Introduction
Acute myocardial infarction is a leading cause of
human mortality worldwide (1–2). In the
clinic, restoration of blood flow is the most effective way to
prevent necrosis of the myocardium. However, myocardial
ischemia-reperfusion (IR) injuries including arrhythmias,
myocardial stunning, and microvascular damage may also occur when
blood flow is restored (1–4).
Two major factors, oxidative stress and inflammatory
response, have vital roles in the mechanisms of necrosis and
apoptosis, and are also involved in the pathogenesis of IR injury
(1–3). Oxidative stress is usually associated
with the formation of reactive oxygen species (ROS), which may
trigger a cytotoxic cascade leading to the activation of various
downstream targets and subsequent inflammation and lipid
peroxidation (2,3). Previous studies have demonstrated that
activation of the phosphoinositide-3-kinase (PI3K)/Akt pathway may
protect against IR-induced myocardial injury and prevent apoptosis
(2,4,5). In
addition to regulating cell survival through the phosphorylation of
various anti-apoptotic proteins, such as Akt and mammalian target
of rapamycin (mTOR), PI3K can also regulate and control several
downstream targets, including B-cell lymphoma (Bcl)-2 and caspases.
Furthermore, the pro-apoptotic protein, P38, which is a member of
the mitogen-activated protein kinase pathway, has a crucial role in
cell growth and survival (6).
Strengthening the antioxidant defenses and inhibiting the apoptotic
pathway may be important for reducing myocardial IR damage.
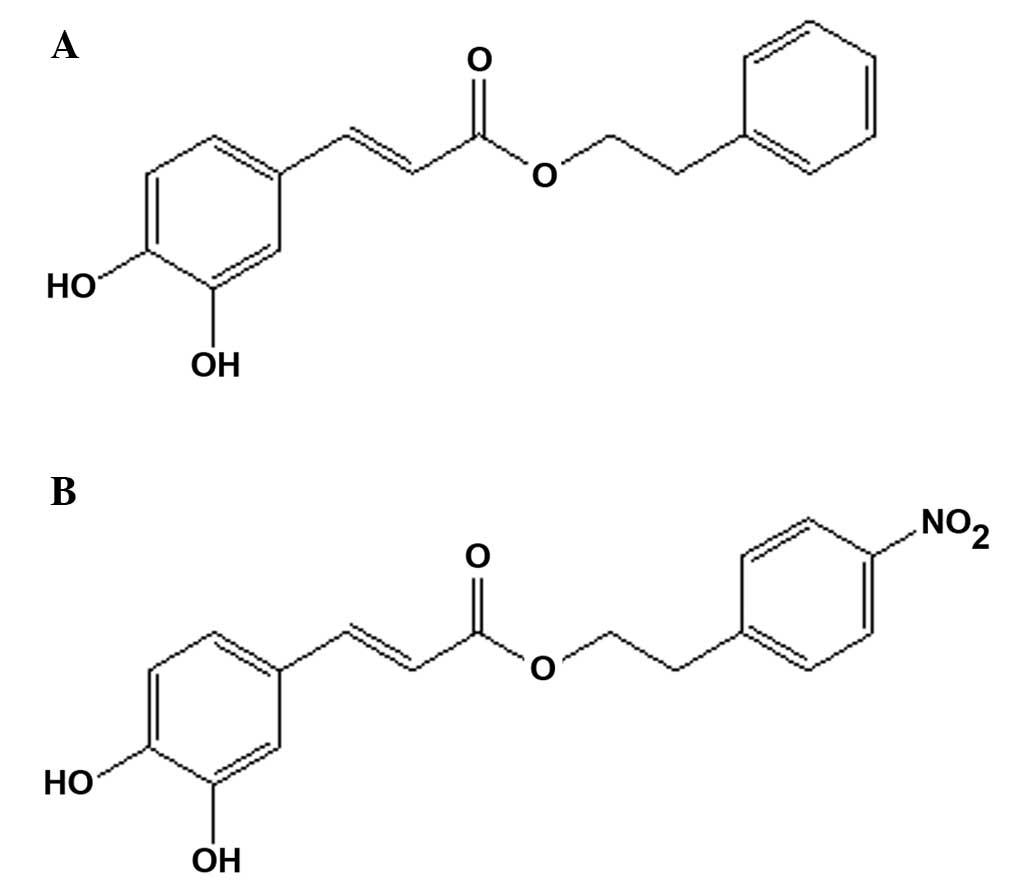
Caffeic acid phenethyl ester (CAPE; Fig. 1A) is a small, flavonoid-like compound
isolated from honey bee propolis, which possesses high
anti-inflammatory, antioxidant, free radical scavenging and
anti-platelet aggregation activity, and has protective effects
against IR injury (1,7,8). The
authors of the present study have previously designed and
synthesized a series of CAPE derivatives (8). Among these derivatives, p-nitro caffeic
acid phenethyl ester (CAPE-NO2; Fig. 1B) possessed the highest activity
levels against platelet aggregation and the highest capability to
increase the white blood cell count, nitric oxide production, and
spleen and thymic indices.
Several therapeutic agents used in the treatment of
cardiac disease, such as nifedipine and imodipine, contain
unsaturated p-nitro and phenyl moieties. In light of the structural
similarities demonstrated between these therapeutic agents and
CAPE-NO2, the authors of the present study hypothesized
that this novel derivative may provide increased protection against
IR-induced myocardial injury in vivo. In the present study,
a rat model of myocardial IR-induced injury was generated in order
to investigate the protective effects and mechanisms of
CAPE-NO2.
Materials and methods
Materials
CAPE and CAPE-NO2 (purity, >99.0%)
were synthesized as previously described (8). 2,3,5-triphenyltetrazolium chloride
(TTC) was purchased from Amresco, LLC (Solon, OH, USA). Diagnostic
assay kits for analyzing creatine kinase (CK), lactate
dehydrogenase (LDH), aspartate transaminase (AST), total superoxide
dismutase (T-SOD), catalase (CAT), glutathione-peroxidase (GSH-Px),
malondialdehyde (MDA) and myeloperoxidase (MPO) were purchased from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Anti-intercellular adhesion molecule (ICAM)-1 (GTX48197-PRO)
antibody was purchased from Dako (Glostrup, Denmark). Antibodies
against PI3K (21890–1-AP), phosphorylated PI3K (p-PI3K;
17894-1-AP), Akt (10176–2-AP), p-Akt (60072–1-lG), mTOR
(10176–2-AP), Bcl-2 (12789–1-AP), Bax (50599–2-lG), cleaved
caspase-3 (25546–1-AP) and P38 (14064–1-AP), and horseradish
peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody
(SA-00001) and Tris-buffered saline were purchased from Proteintech
Group, Inc (Chicago, IL, USA). Anti-p-mTOR (BS-4706) was purchased
from Bioworld Technology, Inc. (St. Louis Park, MN, USA).
Anti-β-actin antibody (AC001-R) and Tween-20 were purchased from
Beijing Dingguo Changsheng Biotechnology Co., Ltd (Beijing, China).
All other chemicals used in the present study were of analytical
grade.
Experimental animals and
treatment
The present study was approved by the Ethics
Committee for Animal Experimentation of Chongqing Medical
University (Chongqing, China). Sprague-Dawley male rats, weighing
260±20g, were purchased from the Experimental Animal Center of
Chongqing Medical University (SCXK(YU)2012-0001). The rats were
maintained under standard conditions of humidity (50%) and
temperature (25±2°C) in a 12 h light and dark cycle. The rats were
fed standard rodent chow, with ad libitum access to water,
and were acclimated for at least one week prior to
experimentation.
The rats were randomly assigned to four groups, with
12 rats in each group: (i) The sham group, rats were intravenously
injected (i.v.) with the vehicle (dimethyl sulfoxide diluted in
0.9% NaCl, v/v=1:104) 10 min prior to and during the sham occlusion
surgery at a dose of 1 ml/kg body weight; (ii) the IR control
group, rats were administered with the vehicle (1 ml/kg body
weight, i.v.) 10 min prior to and during the occlusion; (iii) the
CAPE group, rats were administered with CAPE (10 µg/kg body weight,
i.v.) 10 min prior to and during the occlusion; and (iv) the
CAPE-NO2 group, rats were administered with
CAPE-NO2 (10 µg/kg body weight, i.v.) 10 min prior to
and during the occlusion. CAPE and CAPE-NO2 were
dissolved in the vehicle to a final concentration of 10 µg/ml.
Myocardial IR procedure
The rats were anesthetized with 40 mg/kg sodium
pentobarbital, administered intraperitoneally. Following a
tracheotomy, ventilation was provided using a breathing machine
(respiratory rate, 60 breaths/min; tidal volume, 10–12 ml/kg) and
heart rate was monitored during the procedure using an
electrocardiogram (ECG). The myocardial IR operation was conducted
as described previously (2).
Briefly, the left anterior descending (LAD) coronary artery was
ligated using a 6–0 silk suture. Following this, a medical latex
tube (inner diameter, 1.5 mm) was placed between the ligature and
the LAD. Myocardial ischemia was induced by tightening the ligature
around the latex tube to compress the LAD. Significant ECG changes,
including elevation of the ST segment and widening of the QRS
complex, indicated that the coronary occlusion was successful.
Following 30 min of ischemia, the latex tube was removed in order
to restore the coronary circulation. The sham group underwent the
same procedures, with the exception that the silk suture was left
untied.
Following 2 h reperfusion, all of the rats were
sacrificed using 10% chloral hydrate (0.3–0.4 ml/100 g body weight)
and blood samples were subsequently collected in order to obtain
the serum for further biochemical analysis. The heart tissues were
quickly removed, washed with ice-cold normal saline, blotted dry on
filter paper and weighed. Several sections of the tissues were used
for measuring the infarct size and preparing homogenates; whereas
the remaining tissues were used for immunohistochemical and
histopathological analyses.
Quantification of infarct size
Myocardial infarct size was measured using the TTC
staining method as previously described (3). The rat hearts were maintained at −20°C.
The ventricles were sliced into 5 mm cross sectional rings along
the short axis. The sections were weighed and incubated in 2% TTC
(pH 7.4) for 15 min at 37°C in the dark. The normal tissues were
subsequently stained red, whereas the infarcted tissues remained
unstained (white or pale). The infarcted area was demarcated, and
its size was analyzed using Image-Pro Plus 7.0 software (Media
Cybernetics, Inc., Rockville, MD, USA). As a percentage of left
ventricular mass, infarct size was calculated as the ratio of
infarcted myocardium to the risk region × 100%.
Examination of histopathology
Heart tissue sections (5 mm) were fixed in 10%
buffered formalin for 24 h and subsequently embedded in paraffin.
Following staining with hematoxylin and eosin (Wuhan Goodbio
Technology Co., Ltd., Wuhan, China), the sections were examined
using a Nikon TE2000 light microscope (Nikon Corporation, Tokyo,
Japan).
Preparation of cardiac homogenate
Half of the heart tissues were rinsed in ice-cold
normal saline and homogenized in Tris-HCl buffer (0.01 M, pH 7.4)
at 10,000 rpm at 4°C for 5 min using an electric homogenizer
(Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China) to obtain
10% homogenates. The homogenates were subsequently centrifuged at
10,000 × g at 4°C for 10 min, and the resultant supernatants were
collected in order to measure the levels of CAT, T-SOD, GSH-Px, MPO
and MDA.
Measurement of cardiac marker enzymes
in the serum
Serum samples were isolated from the blood samples
by centrifugation at 650 × g for 15 min. Serum AST, LDH and CK
levels were measured using spectrophotometric kits, according to
the manufacturer's protocol.
Measurement of the antioxidant system
and lipid peroxidation products
The heart homogenates were analyzed for lipid
peroxidation, by proxy of the MDA content, and the levels of
endogenous anti-peroxidative enzymes, including CAT, T-SOD and
GSH-Px, were measured using spectrophotometric kits according to
the respective manufacturer's protocols.
Measurement of inflammatory
mediators
MPO levels were measured using a spectrophotometric
kit. ICAM-1 expression levels were determined via
immunohistochemical staining, according to the manufacturer's
protocol.
Immunohistochemical staining
The formalin-fixed, paraffin-embedded heart tissue
sections (5 mm) were de-paraffinized and subsequently rehydrated.
Endogenous peroxidase activity was blocked using 3%
H2O2, and following microwave antigen
retrieval using 1 mM EDTA-Tris bugger (pH 9.0; Wuhan Goodbio
Technology Co., Ltd.), the sections were incubated with 10% normal
goat serum (Wuhan Boster Biotechnology Co., Ltd., Wuhan, China),
and then overnight at 4°C with rabbit anti-ICAM-1 antibody. The
samples were subsequently washed and HRP-conjugated goat
anti-rabbit IgG (1:5,000; SH-0032; Beijing Dingguo Changsheng
Biotechnology Co., Ltd.) secondary antibodies were applied at 37°C
for 50 min. Finally, diaminobenzidine was used for color
development, and counterstaining was performed using hematoxylin.
Images were obtained using a light microscope (magnification, 100x;
Nikon Corporation) and were subsequently assessed by densitometry
using Image-Pro Plus 6.0 software (Media Cybernetics, Inc.). The
calculated densities correlate with the expression levels of the
protein.
Western blot analysis
Myocardium tissues were homogenized using RIPA lysis
buffer (Nanjing Jiancheng Institute of Biotechnology) at 4°C for 30
min, centrifuged at 12,000 × g for 10 min and the supernatant was
subsequently removed. Proteins were quantified using the BCA kit
(Beyotime Institute of Biotechnology, Haimen, China) and were
subsequently separated by 12% sodium dodecyl sulfate polyacrylamide
gel electrophoresis and transferred to polyvinylidene difluoride
membranes (Nanjing Jiancheng Institute of Biotechnology). Following
this, the membranes were blocked with 5% non-fat dried milk and
incubated with primary antibodies against Akt:1,000), p-Akt
(1:1,000), PI3K (1:500), p-PI3K (1:500), mTOR (1:500), p-mTOR
(1:500), Bcl-2 (1:1,000), Bax (1:500), cleaved caspase-3 (1:500)
and P38 (1:500) and β-actin (1:2,000) overnight at 4°C.
Subsequently, the membranes were washed three times with
Tris-buffered saline with Tween-20 for 10 min, incubated with
HRP-conjugated goat anti-rabbit secondary antibody (1:5,000) for 2
h at room temperature, and washed again. The protein bands were
developed using a chemiluminescent system and subsequently
quantified via densitometric analysis using Quantity One (both
Bio-Rad Laboratories Inc., Hercules, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical significance was determined using one-way analysis of
variance followed by least significant difference multiple
comparison tests using SPSS 18.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Reduction of the myocardial infarct
size
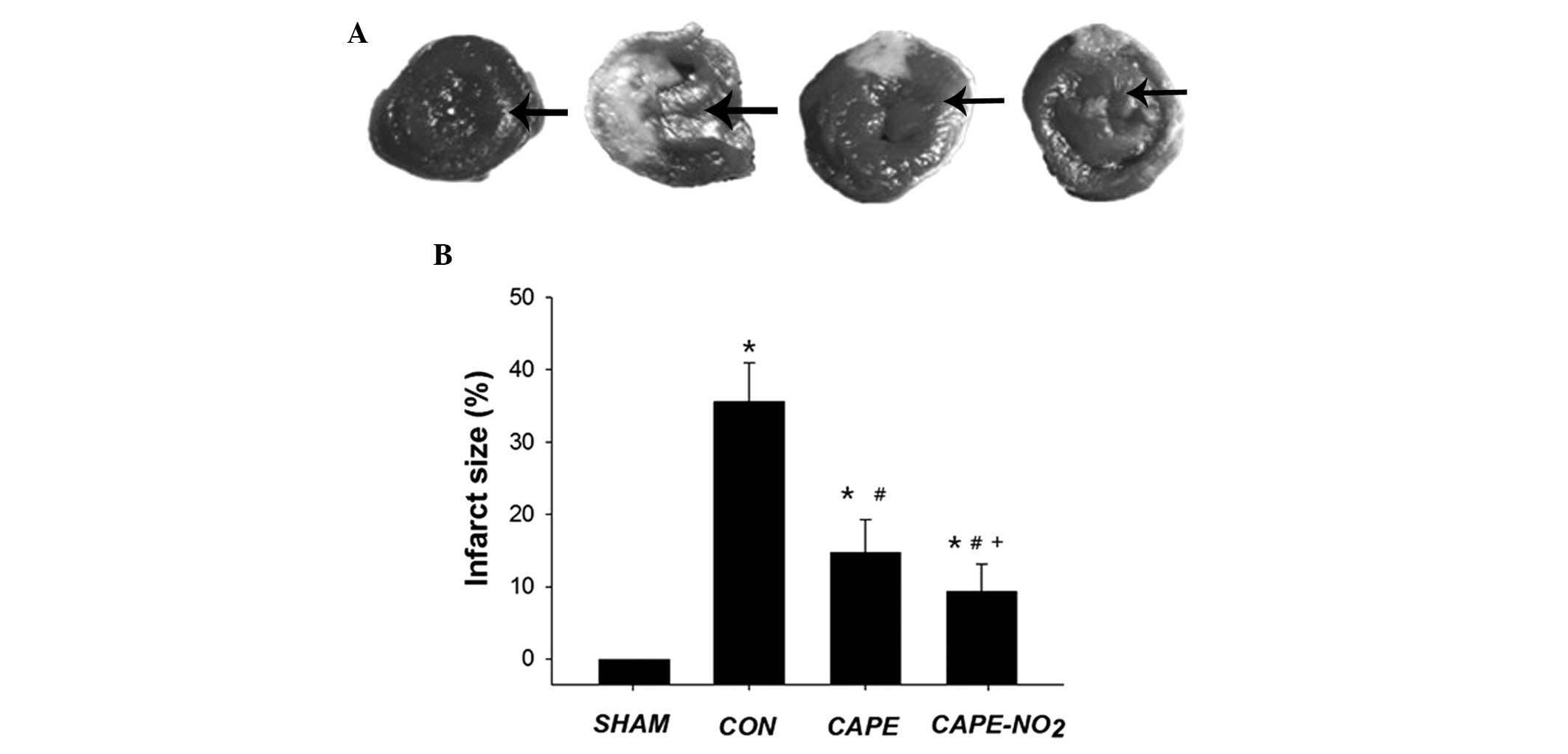
As outlined in Fig. 2A
and B, minimal myocardial tissues with a risk of infarction
were detected in the sham group (infarct size, 1.1±0.4%); whereas
IR resulted in myocardial infarction with a mean infarct size of
35.65±5.4%. Treatment with CAPE and CAPE-NO2 markedly
decreased the infarct size to 14.7±3.14% and 10.32±3.8%,
respectively. These results indicate that CAPE-NO2 may
significantly increase (P<0.05) protection against myocardial IR
injury, as compared with CAPE.
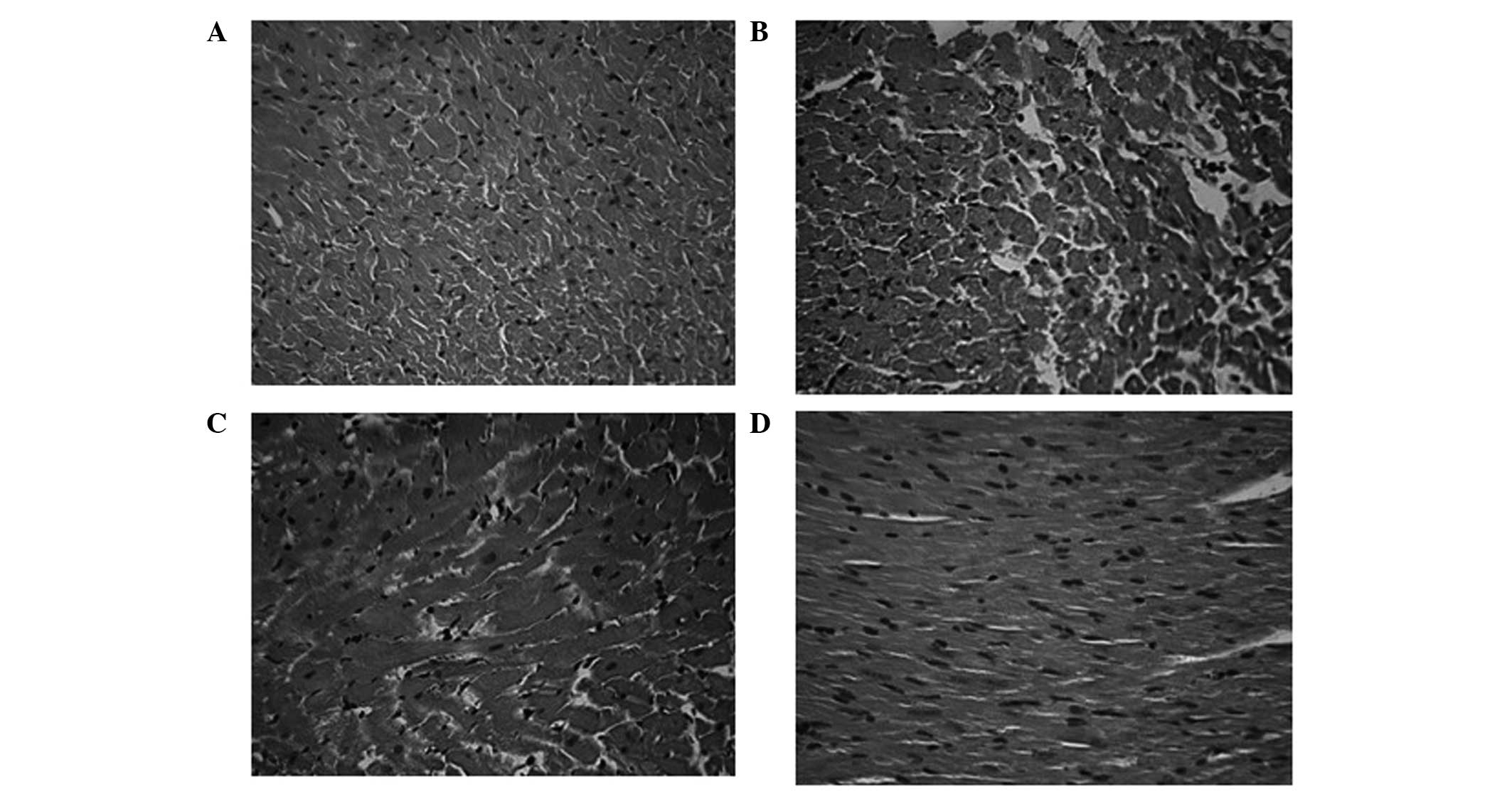
Myocardial necrosis following IR
The effects of CAPE and CAPE-NO2
administration on myocardial histopathological damage were
observed. In the sham group (Fig.
3A), the cardiac muscle fibers were relatively uniform, with
little inflammatory infiltration, edema or cardiac necrosis. In the
IR control group (Fig. 3B), ischemia
resulted in histological changes in cardiac morphology, including
massive cell necrosis and the loss of cardiomyocyte architecture on
the left ventricular wall. Treatment with CAPE-NO2
(Fig. 3C) and CAPE (Fig. 3D) markedly alleviated this
histopathological damage.
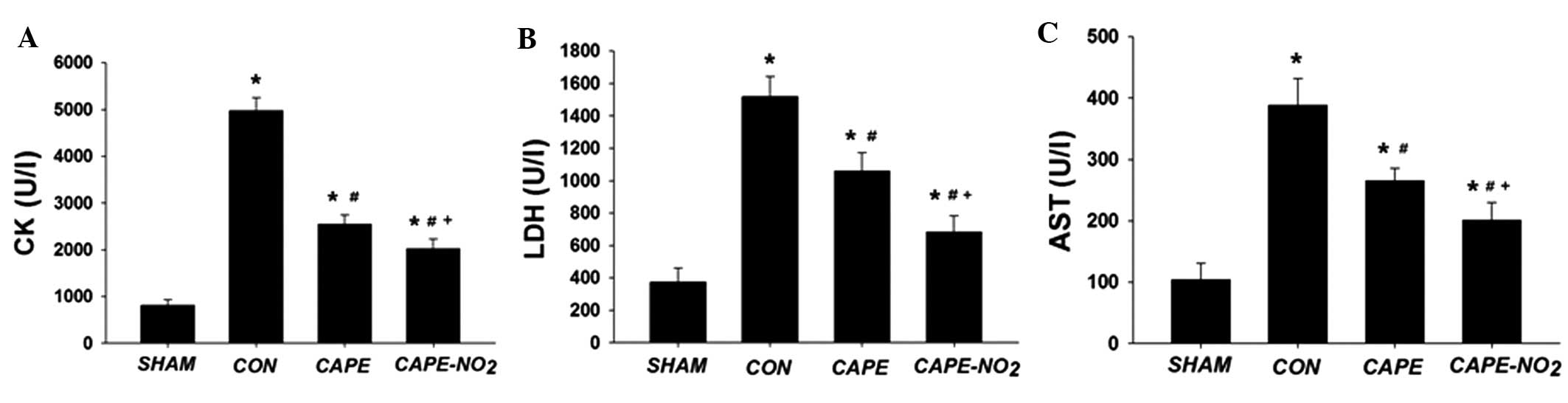
Determination of cardiac marker enzyme
activity levels
As outlined in Fig.
4A–C, the activities of CK, LDH and AST were significantly
increased in the IR control group, as compared with the sham group.
Pretreatment with CAPE and CAPE-NO2 significantly
inhibited the elevation of CK (by 48.6 and 59.3%, respectively),
LDH (by 30.2 and 55.1%, respectively) and AST activity levels (by
31.2 and 48.1%, respectively) (P<0.05 for all differences).
Furthermore, CAPE-NO2 exhibited a significantly superior
protective effect in reducing the activities of CK, LDH and AST
(P<0.05), as compared with CAPE.
Effects of CAPE-NO2 on the
antioxidant system and lipid peroxidation
Ischemia resulted in a significant decrease in CAT,
T-SOD and GSH-Px activities, and an increase in the levels of MDA,
as compared with the sham group (Fig.
5A–D; P<0.05). Conversely, pretreatment with CAPE and
CAPE-NO2, significantly ameliorated these alterations
(P<0.05). Furthermore, treatment with CAPE-NO2
demonstrated greater antioxidant effects, as compared with
CAPE.
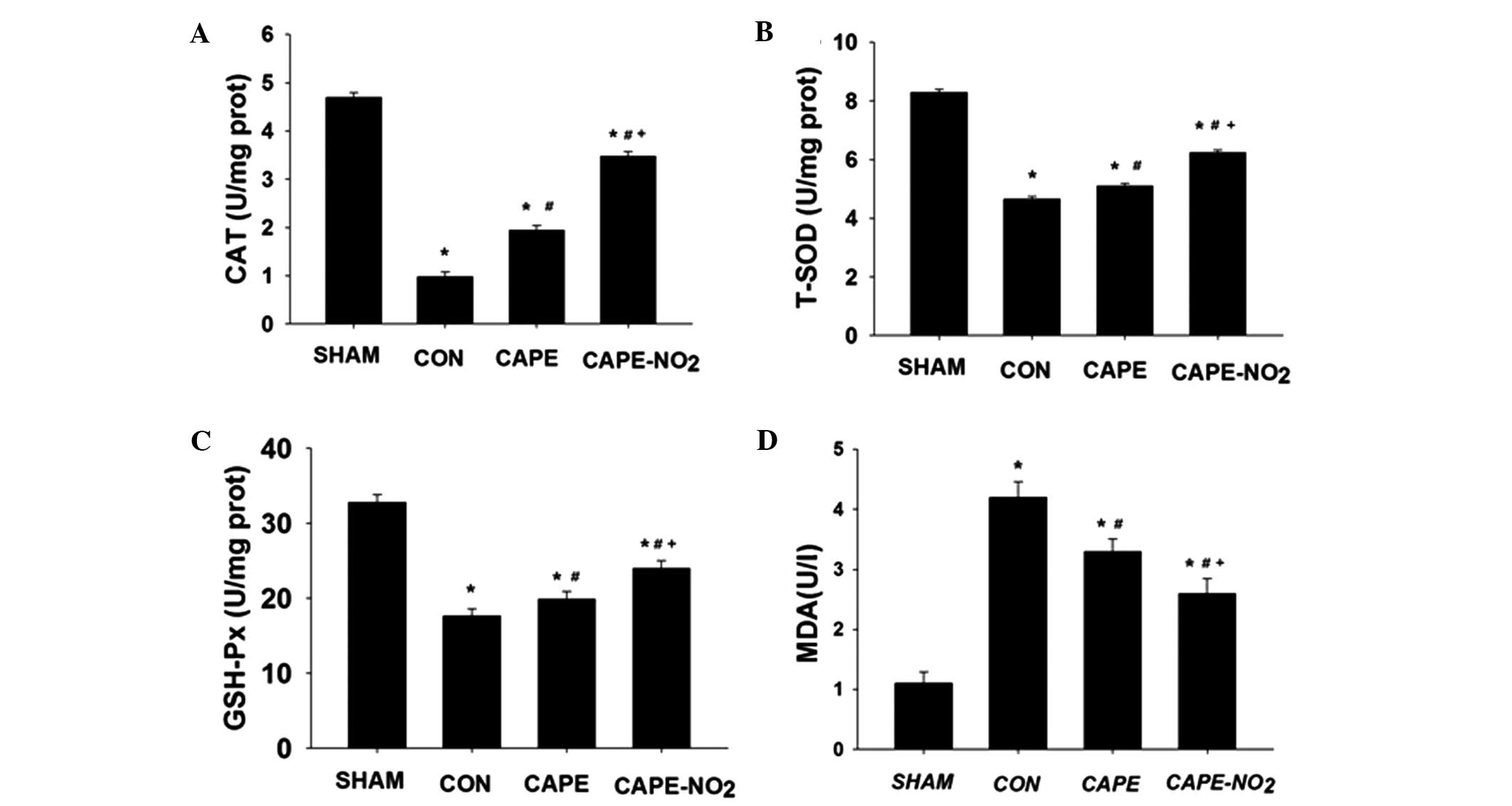 | Figure 5.CAPE and CAPE-NO2 enhanced
the levels of antioxidant enzymes, including (A) CAT, (B) T-SOD,
(C) GSH-Px levels and reduced (D) MDA levels in the various groups.
Data are presented as the mean ± standard deviation (n=12).
*P<0.05 vs. the sham group; #P<0.05 vs. the CON
group; +P<0.05 vs. the CAPE group. CAT, catalase;
T-SOD, total superoxide dismutase; GSH-Px, glutathione-peroxidase;
MDA, malondialdehyde; CON, ischemia-reperfusion control group;
CAPE, caffeic acid phenethyl ester; CAPE-NO2, p-nitro
caffeic acid phenethyl ester. |
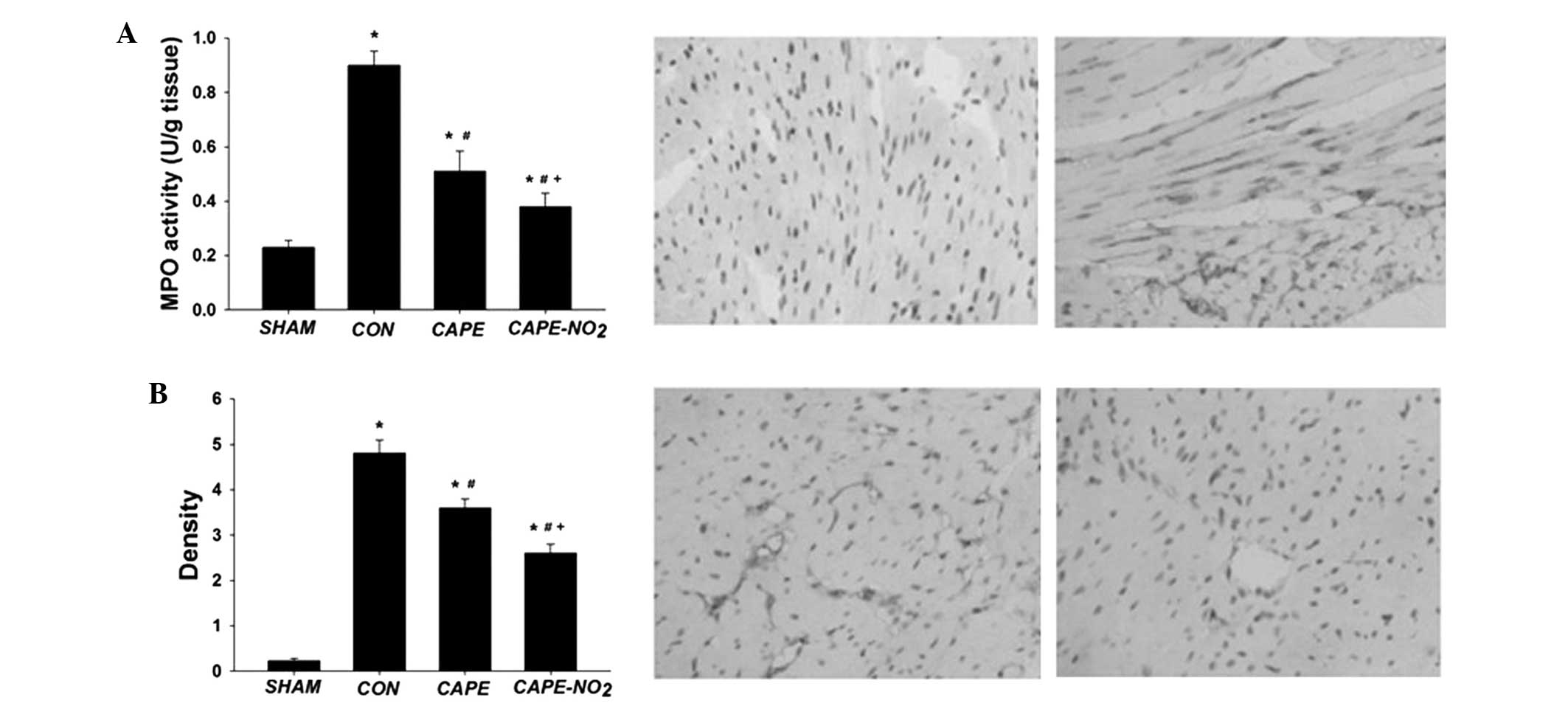
Anti-inflammatory effects
The expression levels of MPO and ICAM-1 were
significantly upregulated in the IR control group, whereas
downregulation was demonstrated in the CAPE and CAPE-NO2
groups (MPO by 43.9 and 57.1%, respectively, and ICAM-1 by 23.56
and 43.4%, respectively; P<0.05) (Fig. 6A and B). These results suggest that
CAPE-NO2 may possess stronger anti-inflammatory activity
as compared with CAPE.
Protein expression levels in cardiac
tissue
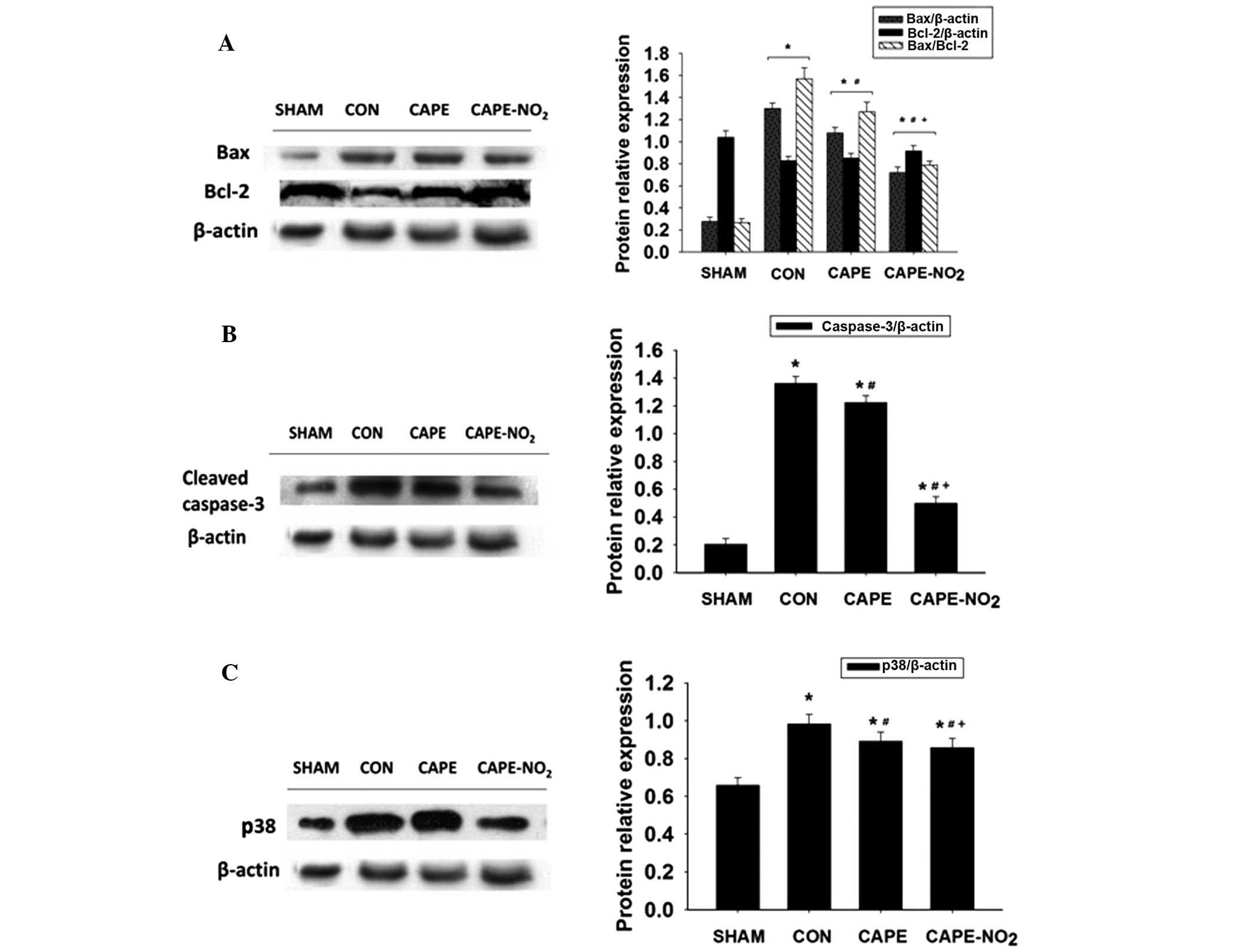
Increased expression levels of Bax,
cleaved-caspase-3 and P38 protein and decreased expression levels
of Bcl-2 were demonstrated in the IR control group, as compared
with the sham group (Fig. 7A–C;
P<0.01). Furthermore, upon CAPE and CAPE-NO2
pretreatment, decreased expression levels of Bax, cleaved-caspase-3
and P38 (P<0.01) and an increase in the expression levels of
Bcl-2 (P<0.01) were demonstrated, as compared with the IR group.
In addition, calculation of the relative ratios of Bax to Bcl-2
(Fig. 7A) demonstrated that
Bax/Bcl-2 in the IR group was significantly increased, as compared
with the sham group. The Bax/Bcl-2 ratios in the CAPE and
CAPE-NO2 groups were markedly lower than those in the IR
group.
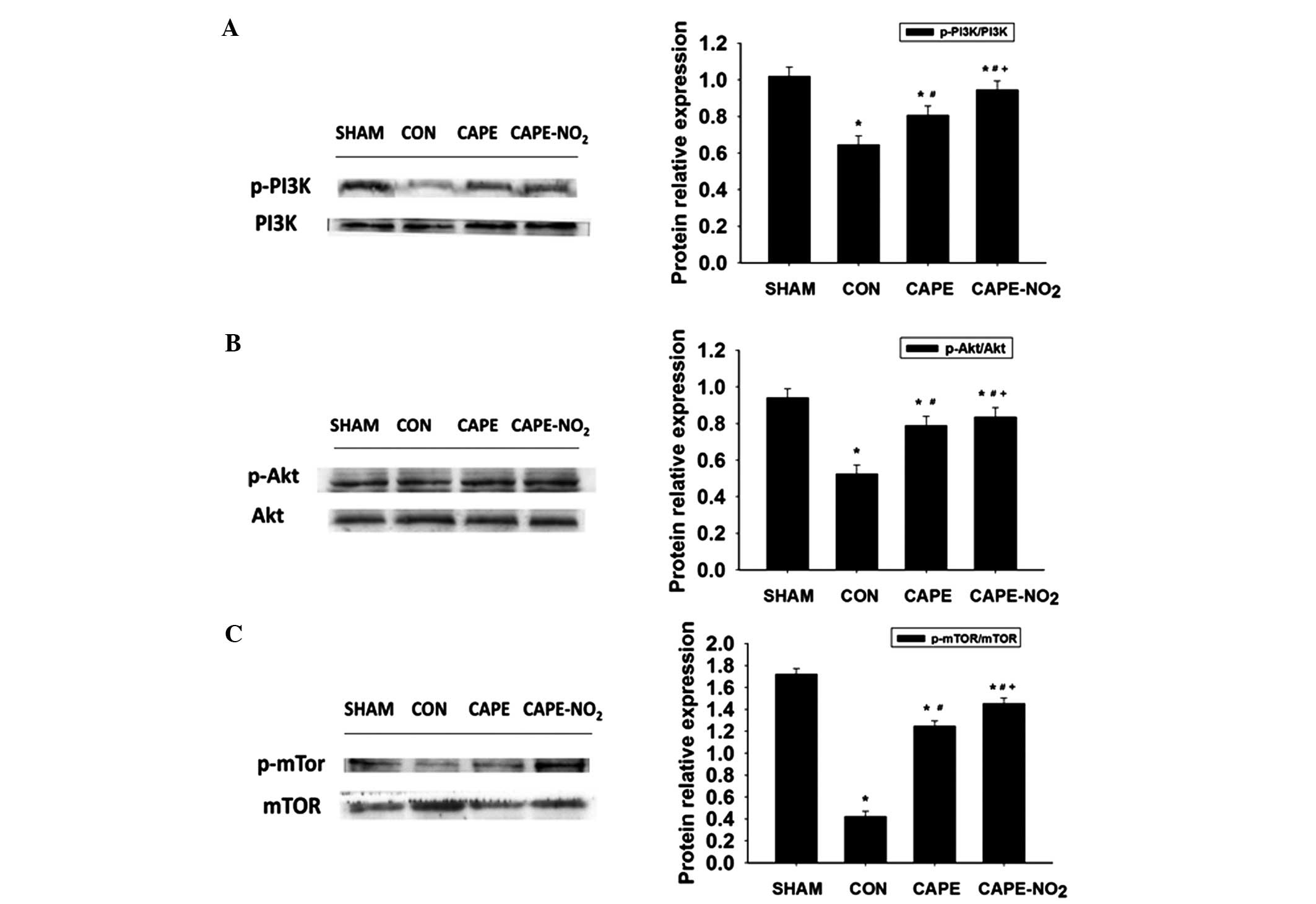
In the IR control group, the protein expression
levels of p-PI3K, p-Akt and p-mTOR were significantly decreased
(P<0.01), as compared with the sham group (Fig. 8A–C). Furthermore, the administration
of CAPE and CAPE-NO2 significantly increased p-PI3K,
p-Akt and p-mTOR expression levels, as compared with the IR group
(P<0.01).
Discussion
The present study demonstrated that
CAPE-NO2 exerted a protective effect on a rat model of
acute myocardial IR injury as indicated by: Significant
histopathological alterations, infarct size reductions, restoration
of antioxidant enzyme activity levels, amelioration of inflammation
and inhibition of apoptosis, as compared with the IR group.
CK, LDH and AST are three myocardial enzymes that
are widely used for ascertaining the degree of myocardial injury,
and their respective activities were significantly increased in the
myocardium of the rats following IR. Pretreatment with
CAPE-NO2 significantly inhibited the elevation of CK,
LDH and AST levels, which suggests that CAPE-NO2 may be
useful as a protective treatment for myocardial IR.
Myocardial infarct size is the gold standard for
evaluating the cardioprotective capability of a therapeutic agent
(9). As hypothesized,
CAPE-NO2 was able to abolish the increase in infarct
size demonstrated in the IR group. Furthermore, the results of the
present study indicated that CAPE-NO2 further increased
myocardial survival and protected cardiac function following
myocardial ischemia, as compared with the CAPE group.
Myocardial IR induces severe cardiac damage via
necrotic and apoptotic myocardial injury. In addition, oxidative
stress resulting from the metabolism of ROS has an integral role in
myocardial injury (10). Free
radicals are capable of altering the structural and functional
integrity of cells through various mechanisms, including lipid
peroxidation, proteolysis, and the shearing of nuclear material. A
dynamic relationship exists between ROS and antioxidants in the
human body (11); however, during
myocardial IR, this balance is challenged by the increased demand
placed upon the antioxidant defense system (2). Practical therapeutic strategies that
may prevent IR-induced myocardial injury include inhibiting the
propagation of the oxidative chain reaction and directly decreasing
the levels of free radicals (1–3).
Furthermore, flavonoid antioxidants are able to protect against
oxidative stress and may potentially be used to treat
cardiovascular disease (3). As a
flavonoid-like compound, CAPE-NO2 has yet to be studied
with regards to its antioxidant activity. The present study
demonstrated that IR-induced myocardial injury is associated with
increased oxidative stress, as indicated by the depletion of
endogenous myocardial antioxidants, including CAT, SOD and GSH-Px.
Conversely, IR injury-induced ROS were directly scavenged by
CAPE-NO2, thereby preventing lipid peroxidation via MDA
and helping to maintain membrane integrity. This effect was further
supported by the decrease in CK, LDH and AST expression levels
demonstrated in the CAPE-NO2 group. Similarly,
CAPE-NO2 effectively blocked the increase in MDA levels,
and increased the cardiac activity levels of CAT, SOD and
GSH-Px.
Inflammation is a vital pathological mechanism which
underlies the propagation of IR-induced myocardial injury (12). MPO and ICAM-1 are inflammatory
mediators and markers which are associated with the development of
certain inflammatory diseases. Antioxidants attenuate inflammatory
responses, suggesting a link between oxidative stress and
inflammation (13). In the present
study, pretreatment with CAPE-NO2 successfully
suppressed myocardial damage, MPO activity levels and ICAM-1
expression levels. Furthermore, the suppression of inflammation
induced by CAPE-NO2 was mediated via the downregulation
of MPO and ICAM-1 expression levels. To the best of our knowledge,
these results suggest for the first time that CAPE-NO2
has anti-inflammatory activity in IR-induced myocardial injury.
In addition to the antioxidant and anti-inflammatory
effects of CAPE-NO2 against IR-induced cardiac injury,
increased anti-apoptotic effects were also observed in accordance
with activation of the PI3K/Akt/mTOR signaling pathway, which is a
potent signaling pathway for survival associated with several
diseases, including IR injury (14–16). The
present study demonstrated that the administration of
CAPE-NO2 significantly increased the expression levels
of p-PI3K, p-Akt and p-mTOR in the myocardium. These data suggested
that CAPE-NO2-induced prevention of myocardial IR injury
may depend, at least in part, on the PI3K/Akt/mTOR pathway. Akt
exerts its protective effects through the phosphorylation of
diverse molecular targets, including the Bcl-2 family (17). Previous studies have suggested that
IR may stimulate the apoptosis of cardiomyocytes via the
downregulation of Bcl-2 with a simultaneous increase in Bax
expression levels (2,4). Furthermore, the Bax/Bcl-2 ratio may be
a critical factor in the cellular threshold for apoptosis (18). Cleaved caspase-3 and P38 are two key
mediators of apoptosis and are often used as apoptotic markers
(6). The activity of caspase-3 is
induced by pro-apoptotic Bax and inhibited by anti-apoptotic Bcl-2
(19). The results of the present
study demonstrated that IR increased apoptosis by promoting the
expression of Bax, cleaved-caspase-3 and P38 and inhibiting the
production of Bcl-2 in cardiomyocytes. Furthermore, CAPE and
CAPE-NO2 attenuated IR-induced myocardial apoptosis by
simultaneously downregulating Bax, cleaved caspase-3 and P38, and
upregulating Bcl-2 in the cardiomyocytes.
In conclusion, the present in vivo study
demonstrated the effectiveness of CAPE-NO2 in
attenuating IR-induced myocardial injury, possibly through a
reduction in oxidative stress, inflammation and apoptotic cell
death, which is in agreement with its potential antioxidant
activity. The present study suggests that CAPE-NO2 may
be an alternative treatment for oxidation-associated heart
diseases.
Acknowledgements
The authors of the present study would like to thank
the National Natural Science Foundation of China (grant no.
21002081) and the Key Program Projects of the Municipal Natural
Science Foundation of Chongqing, China (grant no. CSTC2008AA1001)
for financial support.
References
|
1
|
Parlakpinar H, Sahna E, Acet A, Mizrak B
and Polat A: Protective effect of caffeic acid phenethyl ester
(CAPE) on myocardial ischemia-reperfusion-induced apoptotic cell
death. Toxicology. 209:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu H, Guo X, Chu Y and Lu S: Heart
protective effects and mechanism of quercetin preconditioning on
anti-myocardial ischemia reperfusion (IR) injuries in rats. Gene.
545:149–155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei B, Li WW, Ji J, Hu QH and Ji H: The
cardioprotective effect of sodium tanshinone IIA sulfonate and the
optimizing of therapeutic time window in myocardial
ischemia/reperfusion injury in rats. Atherosclerosis. 235:318–327.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yin Y, Guan Y, Duan J, Wei G, Zhu Y, Quan
W, Guo C, Zhou D, Wang Y, Xi M and Wen A: Cardioprotective effect
of Danshensu against myocardial ischemia/reperfusion injury and
inhibits apoptosis of H9c2 cardiomyocytes via Akt and ERK1/2
phosphorylation. Eur J Pharmacol. 699:219–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quan W, Wu B, Bai Y, Zhang X, Yin J, Xi M,
Guan Y, Shao Q, Chen Y, Wu Q and Wen A: Magnesium lithospermate B
improves myocardial function and prevents simulated
ischemia/reperfusion injury-induced H9c2 cardiomyocytes apoptosis
through Akt-dependent pathway. J Ethnopharmacol. 151:714–721. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forbes-Hernández TY, Giampieri F,
Gasparrini M, Mazzoni L, Quiles JL, Alvarez-Suarez JM and Battino
M: The effects of bioactive compounds from plant foods on
mitochondrial function: A focus on apoptotic mechanisms. Food Chem
Toxicol. 68:154–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou K, Li X, Du Q, Li D, Hu M, Yang X,
Jiang Q and Li Z: A CAPE analogue as novel antiplatelet agent
efficiently inhibits collagen-induced platelet aggregation.
Pharmazie. 69:615–620. 2014.PubMed/NCBI
|
|
8
|
Liu TL, Li D, Du Q, Li C, Xiaohua L and Li
Z: Novel caffeic acid phenethyl ester (CAPE) analogues as
immunoregulatory agents: Synthesis and SAR study. Lat Am J Pharm.
32:329–334. 2013.
|
|
9
|
Parlakpinar H, Ozer MK and Acet A: Effects
of captopril and angiotensin II receptor blockers (AT1, AT2) on
myocardial ischemia-reperfusion induced infarct size. Cytokine.
56:688–694. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Galang N, Sasaki H and Maulik N: Apoptotic
cell death during ischemia/reperfusion and its attenuation by
antioxidant therapy. Toxicology. 148:111–118. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harrison D, Griendling KK, Landmesser U,
Hornig B and Drexler H: Role of oxidative stress in
atherosclerosis. Am J Cardiol. 91:7A–11A. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang WL, Zhang SM, Tang XX and Liu HZ:
Protective roles of cornuside in acute myocardial ischemia and
reperfusion injury in rats. Phytomedicine. 18:266–271. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Campo GM, Avenoso A, Campo S, Nastasi G,
Traina P, D'Ascola A, Rugolo CA and Calatroni A: The antioxidant
activity of chondroitin-4-sulphate, in carbon tetrachloride-induced
acute hepatitis in mice, involves NF-kappaB and caspase activation.
Br J Pharmacol. 155:945–956. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koh PO: Ferulic acid prevents the cerebral
ischemic injury-induced decrease of Akt and Bad phosphorylation.
Neurosci Lett. 507:156–160. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu M, Feng J, Lucchinetti E, Fischer G,
Xu L, Pedrazzini T, Schaub MC and Zaugg M: Ischemic
postconditioning protects remodeled myocardium via the PI3K-PKB/Akt
reperfusion injury salvage kinase pathway. Cardiovasc Res.
72:152–162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhan JK, Wang YJ, Wang Y, Wang S, Tan P,
Huang W and Liu YS: The mammalian target of rapamycin signalling
pathway is involved in osteoblastic differentiation of vascular
smooth muscle cells. Can J Cardiol. 30:568–575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Chen Y, Wu Y, Ha T and Li C: The
cardioprotection induced by lipopolysaccharide involves
phosphoinositide 3-kinase/Akt and high mobility group box 1
pathways. J Biomed Res. 24:324–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen M, Li B, Zhao X, Zuo H, He X, Li Z,
Liu X and Chen L: Effect of diallyl trisulfide derivatives on the
induction of apoptosis in human prostate cancer PC-3 cells. Mol
Cell Biochem. 363:75–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morkuniene R, Arandarcikaite O and
Borutaite V: Estradiol prevents release of cytochrome c from
mitochondria and inhibits ischemia-induced apoptosis in perfused
heart. Exp Gerontol. 41:704–708. 2006. View Article : Google Scholar : PubMed/NCBI
|