Introduction
Testosterone propionate (TP) is known to serve a
crucial function in benign prostatic hyperplasia (BPH) and
prostatic carcinoma (Pca). Pca is common in the developed world and
is the most prevalent cancer among men in the USA. BPH is also a
common disease in men >50 years of age (1). However, the mechanisms through which TP
affects these processes are not clear. Mitotic orientation is
generally perpendicular to the basement membrane in the glandular
epithelium of control castrated rats; however, a recent study
observed that mitotic orientation was parallel to the basement
membrane in the glandular epithelium of castrated rats treated with
TP (2).
TP is a sex hormone that primarily activates the
androgen receptor (AR) (3), a key
regulatory protein that is a critical intermediate in the
intracellular androgen pathway (4).
Chronic androgen stimulation may promote the expression and
secretion of AR from epithelial cells. Additionally, prostate basal
tissue may contain stem cells, and the aging and molecular injury
mechanisms in stem cells are crucially involved in the development
of age-associated diseases, such as BPH and cancer (5). Prostate basal cells are not dependent
on androgen; however, the presence of androgen is able to induce
the proliferation and differentiation of prostate basal cells
(6), which is a key mechanism for
the induction of prostate cell proliferation.
In order to elucidate the details of the
mechanism(s) involved in changes in mitotic orientation in the
prostatic glandular epithelium of castrated rats exposed to
subchronic TP and the associated differential gene expression, an
animal model was established in Sprague-Dawley (SD) rats (2). The aim of the present study was to
determine the changes in mitotic orientation in SD rats that
received long-term TP exposure and to compare the differences in
gene expression between TP-treated and control rats using gene
microarrays. In addition, the role of the Wnt and AR signal
transduction pathways in prostate cell proliferation was evaluated
by determining the expression of a number of associated genes.
Materials and methods
Experimental animals
A total of 20 male 4-month-old SD rats [certificate
no. SCXK 2003-0002] were obtained from Shanghai Laboratory Animal
Center of Chinese Academy Science (Shanghai, China) and housed in a
specific pathogen-free room (temperature, 20–26°C; humidity,
40–70%), with free access to water and food. All procedures and
animal experiments were approved by the Animal Ethical Committee of
Shanghai Institute of Planned Parenthood Research (Shanghai, China)
and complied with the Animal Management Rules of the Chinese
Ministry of Health (document no. 55, 2001).
Establishment of a rat model for
BPH
Castrated rats were treated with subcutaneous
injection of TP for 30 days at a dose of 3.7 mg/kg/day (n=10). Rats
in the control group (n=10) were treated with an equivalent volume
of olive oil (100 µl). The prostate from each test and control rat
was removed following the final treatment. All tissue specimens
were removed under anesthesia (3% Nembutal, 0.2 ml/100 ml;
Sigma-Aldrich, St. Louis, MO, USA), and the specimens were fixed in
formaldehyde solution and processed in paraffin. Paraffin blocks
were cut into 5-µm sections for hematoxylin and eosin (H&E) and
immunohistochemical staining.
Immunohistochemical staining
procedure
Expression of AR was detected in prostate cells
using immunohistochemical staining. Following deparaffination and
rehydration of the sections, slides were placed in sodium citrate
solution (0.01 M, pH 6.0) and heated to 96–100°C for 25 min. After
cooling, sections were incubated in 5% bovine serum albumin
(Beijing Zhongshan Biological Technology Co., Ltd., Beijing, China)
for 20 min. The sections were then incubated for 2 h with primary
rabbit anti-mouse AR monoclonal antibody (1:100; cat. no. BA0004;
Wuhan Boster Biotechnology, Ltd., Wuhan, China) diluted to 1:100 in
phosphate-buffered saline (PBS) at room temperature. Following
primary incubation, the sections were washed in PBS three times and
incubated with horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (1:50; cat. no. BA1051; Wuhan Boster
Biolotechnology Co., Ltd.) for 30 min at 37°C. Subsequently, the
sections were washed with PBS and subjected to treatment with
3,3′-diaminobenzidine color-substrate solution and placed under
cover slips. AR-labeled cells were observed using an Eclipse 50i
light microscope (Nikon Corporation, Tokyo, Japan) in bright field
mode.
Orientation of mitosis
For prostate epithelial cells, a parallel
orientation was defined as the alignment of the chromosome axis and
basement membrane at a 0–45° angle. By contrast, a perpendicular
orientation was defined as the alignment of the chromosome axis and
the basement membrane at a 45–90° angle. The cells were observed
and imaged using the Nikon Eclipse 50i optical microscope, as
described in a previous study (2).
Evaluation of differential gene
expression by rat genome microarray analysis
Evaluation of differential gene expression was
conducted using the Rat Genome 230 2.0 Array (Affymetrix, Inc.,
Santa Clara, CA, USA), which provides comprehensive coverage of the
transcribed rat genome: 31,000 probe sets analyze the expression
level of >30,000 transcripts and variants from >28,000 known
rat genes. Total RNA was extracted using TRIzol reagent (Ambion;
Thermo Fisher Scientific, Inc., Foster City, CA, USA) and its
quality was confirmed using agarose-formaldehyde (Beijing Zhongshan
Biological Technology Co., Ltd.) (7). Subsequently, double-stranded cDNA was
synthesized from 5 µg total RNA using the One-Cycle cDNA Synthesis
kit (Affymetrix, Inc.), according to the manufacturer's
instructions. Biotin-labeled cRNA was transcribed from the cDNA
using the GeneChip® IVT Labeling kit (Affymetrix, Inc.). Labeled
cRNA (15 µg) was then fragmented and hybridized to the Affymetrix
Rat Genome 230 2.0 Array for 16 h at 45°C in an Affymetrix
GeneChip® Hybridization Oven 640 (Affymetrix, Inc.). The hybridized
array was washed and stained in the GeneChip® Fluidics Station 450
(Affymetrix, Inc.), and fluorescent signals were detected using the
Affymetrix GeneChip® Scanner 3000 (Affymetrix, Inc.). These data
were initially documented with Affymetrix GeneChip® operating
software GCOS 1.2 (Affymetrix, Inc.), which generated an expression
report file.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis of gene expression
Total RNA prepared for microarray analysis was also
used for RT-PCR analysis. Total RNA (2 µg) from each sample was
subjected to reverse transcription using a Superscript First-Strand
cDNA Synthesis kit (Henan Huamei Bioengineering Co., Ltd., Henan,
China) according to the manufacturer's instructions. PCR was
subsequently conducted by mixing 1 µl cDNA, 2.5 µl 10X PCR buffer,
1.5 µl 25 mM MgCl2, 2.5 µl 2.5 mM dNTP, 1 µl 10 µM
specific gene primer pairs, 1 µl 10 µM β-actin or GAPDH primer
pairs, 25 µl H2O and 1 U Taq polymerase (Ambion; Thermo
Fisher Scientific, Inc.). The primers used in the PCR reaction are
presented in Table I. PCR products
were then amplified for 30 cycles, with each cycle consisting of the
following steps: Denaturation for 10 sec at 95°C, annealing for 20
sec at 72°C and polymerization for 5 min at 72°C. The PCR products
were resolved using an Affymetrix Hybridization Oven Model 640
(model 800138; Affymetrix, Inc.) according to a DNA standard curve.
Data were analyzed using Light Cycler 480 software version 1.5
(Roche Diagnostics, Basel, Switzerland).
 | Table I.Primers used in reverse
transcription-polymerase chain reaction. |
Table I.
Primers used in reverse
transcription-polymerase chain reaction.
| Gene | Bidirectional primer
sequence | Annealing temperature
(°C) | Product length
(bp) |
|---|
| β-actin | F:
5′-CCTCTATGCCAACACAGTGC-3′ | 58 | 211 |
|
| R:
5′-GTACTCCTGCTTGCTGATCC-3′ |
|
|
| Ran | F:
5′-CGTAGACGGTAAAGCAATGG-3′ | 59 | 149 |
|
| R:
5′-AGTCAAAGAAGCAGCAAACAC-3′ |
|
|
| Dkk3 | F
:5′-ACACCCAACAAGAAGCAACC-3′ | 59 | 174 |
|
| R:
5′-AGGGAAAGAGGGAGAAAGC-3′ |
|
|
| Fas | F:
5′-GACAACAACTGCTCAGAAGG-3′ | 59 | 105 |
|
| F:
5′-TCAGAATAGTGTTTCCTGTCC-3′ |
| Tgm4 | F:
5′-CGAGAACAGGCGTCAAGATA-3′ | 59 | 246 |
|
| R:
5′-GTCTGTAGGTCAAGGGAGCAT-3′ |
|
|
| Wnt2 | F:
5′-GTGCCGACTGGACAACTCCT-3′ | 59 | 224 |
|
| R:
5′-ACCTCAGAATGCCCAAGACA-3′ |
|
|
Statistical analysis
Data were expressed as the mean ± standard
deviation. One-way analysis of variance (ANOVA) and P-values were
used to evaluate significant differences between the groups.
Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA)
was used to analyze image data. Data analysis to determine
differential gene expression profiles was performed by importing
MAS 5.0 intensity data (Affymetrix, Inc.) into the Partek Genomics
Suite Version 6.4 (Partek Incorporated, St. Louis, MO) for ANOVA
based on time in culture. P<0.05 was considered to indicate a
statistically significant difference. Post-hoc Fisher's exact test
adjusted to correct for false discovery rates (P<0.05) was used
to compare data from multiple groups. Only differentially expressed
genes (−2.0 > fold-change >2.0; P<0.05) were included in
each IPA core analysis.
Results
Mitotic orientation of prostate
epithelia in rats
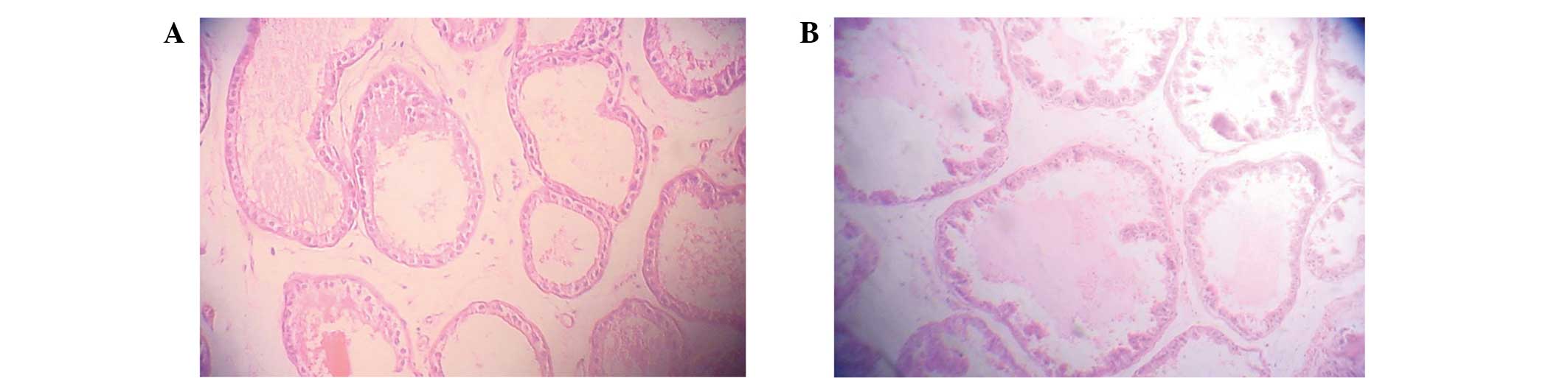
Prostate epithelia from TP-treated rats exhibited
typical prostate proliferation morphology, in which the organ
quotient, volume and area of the prostate glandular cavity were
increased compared with the control group. H&E staining
revealed that the prostate epithelia were highly stylolitic, with
intrusions in the glandular cavity, and the area of the prostate
glandular cavity was large (Fig. 1).
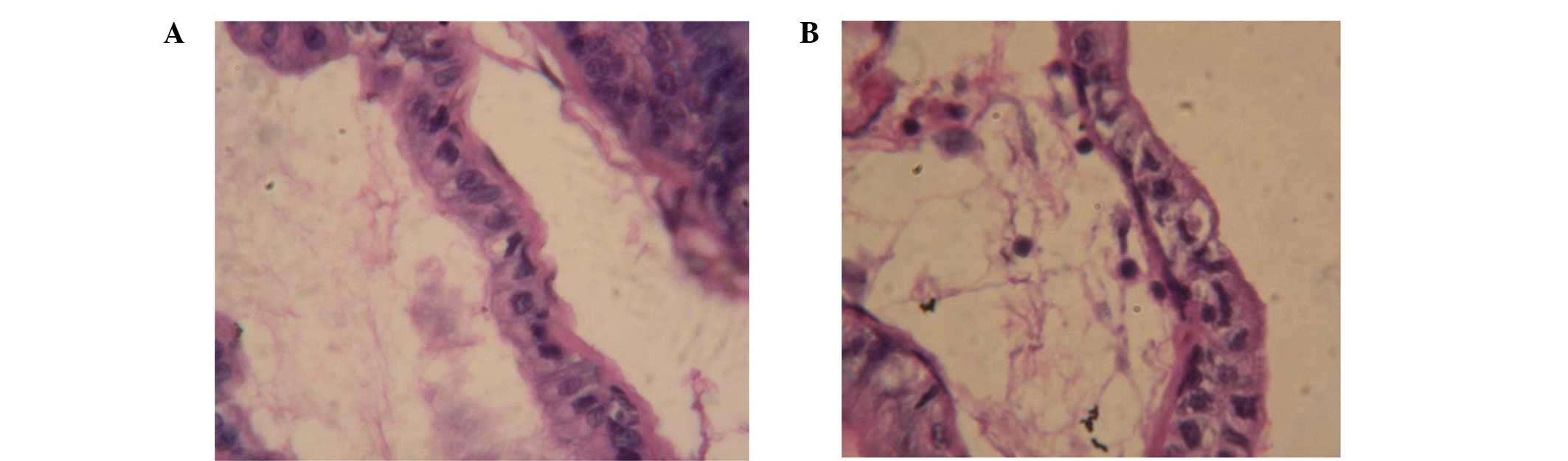
The mitotic orientation of prostate epithelial cells in TP-treated
rats was parallel to the basement membrane, while the orientation
in the control rats was perpendicular to the basement membrane
(Fig. 2). These data demonstrate
that the mitotic orientation of prostate epithelial cells changed
following long-term exposure to TP.
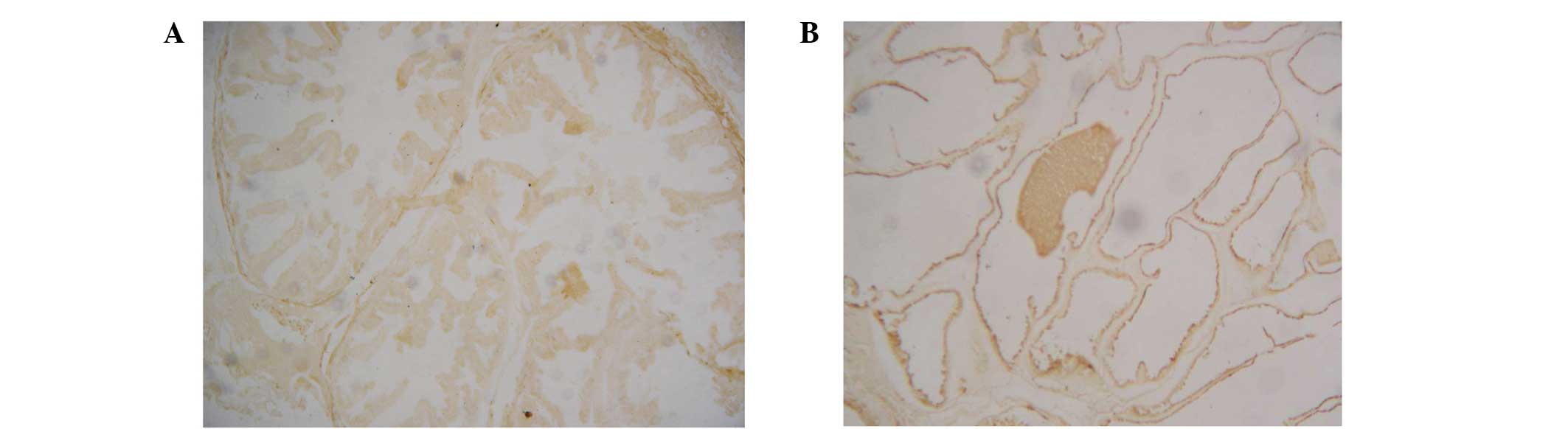
Analysis of AR expression
Notably, immunohistochemical staining revealed an
increased number of AR-positive cells in the prostate epithelia of
the TP-treated rats when compared with the control rats (Fig. 3), indicating that TP treatment
upregulated AR expression.
Results of gene chip microarray
analysis
A rat genome microarray technique was used to
analyze the differences in gene expression between the control and
TP-treated rats. Genes that were altered in the TP group were
assumed to be associated with changes in prostate cell mitotic
orientation and proliferation. A number of the differentially
expressed genes were associated with the AR and Wnt signal
transduction pathways (Table II).
Upregulation was observed in genes that promote cellular
proliferation, including Ran, Tgm4, Odc1 and Wnt2, while
downregulation was observed in genes that inhibit cell
proliferation, such as Dkk3 and Fas.
 | Table II.Wnt- and AR-associated genes that were
up- or downregulated following testosterone propionate treatment in
rats. |
Table II.
Wnt- and AR-associated genes that were
up- or downregulated following testosterone propionate treatment in
rats.
| Probe ID | Gene | Character and
function | Regulation |
|---|
| AT 1367590 | Ran | AR-associated
protein | Up |
| AT 1371022 | Tgm4 | Pca-associated
gene | Up |
| AT 1393927 | Wnt2 | Wnt signaling
transduced element | Up |
| AT 1370328 | Dkk3 | Negative regulation
element of Wnt | Down |
| AT 1397221 | Fas | Apoptosis | Down |
| AT 1370163 | Odc1 | Cell cycle
control | Up |
| AT 1370235 | Dbi | GABA receptor
regulation factor | Up |
| AT 1371150t | Ccnd1 | Cell cycle
control | Up |
| AT 1368785 | Pitx2 | Transcription
regulation factor | Down |
| AT 1369788 | Jun | Transcription
regulation factor | Down |
RT-PCR analysis of selected genes
To confirm the observed changes in gene expression,
RT-PCR analysis was performed for a number of the genes, including
Dkk3, Ran, Fas, Tgm4 and Wnt2 (Table
III). The results of RT-PCR analysis were consistent with the
microarray data.
 | Table III.Reverse transcription-polymerase
chain reaction analysis of the five selected genes and
β-actin. |
Table III.
Reverse transcription-polymerase
chain reaction analysis of the five selected genes and
β-actin.
| Gene | Control | TP |
|---|
| β-actin |
1.95×10−3 |
2.66×10−3 |
| Dkk3 |
2.93×10−4 |
1.18×10−5 |
| Ran |
1.07×10−4 |
2.37×10−4 |
| Fas |
9.47×10−4 |
8.85×10−6 |
| Tgm4 |
1.09×10−4 |
2.46×10−3 |
| Wnt2 |
1.24×10−3 |
1.44×10−4 |
Discussion
To the best of our knowledge, the present study
showed for the first time that the mitotic orientation of rat
prostate epithelial cells changes following exposure to TP for an
extended period. In addition, a microarray analysis of over 28,000
rat genes, followed by verification using RT-PCR, indicated that
the Wnt and AR signal transduction pathways were involved in the
observed changes in the mitotic orientation of prostate epithelial
cells in response to TP treatment.
The present study revealed that the mitotic
orientation of rat prostate epithelial cells is altered following
cell exposure to TP, providing a new method for the study of
TP-dependent prostate cell proliferation. Additionally, the
immunohistochemistry and H&E results of previous studies
demonstrated that the prostate may be a target organ for TP
(8,9). Therefore, changes in cell proliferation
may result from the reorientation of the mitotic complex following
exposure of the prostate to TP. In a previous study, in control
cells, mitotic orientation was parallel to the basement membrane;
therefore, changes in proliferation may occur via the AR signal
transduction pathway (10).
In the present study, TP-treated rats exhibited
upregulation of Wnt2 and other positive regulatory genes,
while negative regulatory genes, such as Dkk3, were
downregulated compared with the control group. The Wnt pathway has
been widely studied (11,12), and is crucially involved in numerous
proliferative diseases, such as Pca. In the classical Wnt signaling
pathway, Wnt2 stimulates cell proliferation and regulates the aging
process of fibroblasts and epithelial cells. In addition, Wnt2
signal reduction has been observed in senescent cells (13). Increased expression of Wnt2 is able
to stimulate prostate epithelial cell proliferation, activate the
Wnt signaling pathway and cause changes in prostate epithelial cell
polarity (13), which may result in
chromosomal changes in prostate epithelial cells.
Dkk3 is a member of the Dkk family, which inhibits
the Wnt signaling pathway by interacting with the LRP5/6 receptor
and promotes lysosome endocytosis (14,15).
Although it has not been as well studied as Wnt, the Dkk3
gene has been shown to be downregulated in prostate tumor cells
(16), suggesting that increased
Dkk3 expression may inhibit tumor cells development
(17). In the present study,
TP-treated rats exhibited downregulated Dkk3 expression
compared with the control group. As it is known that TP induces
BPH, it may be concluded that downregulation of the Dkk3
gene reduces the inhibition of cell proliferation and cell
differentiation, in addition to causing BPH (18).
Tgm4 is a gene involved in the AR signaling
pathway and is expressed in normal and abnormal prostate tissues.
In numerous studies, Tgm4 has been shown to be a candidate
marker for prostate cells (19,20). In
the TP group, Tgm4 upregulation promoted prostate cell
proliferation. Additionally, TP treatment resulted in the
downregulation of Fas, resulting in reduced apoptosis
(21), and therefore enhanced
prostate cell proliferation.
Ran is a member of RAS family and has been shown to
be involved in the androgen signaling pathway in addition to the
regulation of DNA transcription (22). Although numerous studies have
investigated the association between Ran and prostate cancer
(23), more recent studies have
investigated Ran in the context of benign proliferation of prostate
cells (24,25). In the present study, the upregulation
of the Ran gene was confirmed using RT-PCR, and the results
suggested that Ran upregulation may contribute to prostate
cell proliferation in BPH.
AR serves a crucial function in the prostate and is
coregulated by hormone/receptor combinations (26). Previous studies have demonstrated
that numerous coregulatory events are associated with the Ras
family. Furthermore, recent studies have reported that Ras response
element binding protein 1 (RREB-1) is a ligand for AR and
coregulates AR. Therefore, this Ras-associated protein has the
potential to inhibit AR function. The Ras/MAPK kinase pathway is
able to counteract RREB-1, inhibiting the androgen signaling
pathway (27). As a member of the
Ras family, Ran is involved in this inhibitory process and
functions to promote prostate cell proliferation. In addition, Ran
is involved in mitotic spindle organization and biogenesis, which
may affect mitotic orientation of epithelial cells. In Ras-mediated
transformation, Krüppel-like factor 5 regulates the cyclin B1/Cdc2
proteins, in addition to activating the cell cycle, and thereby
promotes cell mitosis (27,28). Thus, collectively, the present data
suggest that TP treatment altered the mitotic orientation of
prostate epithelial cells via a pathway involving Wnt and AR
signaling. Further studies are required to determine the precise
mechanisms through which Krüppel-like factor 5 is involved in this
process.
In conclusion, mitotic reorientation of rat prostate
epithelial cells was altered following exposure to subchronic TP,
which may have promoted prostate cell proliferation via the Wnt and
AR signaling pathways.
Acknowledgements
This study was supported by grants from the New Drug
Major Program of China (no. 2011ZX09301-005), the National Natural
Science Foundation of China (no. 11101077), the Shanghai ‘Science
and Technology Innovation Action Plan’ Experimental Animal Research
Project (nos. 11140901300 and 11140901302) and the Shanghai Key Lab
of Human Performance (Shanghai University of Sport) (grant no.
11DZ2261100). The authors would like to thank Miss Dong-Mei Li, Mr.
Lei Wang, Mr. Gui-Lin He, Professor Xiu-Rong Jiang, Professor
Gui-Ming Liu, Dr Shu-Wu Xie, Dr Zhi-Ling Li and Dr Li Ma for their
technical assistance.
Glossary
Abbreviations
Abbreviations:
|
AR
|
androgen receptor
|
|
TP
|
testosterone propionate
|
|
BPH
|
benign prostate hyperplasia
|
|
H&E
|
hematoxylin and eosin
|
|
Pca
|
prostatic carcinoma
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
SD
|
Sprague-Dawley
|
References
|
1
|
Berry SJ, Coffey DS, Walsh PC and Ewing
LL: The development of human benign prostatic hyperplasia with age.
J Urol. 132:474–479. 1984.PubMed/NCBI
|
|
2
|
Liu XY, Li DM, Zhang XF, Wu JH and Sun ZY:
Mitosis orientation in prostate epithelial cells changed by
endocrine effect. Acta Pharmacol Sin. 29:226–229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gao W: Androgen receptor as a therapeutic
target. Adv Drug Deliv Rev. 62:1277–1284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chatterjee B: The role of the androgen
receptor in the development of prostatic hyperplasia and prostate
cancer. Mol Cell Biochem. 253:89–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beltrami AP, Cesselli D and Beltrami CA:
At the stem of youth and health. Pharmacol Ther. 129:3–20. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Isaacs JT and Coffey DS: Etiology and
disease process of benign prostatic hyperplasia. Prostate Suppl.
2:33–50. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mansour FH and Pestov DG: Separation of
long RNA by agarose-formaldehyde gel electrophoresis. Anal Biochem.
441:18–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vignozzi L, Gacci M, Cellai I, Santi R,
Corona G, Morelli A, Rastrelli G, Comeglio P, Sebastanelli A,
Maneschi E, et al: Fat boosts, while androgen receptor activation
counteracts, BPH-associated prostate inflammation. Prostate.
73:789–800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiang-Yun L, Ying-Wen X, Chen-Jing X,
Jiu-Jiu W, Qi P, Bo G and Zu-Yue S: Possible mechanism of benign
prostatic hyperplasia induced by androgen-estrogen ratios in
castrated rats. Indian J Pharmacol. 42:312–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hartman J, Ström A and Gustafsson JÅ:
Current concepts and significance of estrogen receptor β in
prostate cancer. Steroids. 77:1262–1266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.PubMed/NCBI
|
|
13
|
Ye X, Zerlanko B, Kennedy A, Banumathy G,
Zhang R and Adams PD: Downregulation of Wnt signaling is a trigger
for formation of facultative heterochromatin and onset of cell
senescence in primary human cells. Mol Cell. 27:183–196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Levasseur R, Lacombe D and de Vernejoul
MC: LRP5 mutations in osteoporosis-pseudoglioma syndrome and
high-bone-mass disorders. Joint Bone Spine. 72:207–214. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davidson G, Mao B, del Barco Barrantes I
and Niehrs C: Kremen proteins interact with Dickkopf1 to regulate
anteroposterior CNS patterning. Development. 129:5587–5596. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abarzua F, Sakaguchi M, Takaishi M, Nasu
Y, Kurose K, Ebara S, Miyazaki M, Namba M, Kumon H and Huh NH:
Adenovirus-mediated overexpression of REIC/Dkk-3 selectively
induces apoptosis in human prostate cancer cells through activation
of c-Jun-NH2-kinase. Cancer Res. 65:9617–9622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsieh SY, Hsieh PS, Chiu CT and Chen WY:
Dickkopf-3/REIC functions as a suppressor gene of tumor growth.
Oncogene. 23:9183–9189. 2004.PubMed/NCBI
|
|
18
|
Kawano Y, Kitaoka M, Hamada Y, Walker MM,
Waxman J and Kypta RM: Regulation of prostate cell growth and
morphogenesis by Dickkopf-3. Oncogene. 25:6528–6537. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Wu C, Gu X and Xiong C: A novel
application of cell-free seminal mRNA: Non-invasive identification
of the presence of germ cells or complete obstruction in men with
azoospermia. Hum Reprod. 27:991–997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu B, Harihan A, Rakshit S, Dressler GR
and Wellik DM: The role of Pax2 in mouse prostate development.
Prostate. 72:217–224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thielen JL, Volzing KG, Collier LS, Green
LE, Largaespada DA and Marker PC: Markers of prostate
region-specific epithelial identity define anatomical locations in
the mouse prostate that are molecularly similar to human prostate
cancers. Differentiation. 75:49–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ran L, He W, Zhu X, Zhou Q and Gou X:
Comparison of fluid absorption between transurethral enucleation
and transurethral resection for benign prostate hyperplasia. Urol
Int. 91:26–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Newton CJ, Ran G, Xie YX, Bilko D,
Burgoyne CH, Adams I, Abidia A, McCollum PT and Atkin SL:
Statin-induced apoptosis of vascular endothelial cells is blocked
by dexamethasone. J Endocrinol. 174:7–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Niehrs C and Acebron SP: Mitotic and
mitogenic Wnt signalling. EMBO J. 31:2705–2713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nandan MO, Chanchevalap S, Dalton WB and
Yang VW: Krüppel-like factor 5 promotes mitosis by activating the
cyclin B1/Cdc2 complex during oncogenic Ras-mediated
transformation. FEBS Lett. 579:4757–4762. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Antonarakis ES, Lu C, Luber B, Wang H,
Chen Y, Nakazawa M, Nadal R, Paller CJ, Denmeade SR, Carducci MA,
et al: Androgen receptor splice variant 7 and efficacy of taxane
chemotherapy in patients with metastatic castration-resistant
prostate cancer. JAMA Oncol. 1:582–591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mukhopadhyay NK, Cinar B, Mukhopadhyay L,
Lutchman M, Ferdinand AS, Kim J, Chung LW, Adam RM, Ray SK, Leiter
AB, et al: The zinc finger protein ras-responsive element binding
protein-1 is a coregulator of the androgen receptor: Implications
for the role of the Ras pathway in enhancing androgenic signaling
in prostate cancer. Mol Endocrinol. 21:2056–2070. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi HJ and Zhu BT: Critical role of
cyclin B1/Cdc2 up-regulation in the induction of mitotic
prometaphase arrest in human breast cancer cells treated with
2-methoxyestradiol. Biochim Biophys Acta. 1823:1306–1315. 2012.
View Article : Google Scholar : PubMed/NCBI
|