, and this condition may become a leading
cause of visual impairment (1–3).
Previous studies investigating DR have predominantly been focused
on the identification of pathogenic molecules (14). However, the prevention and treatment
of DR has been investigated (16)
which is particularly relevant to patients with long-standing
diabetes (17).
The results of the present study demonstrated that
GSPE, which contains natural polyphenols, has a protective effect
against DR. Following treatment with GSPE, the retinal morphology
of STZ-induced diabetic rats was markedly improved. In particular,
retinal cells in the GSPE-treated DM group were tightly arranged in
a regular manner, as compared with the DM group, and the nerve
fiber and ganglion cell layers increased in thickness. Furthermore,
STZ-induced diabetic rats exhibited a reduction in body weight and
treatment with GSPE increased the body weight of DM rats to a
certain extent, which has been controversial in previous studies
(9,18,19). The
results of the present study also demonstrated that GSPE was
capable of attenuating oxidative stress in diabetic retinas. SOD
and GSH-Px activity levels increased following GSPE administration,
whereas MDA levels were decreased, which is consistent with
previous findings (20).
Following this, the underlying mechanism of the
protective effect of GSPE was investigated in diabetic retinas.
Although GSPE has been extensively investigated due to its
associations with cardiovascular system disease, nervous system
disease, diabetic nephropathy, rheumatoid arthritis and human
cancers (21,22), there has only been one previous study
investigating the effects of GSPE in the retina (9). Li et al (9) found that GSPE significantly suppressed
the vascular lesions of central regions and decreased capillary
enlargements and neovascularization in diabetic retinas by reducing
advanced glycation end products. Diabetes-associated increases in
advanced glycation end products may induce oxidative stress via
various mechanisms, including enhancement of protein kinase C and
hexosamine and polyol pathways fluxes (23). Nrf2 is an important protective factor
which regulates the progression of DR as a part of the an important
cellular pathway protecting against oxidative stress (12). Since it has previously been
demonstrated that food polyphenols are capable of modulating the
Nrf2 pathway (15), the present
study investigated whether GSPE has a protective effect in DR by
activating the Nrf2 pathway.
The results of the present study indicated that GSPE
exerts protective activity in the retina via the activation of the
Nrf2 pathway. The present study demonstrated that the expression
levels of Nrf2 and its target gene, HO-1, were markedly increased
in the retina following treatment with GSPE. Nrf2 was predominantly
expressed in the nerve fiber, ganglion cell and inner plexiform
layers (Fig. 2A). It is well
established that, as an antioxidation transcription factor, Nrf2
functions exclusively in the nucleus (14). Furthermore, treatment with GSPE
significantly attenuated the apoptosis of retinal cells in the
present study. These results suggested that GSPE may be capable of
activating the Nrf2 pathway, which may protect diabetic retinal
cells against apoptosis.
However, the precise mechanism underlying the
anti-apoptotic effect of GSPE and the Nrf2 pathway remain unclear.
A previous study has demonstrated that the protective effects of
GSPE may be partially attributed to its ability to inhibit
anti-death signaling mediated via proapoptotic transcription
factors and genes, including c-Jun N-terminal kinase (JNK)-1 and
c-Jun (24). Zou et al
(25) have previously demonstrated
that the activation of Nrf2 was capable of preventing oxidative
stress-induced apoptosis by hydroxytyrosol in human retinal pigment
epithelial cells via the JNK-p62/SQSTM1 pathways. Furthermore,
Pehar et al (26)
demonstrated that decreased Nrf2 expression and the downregulation
of the enzymes associated with oxidative stress induces p75
neurotrophin receptor-induced motor neuron apoptosis (26). Furthermore, previous studies have
indicated that activation of HO-1, which is the target gene of
Nrf2, may protect diabetic retinal cells against apoptosis
(27,28). Further studies are required in order
to fully elucidate the anti-apoptotic effect of GSPE, and the
underlying mechanisms.
In conclusion, the results of the present study
suggested that early treatment with GSPE may protect diabetic
retinal cells against diabetic retinopathy by attenuating oxidative
stress-mediated cellular apoptosis, which may be associated with
the activation of the Nrf2 pathway.
Acknowledgements
The present study was supported by the Medicine and
Health Science Technology Development Project of Shandong Province
(grant no. 2014WS0010).
References
|
1
|
Ruta LM, Magliano DJ, Lemesurier R, Taylor
HR, Zimmet PZ and Shaw JE: Prevalence of diabetic retinopathy in
Type 2 diabetes in developing and developed countries. Diabet Med.
30:387–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Man RE, Sasongko MB, Wang JJ, MacIsaac R,
Wong TY, Sabanayagam C and Lamoureux EL: The Association of
Estimated Glomerular Filtration Rate With Diabetic Retinopathy and
Macular Edema. Invest Ophthalmol Vis Sci. 56:4810–4816. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malek M, Khamseh ME, Aghili R, Emami Z,
Najafi L and Baradaran HR: Medical management of diabetic
retinopathy: An overview. Arch Iran Med. 15:635–640.
2012.PubMed/NCBI
|
|
4
|
Barber AJ, Gardner TW and Abcouwer SF: The
significance of vascular and neural apoptosis to the pathology of
diabetic retinopathy. Invest Ophthalmol Vis Sci. 52:1156–1163.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu WK, Liu R, Pei H and Li B: Endoplasmic
reticulum stress-related factors protect against diabetic
retinopathy. Exp Diabetes Res. 2012:5079862012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Williams M, Hogg RE and Chakravarthy U:
Antioxidants and diabetic retinopathy. Curr Diab Rep. 13:481–487.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferreira D and Li XC: Oligomeric
proanthocyanidins: Naturally occurring O-heterocycles. Nat Prod
Rep. 17:193–212. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Houde V, Grenier D and Chandad F:
Protective effects of grape seed proanthocyanidins against
oxidative stress induced by lipopolysaccharides of
periodontopathogens. J Periodontol. 77:1371–1379. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li M, Ma YB, Gao HQ, Li BY, Cheng M, Xu L,
Li XL and Li XH: A novel approach of proteomics to study the
mechanism of action of grape seed proanthocyanidin extracts on
diabetic retinopathy in rats. Chin Med J (Engl). 121:2544–2552.
2008.PubMed/NCBI
|
|
10
|
Kaspar JW, Niture SK and Jaiswal AK:
Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol
Med. 47:1304–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong Q, Mishra M and Kowluru RA:
Transcription factor Nrf2-mediated antioxidant defense system in
the development of diabetic retinopathy. Invest Ophthalmol Vis Sci.
54:3941–3948. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Z, Wei Y, Gong J, Cho H, Park JK, Sung
ER, Huang H, Wu L, Eberhart C, Handa JT, et al: NRF2 plays a
protective role in diabetic retinopathy in mice. Diabetologia.
57:204–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan SM and De Haan JB: Combating oxidative
stress in diabetic complications with Nrf2 activators: How much is
too much? Redox Rep. 19:107–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang S, Park JK and Duh EJ: Novel targets
against retinal angiogenesis in diabetic retinopathy. Curr Diab
Rep. 12:355–363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scapagnini G, Vasto S, Abraham NG, Caruso
C, Zella D and Fabio G: Modulation of Nrf2/ARE pathway by food
polyphenols: A nutritional neuroprotective strategy for cognitive
and neurodegenerative disorders. Mol Neurobiol. 44:192–201. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeong IK and King GL: New perspectives on
diabetic vascular complications: The loss of endogenous protective
factors induced by hyperglycemia. Diabetes Metab J. 35:8–11. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun JK, Keenan HA, Cavallerano JD,
Asztalos BF, Schaefer EJ, Sell DR, Strauch CM, Monnier VM, Doria A,
Aiello LP and King GL: Protection from retinopathy and other
complications in patients with type 1 diabetes of extreme duration:
The joslin 50-year medalist study. Diabetes Care. 34:968–974. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kojima K, Maki K, Tofani I, Kamitani Y and
Kimura M: Effects of grape seed proanthocyanidins extract on rat
mandibular condyle. J Musculoskelet Neuronal Interact. 4:301–307.
2004.PubMed/NCBI
|
|
19
|
Mansouri E, Panahi M, Ghaffari MA and
Ghorbani A: Effects of grape seed proanthocyanidin extract on
oxidative stress induced by diabetes in rat kidney. Iran Biomed J.
15:100–106. 2011.PubMed/NCBI
|
|
20
|
Okudan N, Barışkaner H, Gökbel H, Sahin
AS, Belviranlı M and Baysal H: The effect of supplementation of
grape seed proanthocyanidin extract on vascular dysfunction in
experimental diabetes. J Med Food. 14:1298–1302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding Y, Dai X, Jiang Y, Zhang Z, Bao L, Li
Y, Zhang F, Ma X, Cai X, Jing L, et al: Grape seed proanthocyanidin
extracts alleviate oxidative stress and ER stress in skeletal
muscle of low-dose streptozotocin- and high-carbohydrate/high-fat
diet-induced diabetic rats. Mol Nutr Food Res. 57:365–369. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui XP, Li BY, Gao HQ, Wei N, Wang WL and
Lu M: Effects of grape seed proanthocyanidin extracts on peripheral
nerves in streptozocin-induced diabetic rats. J Nutr Sci Vitaminol
(Tokyo). 54:321–328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamagishi S and Matsui T: Advanced
glycation end products (AGEs), oxidative stress and diabetic
retinopathy. Curr Pharm Biotechnol. 12:362–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bagchi D, Sen CK, Ray SD, Das DK, Bagchi
M, Preuss HG and Vinson JA: Molecular mechanisms of
cardioprotection by a novel grape seed proanthocyanidin extract.
Mutat Res 523–524. 87–97. 2003. View Article : Google Scholar
|
|
25
|
Zou X, Feng Z, Li Y, Wang Y, Wertz K,
Weber P, Weber P, Fu Y and Liu J: Stimulation of GSH synthesis to
prevent oxidative stress-induced apoptosis by hydroxytyrosol in
human retinal pigment epithelial cells: Activation of Nrf2 and
JNK-p62/SQSTM1 pathways. J Nutr Biochem. 23:994–1006. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pehar M, Vargas MR, Robinson KM, Cassina
P, Díaz-Amarilla PJ, Hagen TM, Radi R, Barbeito L and Beckman JS:
Mitochondrial superoxide production and nuclear factor erythroid
2-related factor 2 activation in p75 neurotrophin receptor-induced
motor neuron apoptosis. J Neurosci. 27:7777–7785. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan J, Xu G, Jiang T and Qin Y:
Pharmacologic induction of heme oxygenase-1 plays a protective role
in diabetic retinopathy in rats. Invest Ophthalmol Vis Sci.
53:6541–6556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He M, Pan H, Chang RC, So KF, Brecha NC
and Pu M: Activation of the Nrf2/HO-1 antioxidant pathway
contributes to the protective effects of Lycium barbarum
polysaccharides in the rodent retina after ischemia reperfusion
induced damage. PloS One. 9:e848002014. View Article : Google Scholar : PubMed/NCBI
|