Introduction
Degenerative disc disease is a frequent cause of
lower back pain. A previous study has demonstrated that 75–80% of
people will experience lower back pain with prevalence ranging from
15 to 45%. Severe disc degeneration is associated with a twofold
increase in chronic low back pain (1). Current clinical treatment of this
disease typically combines conservative and surgical treatment,
although each method has been established to independently relieve
symptoms. Surgery presents risks and may worsen the degeneration of
adjacent tissue (2). Progress in
molecular biology has enabled novel treatments, such as gene
therapy, to emerge, and it may be possible to apply these in the
treatment of disc degeneration. Reductions in proteoglycan and type
II collagen levels are commonly associated with disc degeneration
(2). Collagens provide form and
tensile strength, whereas proteoglycans, through interactions with
water, ensure tissue stiffness, viscoelasticity, and resistance to
compression (2). A previous study
has confirmed that single vector-mediated co-transduction of
several genes significantly improves the effect of transgenic
therapy (3). The authors of the
present study have previously demonstrated that the
adeno-associated virus 2 (AAV2)-connective tissue growth factor
(CTGF)-tissue inhibitor of metalloproteinase 1 (TIMP1) vector is
capable of significantly increasing the synthesis of type II
collagen and proteoglycan in disc cells (4). Furthermore, transforming growth factor
(TGF)-β3 increased proliferation and the synthesis of extracellular
matrix macromolecules; therefore, multi-gene therapy of
intervertebral disc degeneration may be more effective. In the
present study, a lentiviral plasmid encoding TGF-β3, CTGF and TIMP1
was evaluated in a rabbit model of annulus fibrosus
puncture-induced intervertebral disc degeneration. Plasmid was
injected into the degenerative lumbar intervertebral discs in order
to investigate whether it could delay the degeneration of the
discs. Plasmids were synthesized using 2A self-cleaving sequences
to ligate the cDNA of TGF-β3, CTGF and TIMP1 in a single open
reading frame derived from lentiviral plasmids. Following
transfection into 293 cells, reverse transcription-quantitative
polymerase chain reaction (RT-PCR) and western blot analysis were
performed to detect the mRNA and protein expression levels of
TGFβ3, CTGF, TIMP1 at various time points following transfection.
The recombinant plasmid, lenti-TGFβ3-P2A-CTGF-T2A-TIMP1, was
constructed successfully, providing a basic model for the packaging
of virus and further study on therapy of intervertebral disc
degeneration (5). The plasmid was
subsequently injected into the degenerative lumbar intervertebral
discs in order to investigate whether it could delay the
degeneration of the discs.
Materials and methods
Animals
A total of 15 healthy, male New Zealand white
rabbits (age, 1 year; weight, 2.5–3 kg) were obtained from the
Experimental Animal Center of the Affiliated Hospital of Qingdao
University (Qingdao, China) and were housed in an animal care
facility at 20–25°C (humidity, 40–50%) with 12-h light/dark cycles
and ad libitum access to food and water. Rabbits were
divided into three groups: Experimental group, control group and
puncture group (n=5 per group). Animal care and experimental
protocols were approved by the Institutional Animal Care and Use
Committee of the Affiliated Hospital of Qingdao University. New
Zealand white rabbits were randomly divided amongst the
experimental, control and puncture groups.
Puncture surgery and plasmid
injection
An established animal model of intervertebral disc
degeneration was created using the annulus fibrosus puncture
technique, and this model was used in all the current experiments
(6,7). Each rabbit was anesthetized with an
intramuscular injection of diazepam (10 mg/kg) and ketamine (80
mg/kg). Under sterile surgical conditions and general anesthesia,
the dorsal skin was prepared aseptically at the central and left
side; the spine was then exposed from an anterolateral,
retroperitoneal approach. The L3-L4, L4-L5 and L5-L6 discs were
sequentially punctured with a 16-gauge needle to a depth of 5 mm.
Four weeks after the puncture surgery, empty lentivirus or
recombinant lentiviral plasmid lenti-TGFβ3-P2A-CTGF-T2A-TIMP1 was
injected into degenerative lumbar intervertebral discs
(representing the control and experimental groups, respectively),
whilst untreated degenerative lumbar intervertebral discs served as
the puncture group. The surgical incisions were closed and standard
postoperative care was performed. An intramuscular injection of
400,000 units penicillin was administered to all animals prior to
and subsequent to surgery, and the animals were permitted to move
freely in their cages following surgery.
mRNA expression of type II collagen
and aggrecan
At 16 and 20 weeks after puncture surgery,
intervertebral discs from each group were collected and homogenized
in TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and total RNA was extracted according to the
manufacturer's protocol. The concentration of total RNA was
assessed using a spectrophotometer (DUR640; Beckman Coulter, Inc.,
Brea, CA, USA) and 1 µg total RNA was transcribed into
complementary (c)DNA using 0.5 µl PrimeScript RTase (2-step), 1 µl
dNTPs (10 Mm), 1 µl random primers (20 µM), 1 µg template RNA,
RNase-free dH2O up to 10 µl, 10 µl PrimeScript buffer
(5X), 4 µl RNase inhibitor (40 U/µl), 5 µl RNase-free
dH2O, 5 µl to a total volume of 20 µl. The reaction was
performed at 42°C for 30 min, 95°C for 5 min and 4°C for 5 min. The
resultant cDNA was used in a PCR, performed with reagents from a
Taq PCR Master Mix kit (Qiagen China Co., Ltd., Shanghai, China).
The primers used for PCR amplification are listed in Table I, along with their GenBank accession
numbers (National Institutes of Health, Bethesda, MD, USA). The
optimal annealing temperatures were 54°C for type II collagen and
aggrecan and 58°C for the internal control gene β-actin. Thermal
cycling was performed using an Eppendorf Mastercyler (535025012;
Eppendorf AG, Hamburg, Germany). Initial denaturation of the
reaction mixture was performed at 95°C for 5 min. PCR amplification
was conducted using the following cycling conditions: Denaturation,
98°C for 30 sec; annealing at the aforementioned temperature for 30
sec; and extension, 72°C for 20 sec, for 37 cycles, as previously
described (8). This was followed by
a final extension step at 72°C for 10 min. PCR products were then
separated by agarose gel electrophoresis using agarose gel that had
been stained with ethidium bromide (E8751; Sigma-Aldrich) prior to
analysis (Sigma-Aldrich, St. Louis, MO, USA). Band intensities of
the target genes were measured by densitometry using an Vilber
Lourmat image analysis system (version 3.1; Vilber Lourmat GmbH,
Eberhardzell, Germany). mRNA levels were normalized and expressed
as a ratio of the band intensity of the sample to that of the
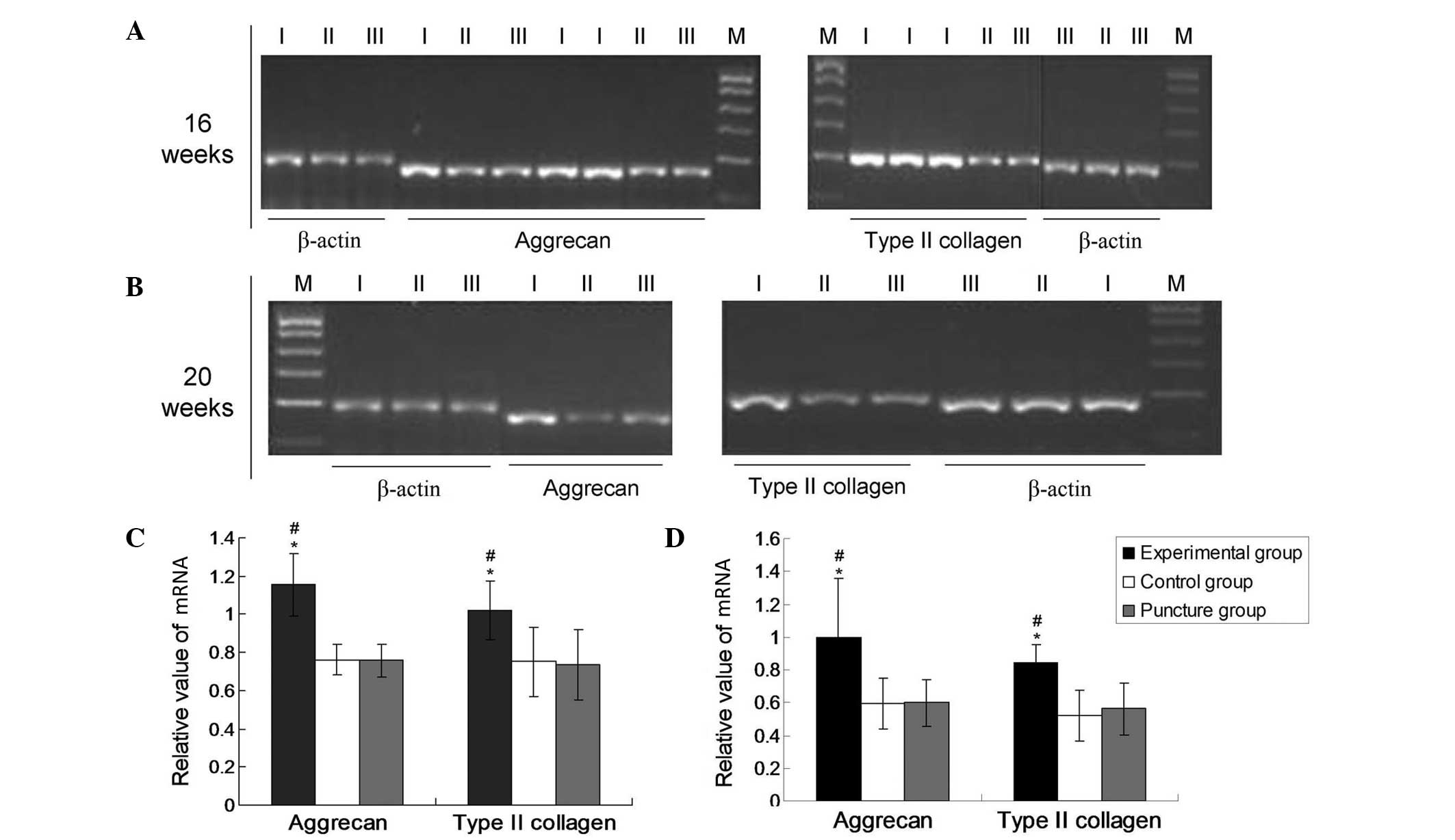
internal reference gene β-actin (Fig.
1). Experiments were performed ≥3 times.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene name | Accession number | Forward sequence
(5′→3′) | Reverse sequence
(5′→3′) | Product size
(bp) |
|---|
| Type II collagen | NM_001195671.1 |
GGCTCCCAGAACATCACCTA |
GATGACAGTCTTGCCCCACT | 198 |
| Aggrecan | XM_008251721.1 |
GACCGAGGTCAGTGGATTGT |
CCAGGTCAGGGATTCTGTGT | 173 |
| β-actin | NM_001101683.1 |
TCCCTGGAGAAGAGCTACGA |
GTGTTGGCGTACAGGTCCTT | 188 |
Western blotting
After 16 and 20 weeks, the nuclei pulposi from each
group were collected, the cells were lysed using 3 ml/g
radioimmunoprecipitation assay lysate (R0278; Sigma-Aldrich) into
the nucleus pulposus tissue and protein was extracted by
ultrasonication in mammalian protein extraction reagent and
protease inhibitor (BioVision, Inc., Hangzhou, China). The mixture
was incubated on ice for 2 h, then centrifuged at 4°C for 10 min at
~20,000 × g. The supernatant was stored at −70°C. Total protein was
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and the separated proteins were transferred by
electroblotting to a polyvinylidene difluoride membrane. The
membrane was then incubated at room temperature for 2 h with
primary antibodies from Bioss, Inc. (Beijing, China), as follows:
Monoclonal rabbit anti-collagen-II (1:100; bs-0709R), monoclonal
rabbit anti-aggrecan (1:100; bs-1223R) and monoclonal mouse
anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:500;
bsm-0978M). Following three washes with phosphate-buffered saline
with Tween 20 (PBST; BioVision, Inc.), the membrane was incubated
with horseradish peroxidase-labeled secondary antibody (1:3,000;
bs-0295G-HRP; Bioss, Inc.) in PBST for 1 h at room temperature.
Following removal of the secondary antibody, a bioluminescent
substrate (EMD Millipore, Billerica, MA, USA) was added and the
membrane was developed using a IS4000MM chemiluminescence imaging
system (Kodak, Rochester, NY, USA). Bands were quantified using
Quantity One software (Bio-Rad Laboratories, Inc.). GAPDH served as
an internal reference protein and each experiment was repeated 12
times.
Magnetic resonance imaging (MRI)
Under general anesthesia of 10 mg/kg ketamine and
0.5 mg/kg diazepam (i.m.), each rabbit was scanned by MRI (Magnetom
Trio 3T; Siemens AG, Munich, Germany) to evaluate the degeneration
of the intervertebral discs at 16 and 20 weeks. All images were
analyzed using the modified Thompson method, as previously
described (9), which categorizes
T2-weighted images according to four levels: Level I, normal high
intensity; level II, mild hypointensity; level III, moderate
hypointensity; and level IV, severe hypointensity.
Statistical analysis
All values are presented as the mean ± standard
deviation. Statistical analyses were performed using SPSS software,
version 15.0 (SPSS, Inc., Chicago, IL, USA). A one-way analysis of
variance was used to compare groups differences between the groups.
MRI data were analyzed using a random sum test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of aggrecan and type II
collagen mRNA
The mRNA expression levels of aggrecan and type II
collagen were normalized to that of the endogenous reference gene,
β-actin. As shown in Fig. 1, the
mRNA expression levels of collagen II and aggrecan were
significantly upregulated in the experimental group after 16 and 20
weeks compared with the levels in the puncture and control groups
(P<0.05). However, no significant differences were revealed in
the expression levels of aggrecan and collagen II between the
puncture and control groups at these time points (P>0.05). In
the experimental group at 16 and 20 weeks, the aggrecan values were
1.0993±0.15032 and 1.0031±0.54409, respectively, and the type II
collagen values were 1.0168±0.11753 and 0.8696±0.15402,
respectively. In the puncture group at 16 and 20 weeks, the
aggrecan values were 0.6848±0.11576 and 0.6017±0.31039,
respectively, and the type II collagen values were 0.6636±0.18730
and 0.5402±0.11997, respectively. In the control group, the
aggrecan values at 16 and 20 weeks were 0.6992±0.10144 and
0.5932±0.33179, respectively and the type II collagen values were
0.6897±0.17477 and 0.5247±0.12953, respectively. These results
demonstrate that lenti-TGFβ3-CTGF-TIMP1 co-transduction
significantly promoted the expression of aggrecan and type II
collagen.
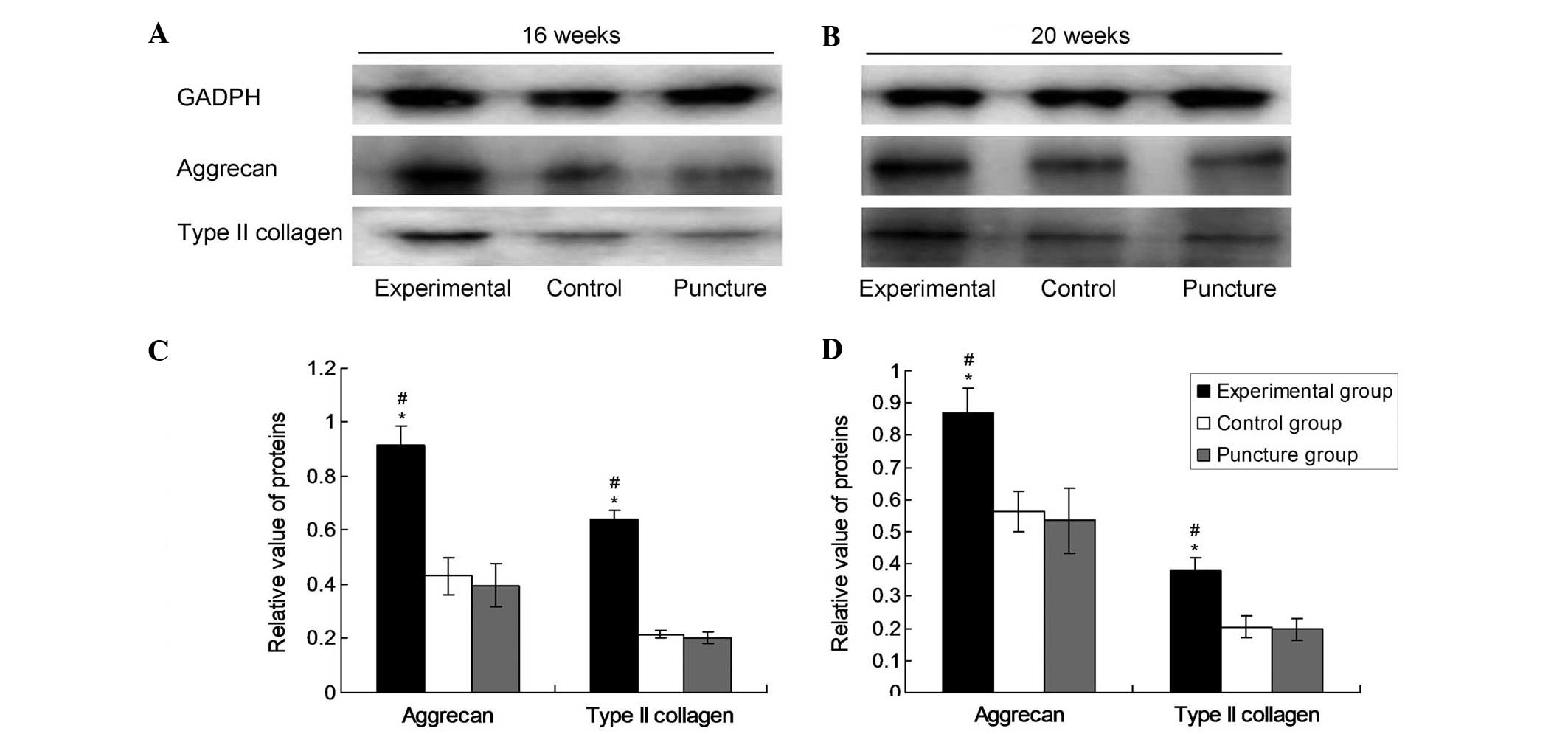
Protein expression of aggrecan and
type II collagen
Densitometric values from western blot analyses were
evaluated by computerized laser densitometry and normalized to that
of the endogenous reference protein GAPDH. As shown in Fig. 2, the levels of aggrecan and type II
collagen protein in the experimental group were significantly
increased compared with those in the control and puncture groups
after 16 and 20 weeks (P<0.05). However, there was no
significant difference in the levels of aggrecan and type II
collagen protein between the puncture group and the control group
(P>0.05). In the experimental group at 16 and 20 weeks, the
aggrecan values were 0.1188±0.00536 and 0.1160±0.01208,
respectively and the type II collagen values were 0.1259±0.01124
and 0.1101±0.01039, respectively. In the puncture group at 16 and
20 weeks, the aggrecan values were 0.0767±0.00918 and
0.0548±0.01045, respectively, and the type II collagen values were
0.0866±0.1118 and 0.0740±0.00937, respectively. In the control
group, the aggrecan values at 16 and 20 weeks were 0.0759±0.00810
and 0.0599±0.01124, respectively and the type II collagen values
were 0.0900±0.00945 and 0.0732±0.01596, respectively. These data
indicate that lenti-TGFβ3-CTGF-TIMP1 co-transduction significantly
promoted the protein expression of aggrecan and type II
collagen.
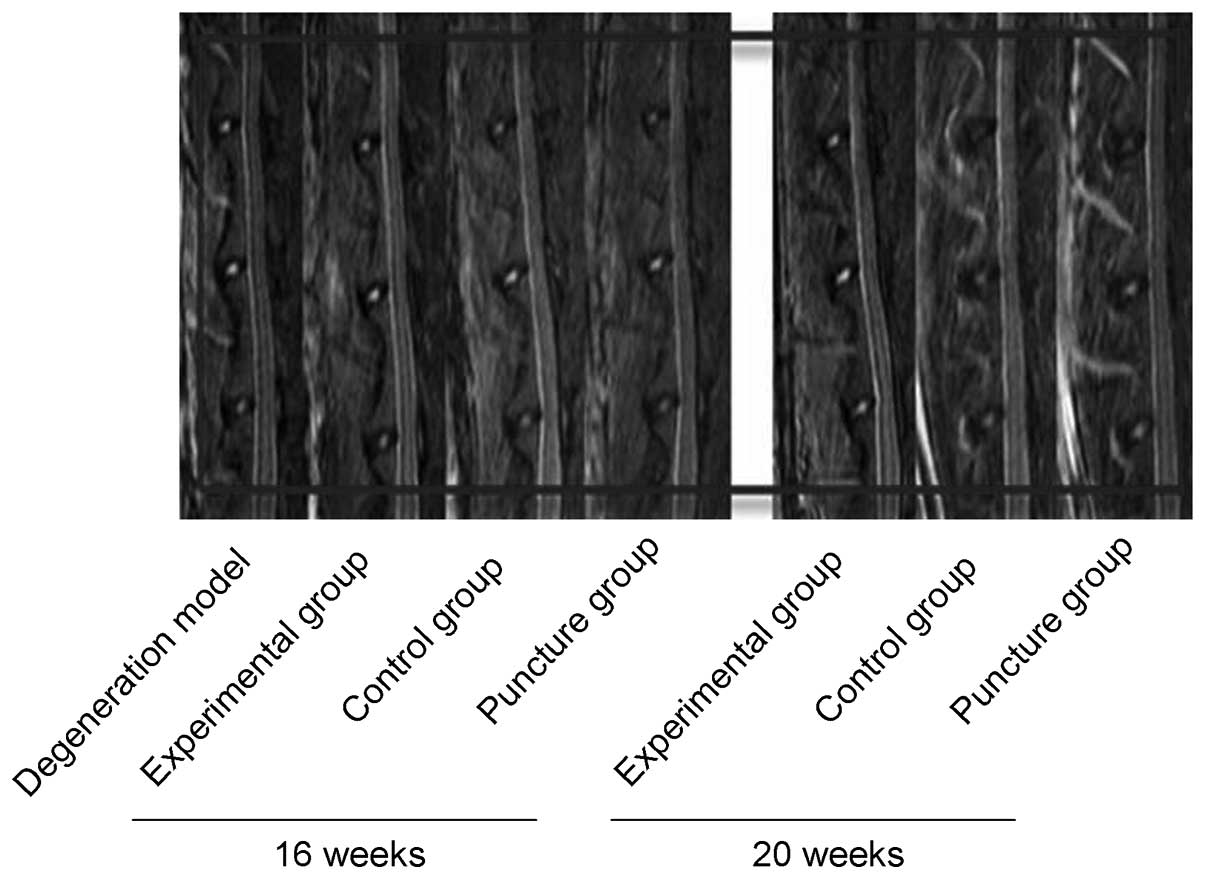
MRI
Comparisons of the degeneration of intervertebral
discs among the three groups were made by MRI scanning at 16 and 20
weeks. Degeneration progressively increased within this time period
in the control and puncture groups (Fig.
3); however, no statistically significant difference was
observed between these two control groups at 16 (n=6) or 20 weeks
(n=9) (P>0.05). By contrast, degeneration in the experimental
group was significantly reduced compared with that in the two
control groups (P<0.05).
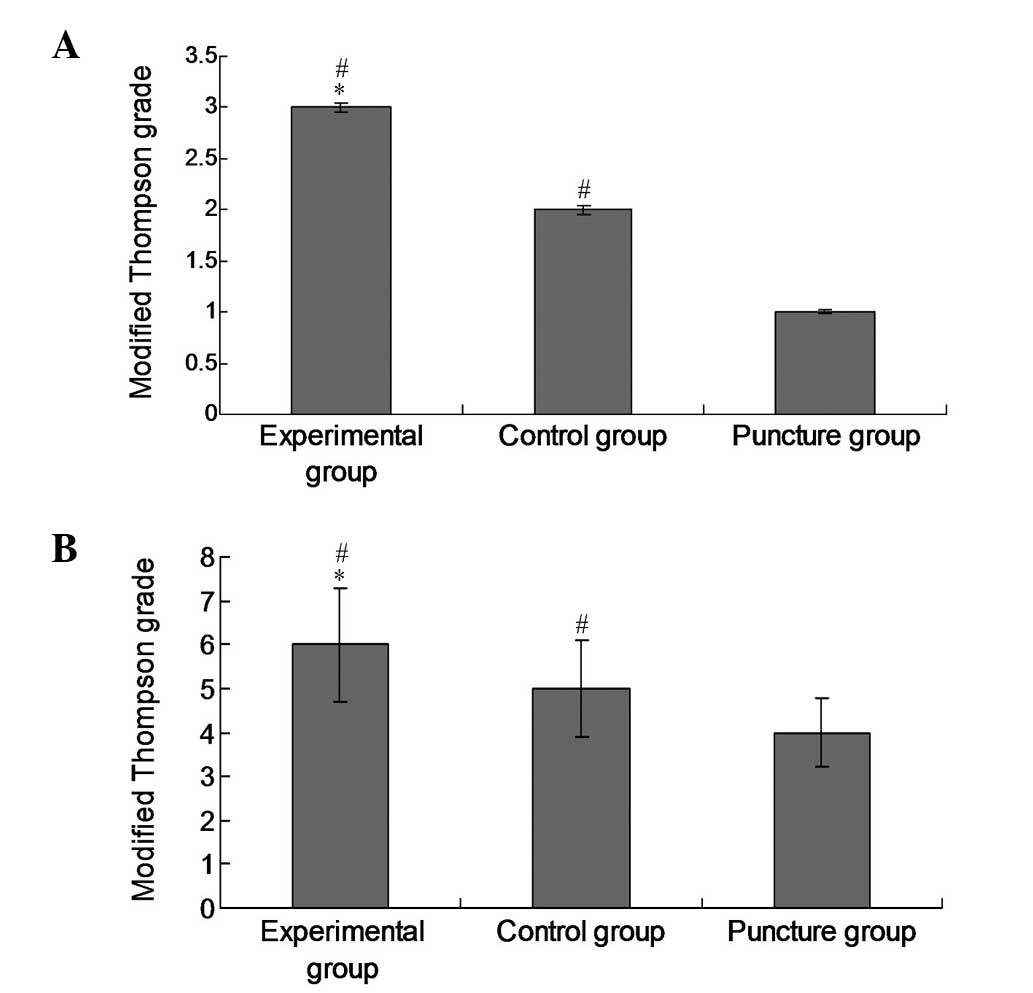
Modified Thompson grades
In order to evaluate the therapeutic effects of the
experimental treatment on intervertebral disc degeneration, the
modified Thompson grade of each group was examined. The modified
Thompson grade of the experimental group was significantly
increased (P<0.05) compared with those of the control and
puncture groups at 16 (Fig. 4A) and
20 weeks (Fig. 4B), which is
indicative of attenuated degeneration following the administration
of lenti-TGFβ3-CTGF-TIMP1.
Discussion
Intervertebral disc degeneration typically results
from the increased activity of matrix metalloproteinases and
decreased secretion of proteoglycan and type II collagen (10,11). The
degenerative disc undergoes complex biochemical changes that
involve a progressive loss of proteoglycan content, leading to
dehydration of the nucleus pulposus (12).
Previous studies have revealed that TGF-β3 can
promote the synthesis of type II collagen by fibroblastic cells in
the degenerating intervertebral disc (13,14),
whilst the expression of TGF-β3 is downregulated in degenerating in
intervertebral discs. Nakanishi et al (15) reported that CTGF enhances
proteoglycan and type II collagen expression in chondrocytes and
inhibits apoptosis. Furthermore, CTGF can inhibit matrix
degradation (16) and induce
angiogenesis through the expression of tissue inhibitor of
metalloproteinases 1 (TIMP1). TIMPs are anticatabolic growth
factors that prevent matrix metalloproteinases from enzymatically
cleaving proteoglycans (17). TIMP1
inhibits the enzymatic activity of matrix metalloproteinase 3 in
the intervertebral disc (18).
Furthermore, Bachmeier et al (17) found that matrix metalloproteinases
exacerbate the degeneration of the intervertebral disc, whilst
their inhibitors can block degenerative processes.
A number of previous studies have reported promising
results using a biological approach in the treatment of
degenerative intervertebral discs (19,20). The
adenoviral vector AAV2-CTGF-TIMP1 was constructed in 2010, with
subsequent successful use in animal experiments (4); single and double vector-mediated
expression of common genes has since been confirmed to
significantly increase the effectiveness of transgenic therapy
(3). However, in order to implement
such improvements in transgenic therapy, vector use must be
optimized. The lentiviral expression system is considered to be one
of the most effective gene therapy vectors available, being able to
transfect almost all mammalian cell types (including mitotic and
amitotic cells) and integrating foreign genes into host
chromosomes, making it a more stable and efficient expression
system than the transient expression achieved using an adenoviral
vector (21–24). Following alteration, the lentiviral
vector can accommodate up to ~10 kb of exogenous genes, which is an
improvement on the limited capacity of the adenoviral vector,
providing favorable conditions for the implementation of a multiple
gene expression system. In 2012, a lenti-TGFβ3-CTGF-TIMP1 gene
expression plasmid was successfully constructed (5); in the present study, an intervertebral
disc degeneration model was generated, and this vector was used to
transduce multiple genes into the degenerative intervertebral disc.
The present study aimed to observe the downstream consequences of
this lentiviral vector on proteoglycan and type II collagen, and
thereby to elucidate the mechanism of action of this therapy during
degeneration of the intervertebral disc. The present study
confirmed that lenti-TGFβ3-CTGF-TIMP1 increased the synthesis of
proteoglycan and type II collagen (demonstrated by reverse
transcription-PCR and western blotting). Furthermore, significant
differences observed using MRI examination provided indirect
evidence that lenti-TGFβ3-CTGF-TIMP1 delayed intervertebral disc
degeneration.
The current study demonstrated the successful use of
a lentiviral vector as a multiple gene expression plasmid in
vivo. This was a novel approach, providing a rationale for
subsequent development of gene therapy, for instance through use of
alternative candidate genes in treatment of intervertebral disc
degeneration.
In the present experiments, a model of
intervertebral disc degeneration was created using the annulus
fibrosus puncture technique. This differs from the complex process
involved in clinical cases, but the intervention measures used in
this model indicate the effectiveness and feasibility of multiple
gene treatment. Compared with the control and puncture groups, mRNA
and protein expression levels of aggrecan and type II collagen were
markedly increased in the experimental group. As compared with the
AAV2-CTGF-TIMP1 gene transduction, aggrecan and type II collagen
mRNA expression was 1.26- and 1.89-fold higher at 16 weeks, and at
20 weeks mRNA expression of aggrecan and type II collagen
(lenti-TGFβ3-CTGF-TIMP1) was 2.11- and 2.54-fold higher, as
compared the expression detected at 24 weeks (AAV2-CTGF-TIMP1)
(4), demonstrating that
lenti-TGFβ3-CTGF-TIMP1 gene transduction can have a stabilizing
role in vivo, which endures for 20 weeks. However, the
potential of its use in complete reversal of disc degeneration
requires additional study. It should be noted that the effects of
the experimental intervention were assessed at an early stage of
intervertebral disc degeneration in the present study, and the
effects of the treatment are likely to differ if it is used at
later stages of the disc degeneration process. In subsequent
studies, it is therefore important to examine the later stages of
disc degeneration.
The present experiments confirm the effectiveness of
co-transduction of multiple genes in the treatment of
intervertebral disc degeneration. It is also important to note that
no apparent side effects were observed in the rabbits in the
present study, side effects may remain a risk in the clinical
setting. For instance, there is a risk of injury when puncturing
the intervertebral disc for the injection of drugs. The suitability
of injection doses, long-term effectiveness and potential side
effects all require additional study prior to the use of gene
therapy clinically; however, the present results demonstrate
promising potential.
In conclusion, the present experimental results
confirm that co-transduction with multiple genes using the
lentiviral plasmid lenti-TGFβ3-P2A-CTGF-T2A-TIMP1 can promote the
synthesis of aggrecan and type II collagen and delay degeneration
of the intervertebral disc in an animal model. Although this
experimental approach has its limitations, the results demonstrate
the potential of this approach in the clinical treatment of
intervertebral disc degeneration.
Acknowledgements
The present study was funded by the National Natural
Science Foundation (grant no. 81171758).
References
|
1
|
Takatalo J, Karppinen J, Niinimäki J,
Tainela S, Näyhä S, Mutanen P, Sequeiros RB, Kylönen E and Tervonen
O: Does lumbar disc degeneration on magnetic resonance imaging
associate with low back symptom severity in young Finnish adults?
Spine. 36:2180–2189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park P, Garton HJ, Gala VC, Hoff JT and
McGillicuddy JE: Adjacent segment disease after lumbar or
lumbosacral fusion: Review of the literature. Spine (Phila Pa
1976). 29:1938–1944. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cui K, Zhou X, Luo J, Feng J, Zheng M,
Huang D, Jiang J, Chen X, Wei Y, Li J and Yang L: Dual gene
transfer of bFGF and PDGF in a single plasmid for the treatment of
myocardial infarction. Exp Ther Med. 7:691–696. 2014.PubMed/NCBI
|
|
4
|
Liu Y, Kong J, Chen BH and Hu YG: Combined
expression of CTGF and tissue inhibitor of metalloprotease-1
promotes synthesis of proteoglycan and collagen type II in rhesus
monkey lumbar intervertebral disc cells in vitro. Chin Med J
(Engl). 123:2082–2087. 2010.PubMed/NCBI
|
|
5
|
Jiang F, Yue B, Ma XX, Zhang GQ, Hu YG and
Chen BH: Construction and detection of lentiviral plasmids
containing human transforming growth factor beta 3, connective
tissue growth factor and tissue inhibitor of metalloproteinases 1
gene. Zhongguo Zuzhi Gongcheng Yanjiu Yu Linchuang Kangfu.
16:699–703. 2012.(In Chinese).
|
|
6
|
Sobajima S, Kompel JF, Kim JS, Wallach CJ,
Robertson DD, Vogt MT, Kang JD and Gilbertson LG: A slowly
progressive and reproducible animal model of intervertebral disc
degeneration characterized by MRI, X-ray and histology. Spine
(Phila Pa 1976). 30:15–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masuda K, Aota Y, Muehleman C, Imai Y,
Okuma M, Thonar EJ, Andersson GB and An HS: A novel rabbit model of
mild, reproducible disc degeneration by an anulus needle puncture:
Correlation between the degree of disc injury and radiological and
histological appearances of disc degeneration. Spine (Phila Pa
1976). 30:5–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsirimonaki E, Fedonidis C, Pneumaticos
SG, Tragas AA, Michalopoulos I and Mangoura D: PKCε signalling
activates ERK1/2, and regulates aggrecan, ADAMTS5, and miR377 gene
expression in human nucleus pulposus cells. PLoS One. 8:e820452013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang H, Wu J, Liu J, Ebraheim M, Castillo
S, Liu X, Tang T and Ebrahein NA: Transplanted mesenchymal stem
cells with pure fibrinous gelatin-transforming growth factor-beta1
decrease rabbit intervertebral disc degeneration. Spine J.
10:802–810. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Antoniou J, Steffen T, Nelson F,
Winterbottom N, Hollander AP, Polle RA, Aebi M and Alini M: The
human lumbar intervertebral disc: Evidence for changes in the
biosynthesis and denaturation of the extracellular matrix with
growth, maturation, ageing, and degeneration. J Clin Invest.
98:996–1003. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leung VY, Chan D and Cheung KM:
Regeneration of intervertebral disc by mesenchymal stem cells:
Potentials, limitations, and future direction. Eur Spine J.
15:406–413. 2006. View Article : Google Scholar
|
|
12
|
Pearce RH, Grimmer BJ and Adams ME:
Degeneration and the chemical composition of the human lumbar
intervertebral disc. J Orthop Res. 5:198–205. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
ten Dijke P, Hansen P, Iwata KK, Pieler C
and Foulkes JG: Identification of another member of the
transforming growth factor type beta gene family. Proc Natl Acad
Sci USA. 85:4715–4719. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Risbud MV, Di Martino A, Guttapalli A,
Seghatoleslami R, Denaro V, Vaccaro AR, Albert TJ and Shapiro IM:
Toward an optimum system for intervertebral disc organ culture:
TGF-beta 3 enhances nucleus pulposus and anulus fibrosus survival
and function through modulation of TGF-beta-R expression and ERK
signaling. Spine (Phila Pa 1976). 31:884–890. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakanishi T, Nishida T, Shimo T, Kobayashi
K, Kubo T, Tamatani T, Tezuka K and Takigawa M: Effects of
CTGF/Hcs24, a product of a hypertrophic chondrocyte-specific gene,
on the proliferation and differentiation of chondrocytes in
culture. Endocrinology. 141:264–273. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McLennan SV, Wang XY, Moreno V, Yue DK and
Twigg SM: Connective tissue growth factor mediates high glucose
effects on matrix degradation through tissue inhibitor of matrix
metalloproteinase type 1: Implications for diabetic nephropathy.
Endocrinology. 145:5646–5655. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bachmeier BE, Nerlich A, Mittermaier N,
Weiler C, Lumenta C, Wuertz K and Boos N: Matrix metalloproteinase
expression levels suggest distinct enzyme roles during lumbar disc
herniation and degeneration. Eur Spine J. 18:1573–1586. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang H, Wu J, Liu J, Ebraheim M, Castillo
S, Liu X, Tang T and Ebraheim NA: Transplanted mesenchymal stem
cells with pure fibrinous gelatin-transforming growth factor-beta1
decrease rabbit intervertebral disc degeneration. Spine J.
10:802–810. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao H, Ni CF, Huang J, Zhao SM, Gu WW,
Jiang H, Chen L and Tan TS: Effects of bone cement on
intervertebral disc degeneration. Exp Ther Med. 7:963–969.
2014.PubMed/NCBI
|
|
20
|
Masuda K: Biological repair of the
degenerated intervertebral disc by the injection of growth factors.
Eur Spine J. 17:S441–S451. 2008. View Article : Google Scholar
|
|
21
|
Miyoshi H, Smith KA, Mosier DE, Verma IM
and Toebett BE: Transduction of human CD34+ cells that
mediate long-term engraftment of NOD/SCID mice by HIV vectors.
Science. 283:682–686. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Naldini L, Blomer U, Gallay P, Ory D,
Mulligan R, Gage FH, Verma IM and Frono D: In vivo gene delivery
and stable transduction of nondividing cells by a lentiviral
vector. Science. 272:263–267. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Santoni Sio F and Naldini L: Short-term
culture of human CD34+ cells for lentiviral gene transfer. Methods
Mol Biol. 506:59–70. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zamule SM, Strom SC and Omiecinski CJ:
Preservation of hepatic phenotype in lentiviral-transduced primary
human hepatocytes. Chem Biol Interact. 173:179–186. 2008.
View Article : Google Scholar : PubMed/NCBI
|