Introduction
Malignant melanoma has become a frequently occurring
malignancy with an annual increase of ~3% (1). The majority (~90%) of patients with
early detected melanoma are curable; however, the efficiency of
clinical drugs in the treatment of patients with advanced
metastatic melanoma is <20% (2),
and the 5-year survival rate is <5%, with a median survival time
of only 2–8 months (3,4). Numerous studies have confirmed that the
poor prognosis of malignant melanoma is primarily attributable to
the high incidence of distant metastasis and a strong capacity for
invasion (5–7). Therefore, the development of more
effective therapies for the inhibition of metastasis presents a
challenge for the treatment of malignant melanoma.
Chemotherapy has a significant role in the treatment
of cancer. However, the majority of chemotherapy drugs also destroy
normal cells, leading to adverse effects (8). Therefore, the identification of natural
compounds with a wide range of anticancer activities, high
selectivity for the destruction of cancer cells and low toxicity of
normal cells is of importance in cancer research. Euphorbia
fischeriana Steud (also known as lang-du), a herbaceous plant
used in traditional Chinese medicine (TCM), has demonstrated
inhibitory effects through its capacity to induce apoptosis,
suppress growth and cause cell cycle arrest when assessed within
several cancer cell lines, including leukemia and prostate cancer
(9,10). Results from preliminary studies have
indicated that Euphorbia fischeriana Steud inhibits the
metastasis of melanoma cells through the regulation of certain
metastasis-related gene expression levels (11,12).
However, the mechanisms involved have yet to be fully
elucidated.
In the present study, the activities of Euphorbia
fischeriana Steud against the highly metastatic B16-F10 mouse
cell line and its association with the phosphoinositide-3-kinase
(PI3K)/protein kinase B (Akt) signaling pathway, were
investigated.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM),
penicillin, streptomycin, fetal bovine serum (FBS),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
trypsin-EDTA and propidium iodide (PI) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Fibronectin was purchased from
BD Biosciences (Franklin Lakes, NJ, USA) and Transwell chambers
from Costar (Corning Inc., NY, USA). Antibodies against phospho
(p)-Akt, phosphatase and tensin homolog (PTEN) and β-actin were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Polyacrylamide and the protein assay kits were obtained from
Bio-Rad Laboratories, Inc. (Hercules, CA, USA). Western blotting
detection reagents were purchased from GE Healthcare Life Sciences
(Chalfont, UK). Phospho (p)-Akt, matrix metalloproteinase-2 (MMP-2)
and β-actin primers, and reverse transcription-polymerase chain
reaction (RT-PCR) kits (Takara RNA PCR Kit) were purchased from
Takara Bio, Inc. (Otsu, Japan).
Extraction of Euphorbia fischeriana
Steud
The roots of Euphorbia fischeriana Steud were
purchased from Lunan Pharmaceutical (Linyi, China). The powdered
roots of Euphorbia fischeriana Steud were extracted by
heating in 88% ethanol at 50°C. Following precipitation, the cooled
solution was filtered and evaporated under reduced pressure to
generate a residue. The extract was then suspended in distilled
water. After a second precipitation step using water, the
supernatant was condensed as an extract of Euphorbia
fischeriana Steud for use in the in vitro experiments.
The extract contained ~0.53% jolkinolide (A and B), 1.06%
fischeriana (A and B) and flavonoids (1.75%).
Cell culture and in vitro growth
assays
The murine melanoma cell line B16-F10 (B16) was
obtained from the American Type Culture Collection (Manassas, VA,
USA). The B16 cell line was cultured (3×103 cells/well)
in DMEM medium containing 10% heat-inactivated FBS, glutamine (2
mM; Hyclone; GE Healthcare Life Sciences), penicillin (100 U/ml;
Sigma-Aldrich) and streptomycin (100 µg/ml; Sigma-Aldrich) at 37°C
in a humidified incubator with 5%atmospheric CO2. In the
treatment groups, the cells were cultured in DMEM supplemented with
10% FBS containing 0.8, 1.2, 1.4, 1.6. 1.8 and 2.0 mg/ml
concentrations of Euphorbia fischeriana Steud, whereas cells
in the control group were treated with 0.1% dimethylsulfoxide
(DMSO; Sigma-Aldrich). The cell growth was evaluated at 24 and 48 h
after treatments with MTT assay kits. Briefly, the murine melanoma
cell line B16 was seeded in 96-well culture plate (3×103
cells/well) and cultivated for 24 h. Euphorbia fischeriana
was then added to final concentrations of 0.8, 1.2, 1.4, 1.2, 1.8
and 2.0 mg/ml. After 24 and 48 h, 10 µl MTT (10 µg/ml) was added to
cells in the plate and incubated for 4 h at 37°C. Then, 150 µl DMSO
was added to each well and incubated for 20 min at room
temperature. Following incubation, the absorbance was measured at
570 nm using a Multiskan MS spectrophotometer (Labsystems,
Stockholm, Sweden). Each experiment was replicated three times.
Flow cytometry for analysis of the
cell cycle and apoptosis
B16 cells were treated with Euphorbia
fischeriana Steud at concentrations of 0 (control, 0.1% DMSO),
0.8, 1.4 or 2.0 mg/ml for 24 h. The treated B16 cells were detached
in phosphate-buffered saline (PBS)/2 mM trypsin-EDTA, centrifuged
at 335 × g for 5 min at 4°C and then resuspended in 250 µl
hypotonic fluorochrome solution (PBS, 50 µg PI, 0.1% sodium citrate
and 0.1% Triton X-100; Sigma-Aldrich) with RNase A (100 U/ml;
Sigma-Aldrich). The DNA content of the cells was analyzed by flow
cytometry (BD FACSCalibur; BD Biosciences) with 20,000 events
analyzed per sample. Cell cycle distribution and apoptosis were
determined on the basis of the DNA content and the sub-G1 cell
population, respectively.
In vitro migration assays
B16 cell migration was evaluated using
fibronectin-coated polycarbonate filters in modified Transwell
chambers. In brief, B16 cells (5×104) were seeded onto the upper
chamber in 200 µl serum-free medium containing Euphorbia
fischeriana Steud at the concentrations of 0 (control, 0.1%
DMSO), 0.8, 1.4 or 2.0 mg/ml; the lower compartment was filled with
a chemo-attractant (0.66 ml DMEM supplemented with 10% FBS).
Following a culture period of 6 h (for the migration assay) at
37°C, the cells transplanted to the lower surface of the filter
were fixed and stained with PI. The cells on the upper side of the
filter were removed using a cotton swab. The migrated cells on the
underside of the filter were counted and recorded for imaging under
a fluorescence microscope (TE2000-U; Nikon Corporation, Tokyo,
Japan). Experiments were replicated three times.
Western blot analysis
To determine the effects of Euphorbia
fischeriana Steud on the expression levels of PTEN and p-Akt in
B16 cells, cells were treated with Euphorbia fischeriana
Steud at various concentrations (0, 0.8, 1.4 or 2.0 mg/ml) for 24
h. The treated cells were washed with ice-cold PBS and suspended in
lysis buffer (Sigma-Aldrich) on ice for 30 min. Lysates were
cleared by centrifugation at 4360 × g for 20 min at 4°C. Equal
amounts of cell extracts (60 µg) were resolved by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, transferred to
nitrocellulose membranes (Sigma-Aidrich), and probed with primary
antibodies to human PTEN (1:1,000; mouse monoclonal; 14642S; Cell
Signaling Technology, Inc.), p-Akt (1:1,000; rabbit polyclonal;
4685S; Cell Signaling Technology, Inc.) and anti-β-actin (1:1,000;
mouse monoclonal; A1978; Sigma-Aldrich) at 4°C overnight. The
membranes were then washed with T-TBS buffer (Takara Bio, Inc.) 3
times for 10 min, and subsequently incubated at room temperature
for 2 h with goat anti-mouse (1:1,000; sc2005; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and goat anti-rabbit
(1:1,000; sc2004; Santa Cruz Biotechnology, Inc.)
horseradish-conjugated secondary antibodies. β-actin was used as a
loading control. After washing with T-TBS buffer 3 times, detection
was performed using an enhanced chemiluminescence system (Amersham
ECL Western Blotting Detection Reagent; GE Healthcare Life
Sciences).
RT-PCR
Total cellular RNA was extracted from the cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) from B16 cells treated with Euphorbia
fischeriana Steud at different concentrations (0/0.1% DMSO
vehicle as control, 0.8, 1.4 or 2.0 mg/ml) for 24 h according to
the manufacturer's protocol, and quantified by spectrophotometry
(NanoDrop 2000c; Thermo Fisher Scientific, Inc.). An RT-PCR kit was
used. Equal amounts of RNA were used for cDNA synthesis in 20 µl
reactions containing primers (Table
I; Bao Biological Engineering Co., Ltd., Dalian, China), 2 µl
total RNA and ddH2O. Cycling conditions comprised of
denaturation for 30 sec at 95°C, followed by 40 cycles of
amplification (at 95°C for 5 sec and 60°C for 30 sec) and a final
elongation step at 72°C for 10 min. To control the PCR reaction
components and the integrity of the RNA, 2 µl of each cDNA sample
was amplified separately by β-actin specific primers. RT-PCR
analyses were carried out in duplicate from ≥3 independent RNA
samples. The experimental data was normalized to the β-actin
expression value, and the relative expression levels were
calculated using the 2−ΔΔCq method (13).
 | Table I.Specific primer and probe
sequences. |
Table I.
Specific primer and probe
sequences.
| Gene | Primer and Probe
Sequence (5′-3′) | Amplicon size
(bp) |
|---|
|
Phosphorylated-Akt |
| 56 |
|
Forward |
CAATTCCGGTCTGAGGAA |
|
Reverse |
CACATGGGAAGTGTTGTCTG |
|
Probe |
CTTCTGACGCGCCTGCCCTC |
| MMP-2 |
| 96 |
|
Forward |
CTGGGAGCATGGAGATGGATA |
|
Reverse |
AAGTGAGAATCTCCCCCAACAC |
|
Probe |
ACATGCCTTTGCCCCGGGCA |
| β-actin |
| 70 |
|
Forward |
GGAAGCACATCATGGGTCAGA |
|
Reverse |
TACGCATCTTCATCTTCCTCCATT |
|
Probe |
TGTGGCAGACTACATGCGCTACC |
Statistical analysis
Data are expressed as mean ± standard deviation and
statistical analysis was conducted using SPSS software, version
13.0 (SPSS, Inc., Chicago, IL, USA). One- or two-way analysis of
variance followed by the Bonferroni post-hoc analysis was performed
to establish whether significant differences existed among groups.
Values between different treatment groups at different times were
compared. Mean concentrations and cell viability or migration (%)
are shown for each group. For all tests, P<0.05 was considered
to indicate a statistically significant difference.
Results
Euphorbia fischeriana Steud inhibits
the growth of B16 cells in a time- and dose-dependent manner
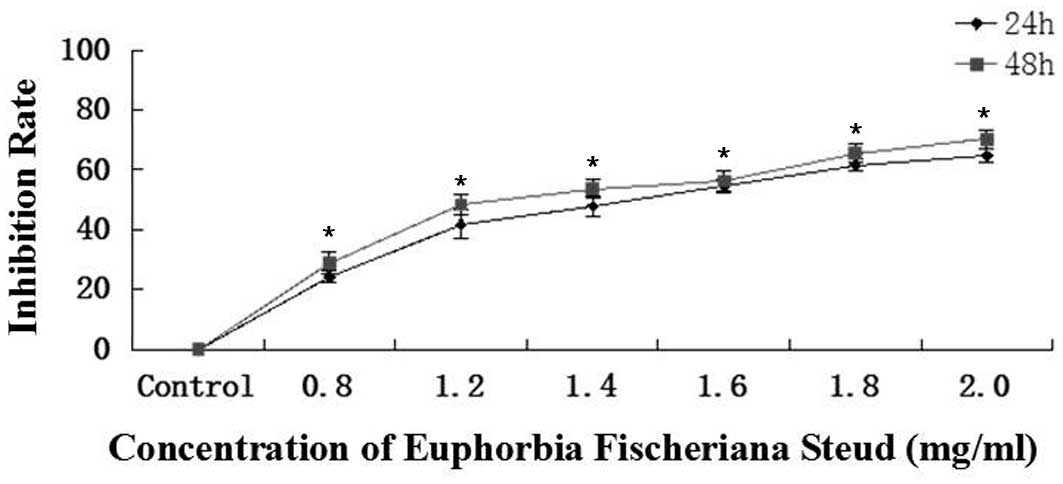
The cell growth inhibition rates for 0.8, 1.2, 1.4,
1.6, 1.8 or 2.0 mg/ml Euphorbia fischeriana Steud at 24 h
were 24.2, 41.7, 47.8, 54.7, 61.5 and 64.7% and at 48 h were 28.7,
48.3, 53.7, 56.2, 65.6 and 70.2%, respectively (Fig. 1). The plots are representative of
three similar experiments performed. The IC50 values at
24 and 48 h were 1.5 and 1.3 mg/ml, respectively. The in
vitro growth assay revealed that Euphorbia fischeriana
Steud suppressed the growth of B16 cells in a dose-and
time-dependent manner following treatment of the cells with
Euphorbia fischeriana Steud at concentrations of 0.8–2.0
mg/ml for 24 and 48 h, respectively.
Euphorbia fischeriana Steud induces
apoptosis and G0/G1 cell cycle arrest in B16 cells in a
dose-dependent manner
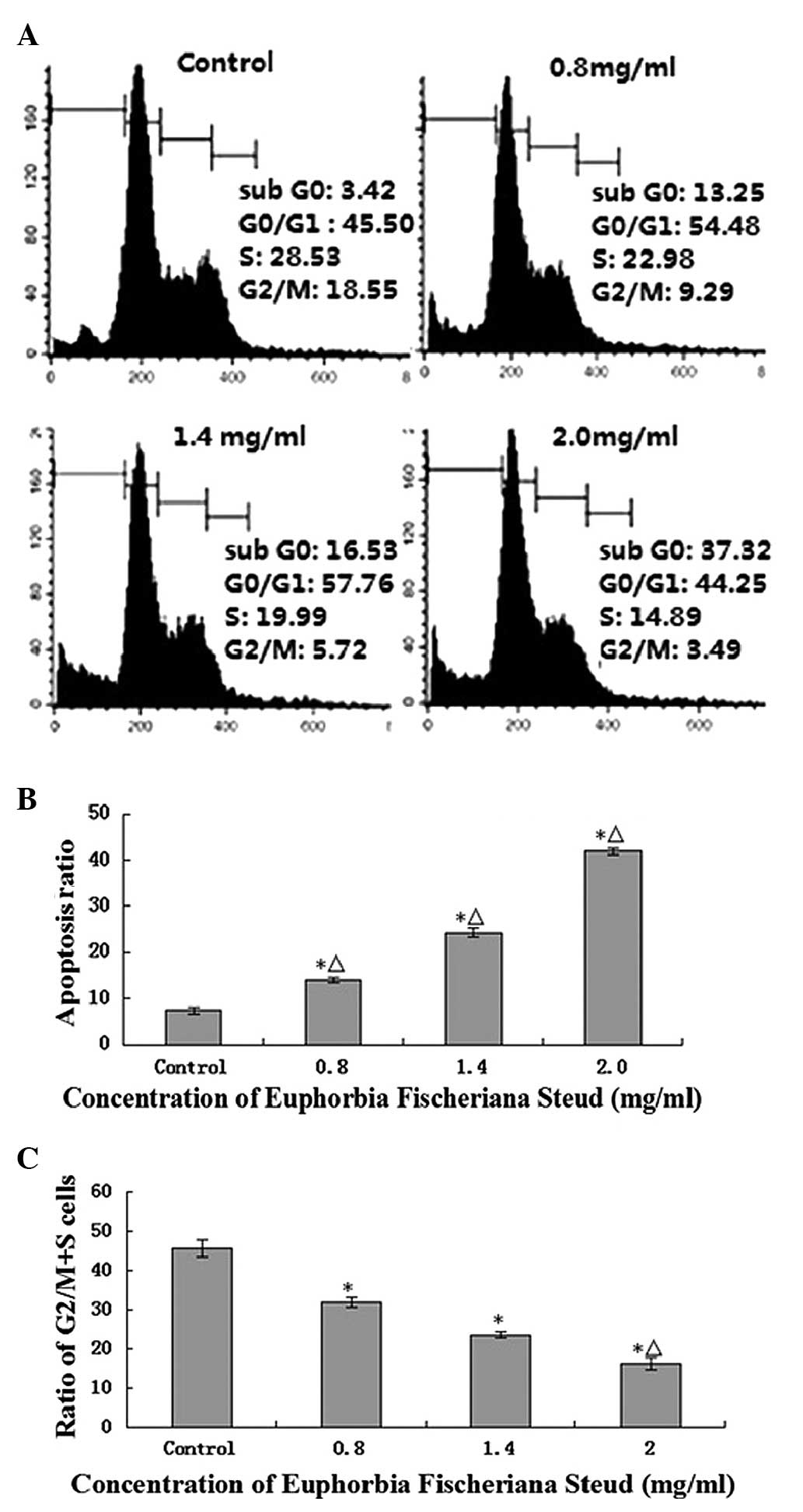
Flow cytometric analysis (Fig. 2A) revealed that Euphorbia
fischeriana Steud (0, 0.8, 1.4 or 2.0 mg/ml) significantly
induced the apoptosis of B16 cells at 24 h with apoptosis rates of
13.97±0.58, 24.26±0.91 and 41.82±0.63% compared with the control,
respectively (P<0.05). Values of 2.0, 1.4 and 0.8 mg/ml group,
compared with the control group, differed significantly (P<0.05;
Fig. 2B). Furthermore, Euphorbia
fischeriana Steud also induced a concentration-dependent G0/G1
arrest and a reduction in the proportion of cells in the G2/M and S
phases (Fig. 2C).
Suppression of migration, upregulation
of PTEN protein expression and downregulation of Akt activation in
B16 cells by Euphorbia fischeriana Steud
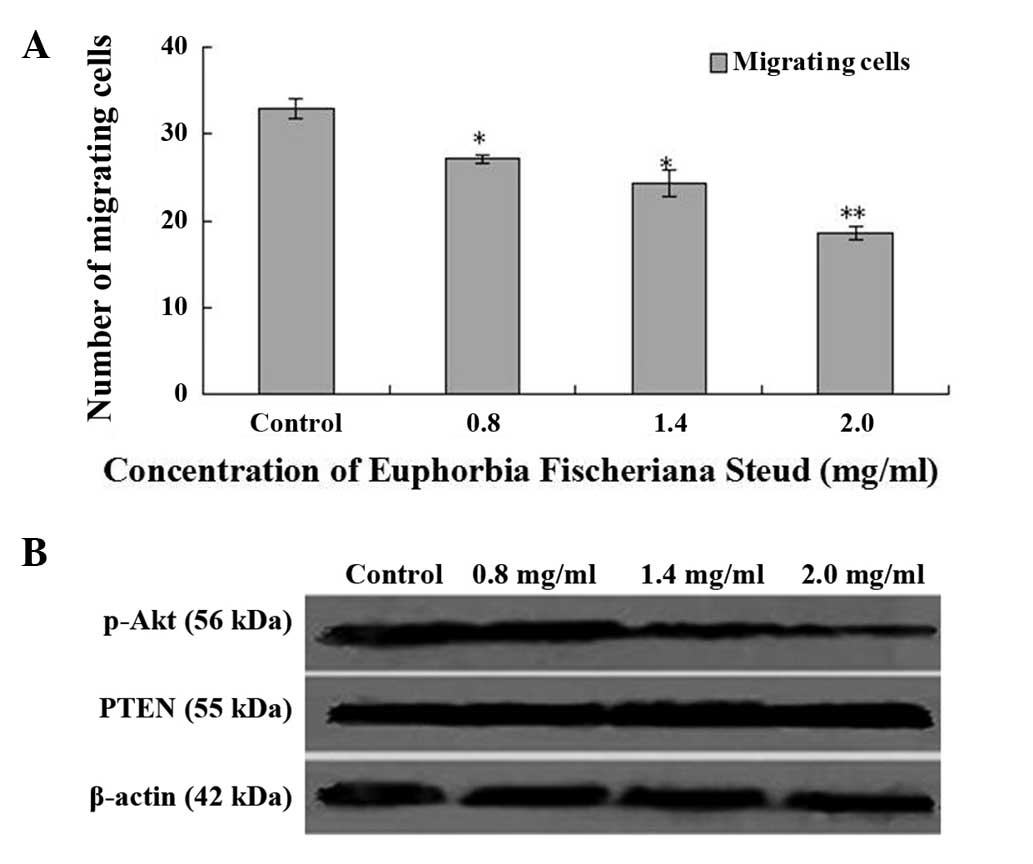
A migration assay in fibronectin-coated Transwell
chambers revealed that, after 24 h of exposure to Euphorbia
fischeriana Steud with concentrations of 0.8, 1.4 or 2.0 mg/ml,
the migration of B16 cells was significantly suppressed with
inhibition rates of 17.58, 26.06 and 43.64%, respectively (Fig. 3A). Western blot analysis confirmed
that, compared with the controls, p-Akt protein expression levels
were reduced, and PTEN protein expression levels were increased in
the cells treated with Euphorbia fischeriana Steud at
concentrations of 0.8, 1.4 and 2.0 mg/ml.
Suppression of the mRNA expression of
p-Akt and MMP-2 in B16 cells by Euphorbia fischeriana Steud
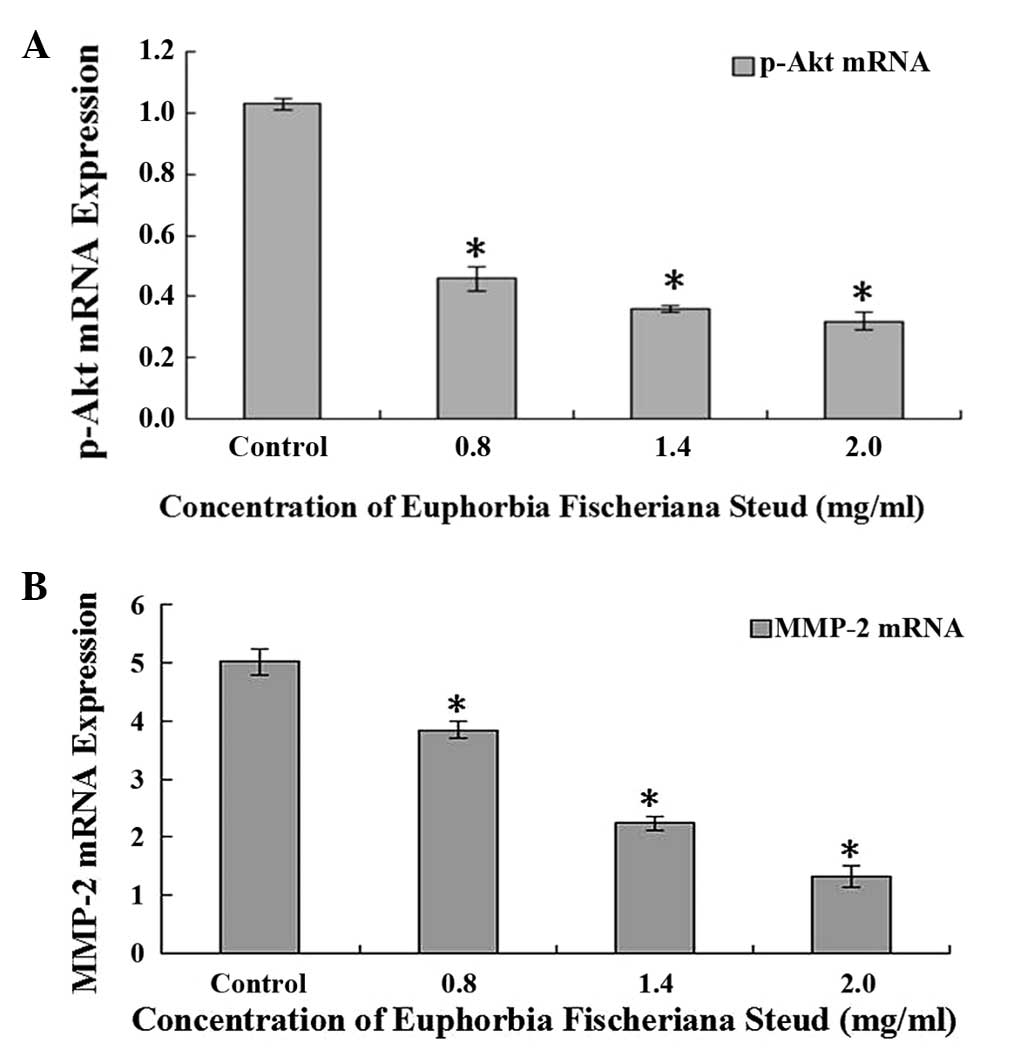
RT-PCR analysis demonstrated that the mRNA
expression of p-Akt and MMP-2 in B16 cells following treatment with
Euphorbia fischeriana Steud at concentrations of 0.8, 1.4 or
2.0 mg/ml Euphorbia fischeriana Steud for 24 h was reduced
in a concentration-dependent manner (Fig. 4).
Discussion
Malignant melanoma is a lethal type of skin cancer,
accounting for 80% of skin cancer mortalities (14). The high degree of malignancy and
occurrence in a dormant state, in addition to a strong tendency for
distant metastasis, invasion and migration, presents a challenge in
the provision of a successful clinical treatment. As a result, the
median survival following malignant melanoma invasion or metastasis
is 2–8 months (15–17). Current clinical chemotherapy drugs
for melanoma demonstrate poor efficacy, with <20% being
effective (18). Furthermore,
adverse effects such as bone marrow suppression and
immunosuppression lead to the treatments being intolerable for
patients (19). Recent developments
in the characterization of certain abnormal cell signal
transduction pathways associated with malignant melanoma have
indicated the importance and feasibility of their use in the
treatment of the aforementioned conditions. Several therapies
targeting abnormal signal pathways involved in malignant melanomas
have recently been used (20,21),
however, their efficacy when administered as a single-agent is poor
and the median progression-free survival remains at only 2.8 months
(22).
Results from recent studies have revealed that an
abnormal absence of the PTEN gene exists in 30–50% of melanoma cell
lines (23,24). Low expression levels of PTEN lead to
an ineffective inhibition of Akt activation, resulting in an
over-activation of the PI3K/Akt pathway, which promotes the
metastasis of tumor cells (25).
Activation of the PI3K/Akt pathway may activate the protein
P70S6K1 through the tuberous sclerosis complex
1/2-mammalian target of rapamycin (mTOR) pathway, and thereby
promote the reconstruction of actin filaments (26,27).
Aberrant activation of mTOR may also increase MMP-2 expression
levels, which can cause degradation of the extracellular matrix and
lead to promotion of tumor cell metastasis (28). Thus, abnormal activation of the
PI3K/Akt pathway in malignant melanoma promotes the migration of
tumor cells through the induction of degradation and enhanced
motility (29–31). In the present study, it was
demonstrated that suppression of the PI3K/Akt pathway greatly
contributes to the inhibition of the proliferation and migration of
melanoma B16 cells.
Euphorbia fischeriana Steud has been used for
the treatment of tumors for numerous years in China. The
aforementioned TCM has attracted considerable attention as it
strongly inhibits the activity of a variety of tumor cells as a
result of a broad spectrum of antitumor properties, while
displaying minimal side effects (12,32). In
the present study, Euphorbia fischeriana Steud was selected
as the raw material for investigation, and water extraction (2 g
crude drug/1 ml extracted liquid) was conducted, with the
predominant chemical constituents of extraction being
Euphorbia lactones, flavonoids, terpenoids, Euphorbia
alcohol and tannins, as identified through pharmacological
analysis. Results from numerous basic research studies have
demonstrated that Euphorbia fischeriana Steud can function
as a strong inhibitor of proliferation and induction of apoptosis
in a variety of tumor cell lines (33–35). In
addition to its capacity to suppress the proliferation of malignant
melanoma B16 cells, Euphorbia fischeriana Steud also
demonstrates the ability to regulate the expression levels of
factors that are associated with melanoma cell transfer (36). In the present study, the cell signal
transduction pathway and molecular mechanisms of Euphorbia
fischeriana Steud associated with the inhibition of metastasis
of malignant melanoma cells, were examined. Results obtained from
studies such as the present one may provide the experimental
evidence required for the development of Euphorbia
fischeriana Steud in the treatment of malignant melanoma.
Furthermore, an understanding of the mechanisms of Euphorbia
fischeriana Steud provide the foundation for the identification
of other novel natural compounds with low toxicity and high
selectivity for the destruction of various cancer cells.
In the present study, it was demonstrated that the
aqueous extract of Euphorbia fischeriana Steud inhibited the
growth and migration of B16 cells in a dose-dependent manner and
induced apoptosis and G0/G1 cell cycle arrest in B16 cells.
Euphorbia fischeriana Steud also suppressed the PI3K/Akt
signaling pathway in B16 cells through the upregulation of PTEN and
downregulation of p-Akt expression levels and also by reducing the
mRNA expression levels of p-Akt and MMP-2. To the best of our
knowledge, no other reports exist detailing the association between
the PI3K/Akt signaling pathway and the inhibitory effects of
Euphorbia fischeriana Steud on the growth and metastasis of
melanoma B16 cells in vitro, and the underlying mechanisms.
The data provided in the current study suggest that Euphorbia
fischeriana Steud may have potential in therapeutic and/or
adjuvant therapeutic applications for the treatment of human
melanoma and other cancers.
In the present study, a series of experiments
directed at examining the association and mechanisms between the
PI3K/Akt signaling pathway and the inhibitory effects of
Euphorbia fischeriana Steud on the growth and metastasis of
melanoma B16 cells were performed. The results demonstrate that
Euphorbia fischeriana Steud inhibited the growth and
metastasis of B16 cells, possibly via modulation of the PI3K/Akt
signaling pathway, with upregulation of PTEN expression levels and
downregulation of p-Akt expression levels.
Acknowledgements
The present study was supported by grants from the
Project of Army Medical Research in Science and Technology (grant
no. 06G034), the Nature Science Foundation of China (grant no. NSFC
81370730 and 81571512), the Department of Science and Technology in
Shandong province (grant no. ZR2015JL027) and the International
Excellent Talent Visitor Scholar Program in 2015.
Glossary
Abbreviations
Abbreviations:
|
PI3K
|
phosphatidyl inositol 3-kinase
|
|
Akt
|
protein kinase B
|
|
mTOR
|
mammalian target of rapamycin
|
|
PTEN
|
phosphatase and tensin homolog deleted
on chromosome ten
|
|
MMP-2
|
matrix metalloproteinase
|
References
|
1
|
Tsao H, Chin L, Garraway LA and Fisher DE:
Melanoma: From mutations to medicine. Genes Dev. 26:1131–1155.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mateus C and Robert C: Major therapeutic
advances in the treatment of metastatic melanoma. Bull Cancer.
99:619–625. 2012.(In French). PubMed/NCBI
|
|
3
|
Rosenberg E, Horev A and Neulander EZ:
Amelanotic malignant melanoma of the penis. A case report and
literature review. Arch Ital Urol Androl. 84:42–43. 2012.PubMed/NCBI
|
|
4
|
Mikhnin AE, Tarkov SA and Frolova OS:
Cutaneous melanoma in the head and neck region: Current knowledge.
Vopr Onkol. 58:19–25. 2012.(In Russian). PubMed/NCBI
|
|
5
|
Grossmann AH, Grossmann KF and Wallander
ML: Molecular testing in malignant melanoma. Diagn Cytopathol.
40:503–510. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaufman HL: Vaccines for melanoma and
renal cell carcinoma. Semin Oncol. 39:263–275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lacy KE, Karagiannis SN and Nestle FO:
Advances in the treatment of melanoma. Clin Med. 12:168–171. 2012.
View Article : Google Scholar
|
|
8
|
Bloomfield JG and Tanay MA: Chemotherapy
in the community: The importance of patient assessment. Br J
Community Nurs. 17:278–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun YX and Liu JC: Chemical constituents
and biological activities of Euphorbia fischeriana Steud.
Chem Biodivers. 8:1205–1214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang YB, Huang R, Wang HB, Jin HZ, Lou LG
and Qin GW: Diterpenoids from the roots of Euphorbia
fischeriana. J Nat Prod. 69:967–970. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith ME and Bauer-Wu S: Traditional
chinese medicine for cancer-related symptoms. Semin Oncol Nurs.
28:64–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu J, Li X, Liu J, Ma L, Li X and Fønnebø
V: Traditional chinese medicine in cancer care: A review of case
reports published in Chinese literature. Forsch Komplementmed.
18:257–263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tremante E, Ginebri A, Lo Monaco E,
Frascione P, Di Filippo F, Terrenato I, Benevolo M, Mottolese M,
Pescarmona E and Visca P: Melanoma molecular classes and prognosis
in the postgenomic era. Lancet Oncol. 13:e205–e211. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Espinosa E, Berrocal A, López Martín JA,
González Cao M, Cerezuela P, Mayordomo JI and Martín Algarra S:
Grupo Español de Melanoma (GEM): Advances in cutaneous melanoma.
Clin Transl Oncol. 14:325–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sanford M: Ipilimumab: In previously
treated patients with advanced melanoma. Bio Drugs. 26:185–193.
2012.
|
|
17
|
Trinh VA and Hwu WJ: Ipilimumab in the
treatment of melanoma. Expert Opin Biol Ther. 12:773–782. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stratigos AJ, Forsea AM, van der Leest RJ,
de Vries E, Nagore E, Bulliard JL, Trakatelli M, Paoli J, Peris K,
Hercogova J, et al: Euromelanoma: A dermatology-led European
campaign against nonmelanoma skin cancer and cutaneous melanoma.
Past, present and future. Br J Dermatol. 167:99–104. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smalley KS and McArthur GA: The current
state of targeted therapy in melanoma: This time it's personal.
Semin Oncol. 39:204–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mathew R and Messina JL: Recent advances
in pathologic evaluation and reporting of melanoma. Semin Oncol.
39:184–191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Silva E: Adjunct primer for the use of
national comprehensive cancer network guidelines for the surgical
management of cutaneous malignant melanoma patients. World J Surg
Oncol. 6:542012. View Article : Google Scholar
|
|
22
|
Flaherty KT, Hodi FS and Fisher DE: From
genes to drugs: Targeted strategies for melanoma. Nat Rev Cancer.
12:349–361. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aguissa-Touré AH and Li G: Genetic
alterations of PTEN in human melanoma. Cell Mol Life Sci.
69:1475–1491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu H, Goel V and Haluska FG: PTEN
signaling pathways in melanoma. Oncogene. 22:3113–3122. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Madhunapantula SV and Robertson GP: The
PTEN-AKT3 signaling cascade as a therapeutic target in melanoma.
Pigment Cell Melanoma Res. 22:400–419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meier F, Schittek B, Busch S, Garbe C,
Smalley K, Satyamoorthy K, Li G and Herlyn M: The RAS/RAF/MEK/ERK
and PI3K/AKT signaling pathways present molecular targets for the
effective treatment of advanced melanoma. Front Biosci.
10:2986–3001. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Davies MA: The role of the PI3K-AKT
pathway in melanoma. Cancer J. 18:142–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Willems L, Tamburini J, Chapuis N, Lacombe
C, Mayeux P and Bouscary D: PI3K and mTOR signaling pathways in
cancer: New data on targeted therapies. Curr Oncol Rep. 14:129–138.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bartholomeusz C and Gonzalez-Angulo AM:
Targeting the PI3K signaling pathway in cancer therapy. Expert Opin
Ther Targets. 16:121–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Y, Wang BC and Xiao Y: PI3K: A
potential therapeutic target for cancer. J Cell Physiol.
227:2818–2821. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu B and Zhou X: The study of PI3K/AKT
pathway in lung cancer metastasis and drug resistance. Zhongguo Fei
Ai Za Zhi. 14:689–694. 2011.(In Chinese). PubMed/NCBI
|
|
32
|
Lin H, Liu J and Zhang Y: Developments in
cancer prevention and treatment using traditional chinese medicine.
Front Med. 5:127–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang JY, Xu L, Zhang RX and Lao L:
Traditional chinese medicine for cancer pain. Zhong Xi Yi Jie He
Xue Bao. 9:129–134. 2011.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou TX, Bao GH, Ma QG, Che CT, Lv Y, Wang
C and Zheng QT: Langduin C, a novel dimeric diterpenoid from the
roots of Euphorbia fischeriana. Tetrahedron Lett.
44:135–137. 2003. View Article : Google Scholar
|
|
35
|
Wang JH, Zhang K, Niu HY, Shu LH, Yue DM,
Li D and He P: Jolkinolide B from Euphorbia fischeriana
Steud induces in human leukemic cells apoptosis via JAK2/STAT3
pathways. Int J Clin Pharmacol Ther. 51:170–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pon D, Wang Z, Le KN and Chow MS:
Harnessing traditional Chinese medicine to improve cancer therapy:
Issues for future development. Ther Deliv. 1:335–344. 2010.
View Article : Google Scholar : PubMed/NCBI
|