Introduction
Subarachnoid hemorrhage (SAH) is a severe,
life-threatening type of stroke caused by bleeding into the space
surrounding the brain. Up to 50% of all cases of SAH are fatal and
10–15% of stroke victims succumb to the condition prior to hospital
admission. Patients that survive often exhibit neurological or
cognitive impairment. A common and severe complication following
aneurysmal SAH is delayed cerebral ischemia (DCI), which occurs in
~30% of patients surviving the ictus of the hemorrhage. DCI may be
reversible, but in certain cases progresses to a cerebral
infarction, which is associated with an increased risk of severe
disability and mortality (1–3). Therefore, delayed cerebral vasospasm
(DCVS) is a common and potentially fatal complication in patients
that have survived an SAH (4–6).
Cerebral vasospasm is the prolonged, intense vasoconstriction of
the larger conducting arteries in the subarachnoid space, which is
initially surrounded by a clot. During the first few days following
aneurysmal rupture, significant narrowing of the cerebral
vasculature develops gradually. This spasm usually reaches its
maximum level within 1 week of the hemorrhagic event. Vasospasm is
among the leading causes of mortality following an aneurysmal
rupture, along with the effect of the initial hemorrhage and
subsequent bleeding (7,8).
It has been reported that an inflammatory response
may be crucial to the development of DCVS (9). Cerebral vasospasm following an
aneurysmal SAH, which is considered to be caused by sustained
contraction of smooth muscle cells of the major cerebral arteries,
induces cerebral ischemia and affects subsequent mortality and
morbidity (10). Currently, the most
common drug in use for reducing the incidence of DCI and the poor
outcome following an SAH is nimodipine (11). As the effects of nimodipine are
relatively modest, considerable research efforts have focused on
investigating and developing novel drugs for the prevention and
treatment of this complication. Among the suggested therapeutic
options is treatment with statins. Numerous intracellular
signalling transduction pathways are considered to be implicated in
the sustained contraction of smooth muscle cells during cerebral
vasospasm. The critical event in this response is the recruitment
of circulating leukocytes into the inflammatory site (12–14).
Genes associated with inflammation, particularly certain cytokines,
are highly expressed in spastic arteries, which suggests that the
inflammatory response may cause sustained contraction of the
cerebral arteries (4).
Cerebral vasospasm is among the most common and
severe complications of SAH, and exhibits a complex pathogenesis.
The initial processes that occur in SAH involve the activation of
genes associated with angiogenesis, inflammation and extracellular
matrix remodeling (15–17). Tumor necrosis factor (TNF)-α,
interleukin (IL)-1β, IL-6 and nuclear factor (NF)-κB are the
primary contributors to the inflammatory response (18–20).
TNF-α (also known as cachexin or cachectin) is an adipokine that is
involved in systemic inflammation, and is a member of a group of
cytokines that stimulate the acute phase reaction. TNF-α is
produced primarily by activated macrophages, as well as by a number
of other cell types, such as CD4+ lymphocytes, natural
killer cells and neurons.
The primary role of TNF-α is the regulation of
immune cells. TNF-α is an endogenous pyrogen, which can induce
fever, cachexia, apoptotic cell death and inflammation. In
addition, it can inhibit tumorigenesis and viral replication, and
respond to sepsis via IL-1β- and IL-6-producing cells. Furthermore,
TNF-α is able to promote the activation of NF-κB, a heterodimeric
transcription factor that translocates to the nucleus and mediates
the transcription of a vast array of proteins involved in cell
survival and proliferation, anti-apoptotic factors and inflammatory
responses (21–23). These processes increase in the
presence of proangiogenic factors and the expression of
proinflammatory genes. In addition, multiple factors and mechanisms
are considered to be active in the inflammatory response. Recently,
simvastatin and taurine have been demonstrated to exert
anti-inflammatory effects. The anti-inflammatory effects of
simvastatin have been demonstrated in patients with chronic heart
failure (24), while those of
taurine have been shown in a rat model of stroke (25). In the current study, simvastatin was
administered in a rabbit model of SAH to prevent DCVS, and the
underlying molecular biological mechanisms were investigated with
the aim of identifying a potential method for preventing DCVS.
Materials and methods
Animal treatment
In total, 48 New Zealand male white rabbits (weight
range, 1.8–2.2 kg; mean age, 2 months) were randomly assigned to
four groups (n=12 per group), as follows: SAH, SAH + simvastatin,
SAH + taurin and control groups. In the SAH groups, a DCVS model
was established using the double hemorrhage method by injecting
autologous arterial blood into the cisterna magna (12). The SAH + simvastatin group was
administered oral simvastatin (5 mg/kg) daily between days 0–6. The
SAH + taurine group was administered oral taurine (50 mg/kg) daily
between days 0–6. Starch (50 mg/kg) was administered orally to the
animals in the other two groups (control and SAH groups). The
control group mice were not subjected to any other injections or
treatment.
Observation of structure using
histochemistry
The rabbits were anesthetized by intramuscular
injection of xylazine sailaqin (ml/kg; Jilin Huamu Animal Health
Products Co., Ltd, Changchun, China), then rapidly sacrificed. The
spastic vertebrobasilar arteries were rapidly removed for
hematoxylin and eosin staining (Sigma-Aldrich, St. Louis, MO, USA).
The internal diameter and internal diameter / wall thickness of the
basilar artery (BA) were measured.
Immunohistochemistry
Paraffin-embedded artery specimens were cut into
4-µm sections and baked at 65°C for 30 min. The sections were
deparaffinized with xylene, rehydrated, submerged in
ethylenediaminetetraacetic acid (EDTA; pH 8.0), autoclaved for
antigen retrieval, treated with 3% hydrogen peroxide, and then
incubated with 1% fetal bovine serum. Primary goat anti-mouse
polyclonal antibodies against TNF-α (sc-1349; 1:200), IL-1β
(sc-1251; 1:500), IL-6 (sc-1265; 1:200; Santa Cruz Biotechnology,
Inc., La Jolla, CA, USA) and mouse monoclonal NF-κB (N8523; 1:500;
Sigma-Aldrich) were added and the sections were incubated overnight
at 4°C. Next, a horseradish peroxidase-labeled secondary antibody
was applied, and the sections were incubated for 30 min at room
temperature, and then for 5 min at room temperature with
diaminobenzidine. The sections were then counterstained with
hematoxylin and eosin, and mounted with Permount mounting medium
(Yansheng Biotechnology Company, Shanghai, China). Subsequently,
the sections were visualized and photographed using a light
microscope. The degree of immunostaining was scored separately by
two independent investigators and the scores were determined based
on the proportion of positively stained cells (17). The scores from the two investigators
were averaged for further comparison of expression.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
TRIzol was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). Primers were designed using
Premier Primer 5, modified by Oligo 6 (Premier Biosoft
International, Palo Alto, CA, USA) and synthesized by Invitrogen
(Beijing, China). The PrimeScript RT-PCR kit, and SYBR Premix ExTaq
(Perfect Real-Time) were purchased from Fermentas (Thermo Fisher
Scientific, Waltham, MA, USA). The Stratagene Mx3000P qPCR system
(Agilent Technologies, La Jolla, CA, USA) was used for qPCR, in
order to analyze the TNF-α, IL-1β, IL-6, NF-κB and β-actin
expression levels. Cerebral vasospasm scores of the BA were
collected, and then total RNA was extracted using the TRIzol
reagent and reverse-transcribed into cDNA according to the
instructions provided with the PrimeScript RT-PCR kit. The primers
used were as follows: β-actin forward, 5′-ATCGTGCGGGACATCAA-3′, and
reverse, 5′-AGGAAGGAGGGCTGGAA-3′; TNF-α forward,
5′-AAACCCGCAAGTGGAG-3′, and reverse, 5′-AGAACCTGGGAGTAGATGAG-3′;
IL-1β forward, 5′-GCAGGGTAGGTTTATCGTCTTT-3′, and reverse,
5′-GCAGGGTAGGTTTATCGTCTTT-3′; IL-6 forward,
5′-CTGGCGGAAGTCAATCTG-3′, and reverse, 5′-ATAGTGTCCTAACGCTCATC-3′;
and NF-κB forward, 5′-CCCAGCCATTTGCACACCTCAC-3′, and reverse,
5′-TTCAGAATTGCCCGACCAGTTTTT-3′. The qPCR reaction was performed
using a ROXII kit (Takara Bio, Dalian, China), in a 25-µl reaction
mixture containing the following: Master mix (12.5 µl); ROXII dye
(5 µM; 0.5 µl each), forward and reverse primers (10 nmol; 0.5 µl
each); sample cDNA (1 µl); and MilliQ H2O (10 µl). The
amplification conditions were as follows: For β-actin, 95°C for 5
min, then 40 cycles at 95°C for 30 sec, 56.9°C for 40 sec, and 72°C
for 30 sec; for TNF-α, 95°C for 5 min, then 40 cycles at 95°C for
30 sec, 55°C for 40 sec, and 72°C for 30 sec; for IL-1β, 95°C for 5
min, then 40 cycles at 95°C for 35 sec, 55°C for 40 sec, and 72°C
for 35 sec; for IL-6, 95°C for 5 min, then 40 cycles at 95°C for 40
sec, 53°C for 40 sec, and 72°C for 40 sec; and for NF-κB, 95°C for
5 min, then 40 cycles at 95°C for 30 sec, 55°C for 40 sec, and 72°C
for 30 sec. The automatic dissociation curve conditions were added
for all the samples. The relative mRNA expression levels of the
genes were calculated from the cycle threshold value using the
2−∆∆Cq threshold method for quantification.
Electrophoretic mobility shift assay
(EMSA)
NF-κB promoter was additionally analyzed using an
EMSA kit (Pierce Biotechnology, Inc., Rockford, IL, USA), following
a standard laboratory procedure (19). Briefly, end-labeled double-stranded
oligonucleotide probes for the EMSA were prepared using T4
polynucleotide kinase and adenosine triphosphate γ-32P (Board of
Radiation and Isotope Technology, Hyderabad, India). The
DNA-protein binding was conducted in a 25-µl reaction mixture using
4 µg tissue extract in a binding buffer containing 20 mM HEPES (pH
7.5), 60 mM KCl, 0.2 mM EDTA, 10% glycerol, 1 mM DTT and 0.25 µl
protease inhibition cocktail (all from Sigma-Aldrich). The nuclear
extract from tissues was mixed with all the components, with the
exception of an appropriate end-labeled probe, and incubated for 10
min. For competition experiments, a 50-fold excess of identical
unlabeled probe was added 15 min prior to the addition of the
end-labeled probe. In order to analyze the supershift, a mouse
monoclonal anti-NF-κB antibody (1:500; N8523; Sigma-Aldrich) was
added 15 min prior to the addition of the end-labeled probe.
Subsequently, 50 fM end-labeled probe was added and incubated for
20 min. All incubation steps were performed on ice. Following
incubation, DNA-protein complexes were resolved on a 5%
non-denaturing polyacrylamide gel in 0.5X Tris-boric acid-EDTA
buffer at 4°C and 30 mA. The oligo sequences for the NF-κB promoter
used in the EMSA were 5′-AGTTGAGGGGACTTTCCCAGGC-3′ (forward) and
5′-TGAACTCCCCTGAAAGGGTCCG-3′ (reverse). A double-stranded probe was
constructed by mixing an equal quantity of two oligos in
Tris-HCl-EDTA (pH 8.0), followed by heating at 65°C for 10 min. The
mixture was then gradually cooled to room temperature over 30
min.
Statistical analysis
Statistical analysis was performed using the SPSS
software, version 20.0 (IBM SPSS, Armonk, NY, USA) for t-tests. For
immunohistochemical analysis, an Automatic Analytic System, version
2.0 was used to record the percentage of positive cells and the
Alpha Imager 1220 documentation and analysis system, version 5.50
(both from Emerald Green Biotech Co., Ltd., Hangzhou, China) was
used to measure the integral optical density of NF-κB. P<0.01
was considered to indicate a statistically significant
difference.
Results
Histochemistry
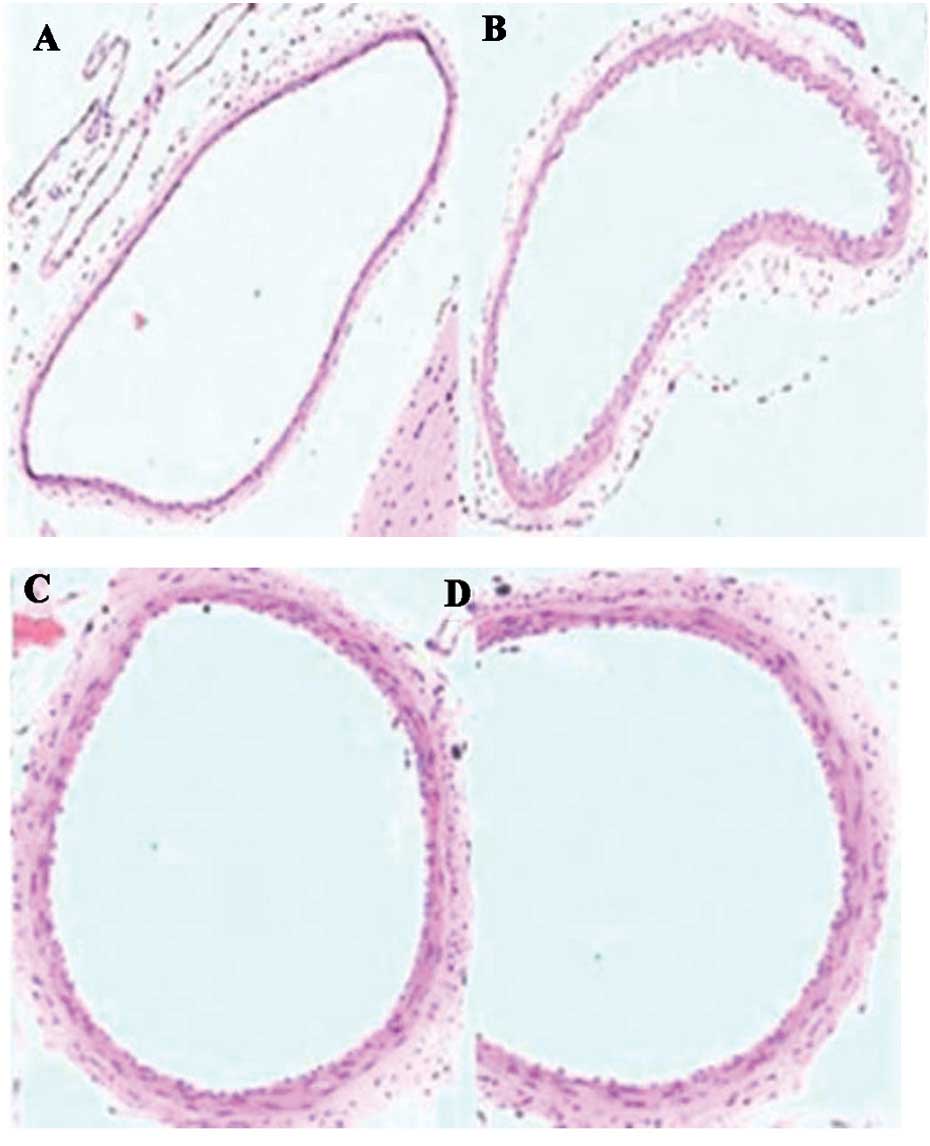
The results of histochemical analysis are presented
in Table I and Fig. 1. The basilar artery (BA) walls in the
SAH + simvastatin and SAH + taurine groups exhibited reduced
narrowing and corrugation of the tunica elastica interna compared
with the SAH group (P<0.05).
 | Table I.Internal diameter and internal D/T of
the basilar artery on day 7 after SAH. |
Table I.
Internal diameter and internal D/T of
the basilar artery on day 7 after SAH.
| Group | Vessel diameter
(µm) | D/T |
|---|
| SAH | 413.8±45.2 | 10.73±4.85 |
| SAH +
simvastatin | 534.6±54.0 | 18.75±4.72 |
| SAH + taurine | 523.6±48.0 | 17.58±4.56 |
| Control | 588.9±58.0 | 25.37±6.46 |
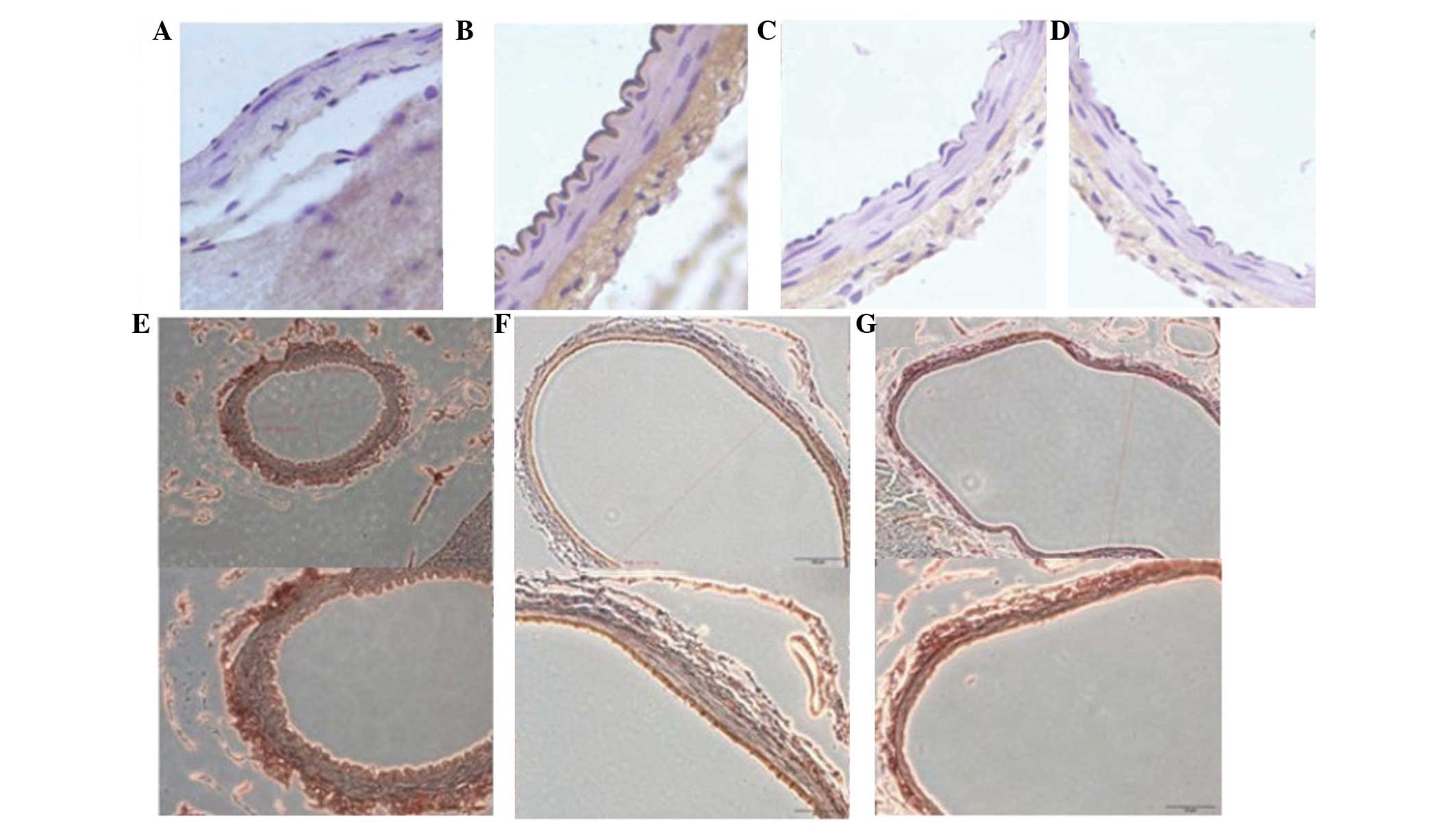
Immunohistochemistry
The expression of TNF-α, IL-1β and IL-6 was detected
using immunohistochemistry after all animals were sacrificed on day
7. The immunohistochemistry results revealed that cerebral
vasospasm of the BA in the SAH + simvastatin and SAH + taurine
groups was alleviated, with reduced expression of TNF-α, IL-1β,
IL-6 and NF-κB, compared with the SAH group (P<0.05; Table II). Furthermore, there was no
statistically significant difference between the SAH + simvastatin
and SAH + taurine groups (P>0.05; Fig. 2).
 | Table II.Protein expression on day 7 after SAH
as determined using immunohistochemistry. |
Table II.
Protein expression on day 7 after SAH
as determined using immunohistochemistry.
| Group | TNF-α | IL-1β | IL-6 | NF-κB |
|---|
| SAH | 0.4109±0.0934 | 0.4396±0.0981 | 0.4020±0.0659 | 0.4430±0.0623 |
| SAH +
simvastatin |
0.2531±0.0723a |
0.2362±0.1139a |
0.2769±0.0872a |
0.2238±0.0953a |
| SAH + taurine |
0.2621±0.0798a |
0.2351±0.1142a |
0.2745±0.0853a |
0.2312±0.0827a |
| Control | 0.2181±0.0870 | 0.1967±0.1056 | 0.2842±0.0528 | 0.2942±0.0645 |
RT-qPCR
The expression levels of TNF-α, IL-1β and IL-6 mRNA
were detected using RT-qPCR after all animals were sacrificed on
day 7. The RT-qPCR results indicated that cerebral vasospasm of the
BA in the SAH + simvastatin and SAH + taurine groups was
alleviated, with reduced expression of TNF-α, IL-1β, IL-6 and NF-κB
compared with the SAH group (P<0.05; Table III). There was no significant
difference between the SAH + simvastatin and SAH + taurine groups.
These results indicated that simvastatin or taurine exhibited an
anti-inflammatory effect in treatment of SAH, and NF-κB was also
involved.
 | Table III.mRNA expression levels on day 7 after
SAH by RT-qPCR. |
Table III.
mRNA expression levels on day 7 after
SAH by RT-qPCR.
| Group | TNF-α | IL-1β | IL-6 | NF-κB |
|---|
| SAH | 0.5431±0.2011 | 0.6324±0.1762 | 0.5002±0.1590 | 0.6542±0.1542 |
| SAH +
simvastatin |
0.3526±0.1745a |
0.3816±0.0908a |
0.3224±0.1837a |
0.3513±0.1845a |
| SAH + taurine |
0.4050±0.1296a |
0.3125±0.1285a |
0.3671±0.1379a |
0.3565±0.1388a |
| Control | 0.3060±0.1672 | 0.3595±0.1261 | 0.2876±0.1535 | 0.2751±0.1452 |
EMSA
The results of the EMSA are presented in Table IV. Following treatment with
simvastatin or taurine, the NF-κB activity was significantly
decreased compared with the SAH group (P<0.01), and the value of
NF-κB activity was comparable to that in the control group
(P>0.05). These results suggest that simavastin and/or taurine
are able to alleviate the SAH via the regulation of NF-κB
activity.
 | Table IV.Activity of NF-κB as determined by
EMSA (IOD). |
Table IV.
Activity of NF-κB as determined by
EMSA (IOD).
| Group | NF-κB |
|---|
| SAH | 16,782±4,095 |
| SAH +
simvastatin |
6,689±1,319a |
| SAH + taurine |
6,796±1,365a |
| Control | 5,927±1,945 |
Discussion
Vasospasm, in which the blood vessels constrict and
restrict blood flow, is a severe complication of SAH and may result
in ischemic brain injury (referred to as delayed ischemia) and
permanent brain damage due to insufficient oxygen (26). Vasospasm may be fatal if severe.
Delayed ischemia is characterized by new neurological symptoms and
may be confirmed by transcranial Doppler or cerebral angiography.
Among all the patients admitted with SAH, ~33% exhibit delayed
ischemia, and 50% of these patients experience permanent
neurological damage as a result (26–28). It
is possible to screen for the development of vasospasm using a
transcranial Doppler every 24–48 h. A blood flow velocity of
>120 cm/sec is suggestive of vasospasm (28). The pathogenesis underlying vasospasm
is complicated. The initial processes that lead to SAH involve the
activation of genes involved in angiogenesis, inflammation and
extracellular matrix remodeling. Modern pharmacology has
demonstrated that taurine has sedating, anti-inflammatory and
antioxidative stress properties (29).
Immune regulation and induction of various
inflammatory and growth regulatory genes, including IL-1β, IL-6,
TNF-α and granulocyte-macrophage colony-stimulating factor
(GM-CSF), require the activation of transcription factors, such as
NF-κB, activated transcription factor (ATF-2), c-Fos and cAMP
response element-binding protein (CREB) (30). Previous studies have reported that
taurine treatment significantly reduces the secretion of the
aforementioned proinflammatory cytokines and the expression of the
IL-1β, IL-6, TNF-α, GM-CSF, IL-12 and p40 genes (31,32). At
concentrations of 2.5, 5 and 10 mg/ml, taurine inhibits the
collagen matrix invasion of B16F-10 melanoma cells in a
dose-dependent manner. A previous study demonstrated that taurine
is able to inhibit matrix metalloproteinase production using
zymographic analysis (33).
Furthermore, the study revealed that the nuclear translocation of
p65, p50, c-Rel subunits of NF-κB and other transcription factors,
such as ATF-2, c-Fos and CREB, were inhibited by treatment with
taurine (33). TNF-α, IL-1β, IL-6
and NF-κB are dominant factors in the regulation of inflammation,
and the cytokines IL-6 and TNF-α are produced by various cells,
including adipose tissue (34) and
macrophages (35). Overexpression of
TNF-α occurs in the adipose tissues of obese animals and humans
(36). TNF-α may stimulate
plasminogen activator inhibitor (PAI)-1 secretion in human adipose
tissue fragments, and circulating plasma IL-6 is associated with
PAI-1 plasma levels. Statins have been used in the prevention of
vasospasm, on the basis of the hypothesis that the actions of
statins may upregulate endothelial nitric oxide synthase (eNOS)
(37). Impairment of eNOS,
endothelium-dependent relaxation and cerebrovascular autoregulation
all occur in vasospastic cerebral arteries following SAH. Statins
improve endothelial function and increase the mRNA and protein
expression of eNOS, and enzymatic activity three-fold. The results
of previous animal models of SAH suggest that statins may
ameliorate cerebral vasospasm (38,39). It
has been hypothesized that patients chronically treated with
statins may exhibit a decreased risk of symptomatic vasospasm after
SAH. Rasmussen et al (40)
studied a TNF-α-activated human umbilical vein endothelial cell
model, in which fluvastatin inhibited the activation and release of
the active p50 subunit of NF-κB and inhibited NF-κB activity.
Dysregulation of NF-κB, phosphorylated-extracellular
signal-regulated kinases and Bax may trigger apoptosis, cell cycle
arrest and oxidative stress in vascular endothelium. Taurine
reduces ischemic brain damage by suppressing the inflammation
associated with poly(ADP-ribose) polymerase (PARP) and NF-κB in a
rat model of stroke, and taurine significantly reduced the levels
of TNF-α, IL-1β, inducible NOS and intracellular adhesion
molecule-1 (25). An inflammatory
reaction associated with the PARP and NF-κB-induced expression of
inflammatory mediators may be a mechanism underlying the effects of
taurine against ischemic stroke. Zelvyte et al (41) reported that pravastatin is able to
effectively suppress NF-κB expression in human monocytes. In
addition, numerous studies have indicated that statins may inhibit
the expression of NF-κB (42,43).
In the current study, simvastatin and taurine were
administered to rabbits in an SAH model to evaluate their
preventative and therapeutic effects against DCVS. Furthermore, the
mechanism by which statins reduce DCVS at the molecular level was
investigated in an effort to identify a novel method for the
treatment of DCVS. In conclusion, simvastatin and taurine were able
to reduce DCVS following SAH in the rabbit model, which may be
associated with the anti-inflammatory effect of statins.
Acknowledgements
This study was supported by a grant from the
National Natural Science Fund (no. 81271313).
References
|
1
|
Kerz T, Victor A, Beyer C, Trapp I, Heid F
and Reisch R: A case control study of statin and magnesium
administration in patients after aneurysmal subarachnoid
hemorrhage: Incidence of delayed cerebral ischemia and mortality.
Neurol Res. 30:893–897. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suzuki H, Kanamaru K, Shiba M, Fujimoto M,
Kawakita F, Imanaka-Yoshida K, Yoshida T and Taki W: Tenascin-C is
a possible mediator between initial brain injury and
vasospasm-related and -unrelated delayed cerebral ischemia after
aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl.
120:117–121. 2015.PubMed/NCBI
|
|
3
|
da Costa L, Fisher J, Mikulis DJ,
Tymianski M and Fierstra J: Early identification of brain tissue at
risk for delayed cerebral ischemia after aneurysmal subarachnoid
hemorrhage. Acta Neurochir Suppl. 120:105–109. 2015.PubMed/NCBI
|
|
4
|
Hirashima Y, Endo S, Kato R and Takaku A:
Prevention of cerebrovasospasm following subarachnoid hemorrhage in
rabbits by the platelet-activating factor antagonist, E5880. J
Neurosurg. 84:826–830. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dumont AS, Dumont RJ, Chow MM, Lin CL,
Calisaneller T, Ley KF, Kassell NF and Lee KS: Cerebral vasospasm
after subarachnoid hemorrhage: putative role of inflammation.
Neurosurgery. 53:123–133; discussion 133–125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin CL, Dumont AS, Calisaneller T, Kwan
AL, Hwong SL and Lee KS: Monoclonal antibody against E selectin
attenuates subarachnoid hemorrhage-induced cerebral vasospasm. Surg
Neurol. 64:201–205; discussion 205–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim E, Kim HC, Park SY, Lim YJ, Ro SH, Cho
WS, Jeon YT, Hwang JW and Park HP: Effect of red blood cell
transfusion on unfavorable neurological outcome and symptomatic
vasospasm in patients with cerebral aneurysmal rupture: Old versus
fresh blood. World Neurosurg. Aug 28–2015.(Epub ahead of
print).
|
|
8
|
Nickele C, Muro K, Getch CC, Walker MT and
Bernstein RA: Severe reversible cerebral vasoconstriction syndrome
mimicking aneurysmal rupture and vasospasm. Neurocrit Care.
7:81–85. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Salunke P, Patra DP and Mukherjee KK:
Delayed cerebral vasospasm and systemic inflammatory response
syndrome following intraoperative rupture of cerebral hydatid cyst.
Acta Neurochir (Wien). 156:613–614. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Zheng T, Altura BT and Altura BM:
Antioxidants prevent elevation in [Ca(2+)](i) induced by low
extracellular magnesium in cultured canine cerebral vascular smooth
muscle cells: Possible relationship to Mg(2+) deficiency-induced
vasospasm and stroke. Brain Res Bull. 52:151–154. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heffren J, McIntosh AM and Reiter PD:
Nimodipine for the prevention of cerebral vasospasm after
subarachnoid hemorrhage in 12 children. Pediatr Neurol. 52:356–360.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Munakata A, Ohkuma H and Shimamura N:
Effect of a free radical scavenger, edaravone, on free radical
reactions: Related signal transduction and cerebral vasospasm in
the rabbit subarachnoid hemorrhage model. Acta Neurochir Suppl.
110:17–22. 2011.PubMed/NCBI
|
|
13
|
Nishziawa S: Roles of signal transduction
mechanisms in cerebral vasospasm following subarachnoid hemorrhage:
Overview. Acta Neurochir Suppl. 110:27–30. 2011.PubMed/NCBI
|
|
14
|
Zubkov AY, Nanda A and Zhang JH: Signal
transduction pathways in cerebral vasospasm. Pathophysiology.
9:47–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jośko J, Hendryk S, Jedrzejowska-Szypułka
H, Słowiński J, Gwóźdź B, Lange D, Snietura M, Zwirska-Korczala K
and Jochem J: Cerebral angiogenesis after subarachnoid hemorrhage
(SAH) and endothelin receptor blockage with BQ-123 antagonist in
rats. J Physiol Pharmacol. 52:237–248. 2001.PubMed/NCBI
|
|
16
|
Zhou ML, Zhu L, Wang J, Hang CH and Shi
JX: The inflammation in the gut after experimental subarachnoid
hemorrhage. J Surg Res. 137:103–108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sarrafzadeh A, Copin JC, Bengualid DJ,
Turck N, Vajkoczy P, Bijlenga P, Schaller K and Gasche Y: Matrix
metalloproteinase-9 concentration in the cerebral extracellular
fluid of patients during the acute phase of aneurysmal subarachnoid
hemorrhage. Neurol Res. 34:455–461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Martin R, Hoeth M, Hofer-Warbinek R and
Schmid JA: The transcription factor NF-kappa B and the regulation
of vascular cell function. Arterioscler Thromb Vasc Biol.
20:E83–E88. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song YS, Lee YS and Chan PH: Oxidative
stress transiently decreases the IKK complex (IKKalpha, beta, and
gamma), an upstream component of NF-kappaB signaling, after
transient focal cerebral ischemia in mice. J Cereb Blood Flow
Metab. 25:1301–1311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang CY, Fujimura M, Noshita N, Chang YY
and Chan PH: SOD1 down-regulates NF-kappaB and c-Myc expression in
mice after transient focal cerebral ischemia. J Cereb Blood Flow
Metab. 21:163–173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu W, Guan Y, Zhao G, Fu XJ, Guo TZ, Liu
YT, Ren XL, Wang W, Liu HR and Li YQ: Elevated IL-6 and TNF-α
levels in cerebrospinal fluid of subarachnoid hemorrhage patients.
Mol Neurobiol. Jun 11–2015.(Epub ahead of print). View Article : Google Scholar
|
|
22
|
Young AM, Karri SK, You W and Ogilvy CS:
Specific TNF-alpha inhibition in cerebral aneurysm formation and
subarachnoid hemorrhage. Curr Drug Saf. 7:190–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanafy KA, Grobelny B, Fernandez L, Kurtz
P, Connolly ES, Mayer SA, Schindler C and Badjatia N: Brain
interstitial fluid TNF-alpha after subarachnoid hemorrhage. J
Neurol Sci. 291:69–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pinchuk TV, Fedulaev YN, Khairetdinova GA,
Denisova NN, Chura OV and Logunova IY: Anti-inflammatory effects of
simvastatin in patients with chronic heart failure. Bull Exp Biol
Med. 157:552–554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun M, Zhao Y, Gu Y and Xu C:
Anti-inflammatory mechanism of taurine against ischemic stroke is
related to down-regulation of PARP and NF-κB. Amino Acids.
42:1735–1747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seiyama A, Yoshikawa N and Imamura Y:
Ischemic pretreatment delays ischemic brain vasospasm injury in
gerbils. Adv Exp Med Biol. 812:247–252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang H, Zhang B and Li S, Liang C, Xu K
and Li S: Whole brain CT perfusion combined with CT angiography in
patients with subarachnoid hemorrhage and cerebral vasospasm. Clin
Neurol Neurosurg. 115:2496–2501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suarez JI, Tarr RW and Selman WR:
Aneurysmal subarachnoid hemorrhage. N Engl J Med. 354:387–396.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miyazaki T and Matsuzaki Y: Taurine and
liver diseases: A focus on the heterogeneous protective properties
of taurine. Amino Acids. 46:101–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Juvela S: Nonsteroidal anti-inflammatory
drugs as risk factors for spontaneous intracerebral hemorrhage and
aneurysmal subarachnoid hemorrhage. Stroke. 34:e34–e36. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Joo K, Lee Y, Choi D, Han J, Hong S, Kim
YM and Jung Y: An anti-inflammatory mechanism of taurine conjugated
5-aminosalicylic acid against experimental colitis: Taurine
chloramine potentiates inhibitory effect of 5-aminosalicylic acid
on IL-1beta-mediated NFkappaB activation. Eur J Pharmacol.
618:91–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marcinkiewicz J, Kurnyta M, Biedroń R,
Bobek M, Kontny E and Maśliński W: Anti-inflammatory effects of
taurine derivatives (taurine chloramine, taurine bromamine, and
taurolidine) are mediated by different mechanisms. Adv Exp Med
Biol. 583:481–492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Quinn MR, Barua M, Liu Y and Serban V:
Taurine chloramine inhibits production of inflammatory mediators
and iNOS gene expression in alveolar macrophages; a tale of two
pathways: Part I, NF-kappaB signaling. Adv Exp Med Biol.
526:341–348. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Buechler C, Ullrich H, Aslanidis C, Bared
SM, Lingenhel A, Ritter M and Schmitz G: Lipoprotein (a)
downregulates lysosomal acid lipase and induces interleukin-6 in
human blood monocytes. Biochim Biophys Acta. 1642:25–31. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Coppack SW: Pro-inflammatory cytokines and
adipose tissue. Proc Nutr Soc. 60:349–356. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hotamisligil GS, Arner P, Caro JF,
Atkinson RL and Spiegelman BM: Increased adipose tissue expression
of tumor necrosis factor-alpha in human obesity and insulin
resistance. J Clin Invest. 95:2409–2415. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Asahi M, Huang Z, Thomas S, Yoshimura S,
Sumii T, Mori T, Qiu J, Amin-Hanjani S, Huang PL, Liao JK, et al:
Protective effects of statins involving both eNOS and tPA in focal
cerebral ischemia. J Cereb Blood Flow Metab. 25:722–729. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kramer AH: Statins in the management of
aneurysmal subarachnoid hemorrhage: An overview of animal research,
observational studies, randomized controlled trials and
meta-analyses. Acta Neurochir Suppl. 110:193–201. 2011.PubMed/NCBI
|
|
39
|
Sabri M and Macdonald RL: Statins: A
potential therapeutic addition to treatment for aneurysmal
subarachnoid hemorrhage? World Neurosurg. 73:646–653. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rasmussen LM, Hansen PR, Nabipour MT,
Olesen P, Kristiansen MT and Ledet T: Diverse effects of inhibition
of 3-hydroxy-3-methylglutaryl-CoA reductase on the expression of
VCAM-1 and E-selectin in endothelial cells. Biochem J. 360:363–370.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zelvyte I, Dominaitiene R, Crisby M and
Janciauskiene S: Modulation of inflammatory mediators and PPARgamma
and NFkappaB expression by pravastatin in response to lipoproteins
in human monocytes in vitro. Pharmacol Res. 45:147–154. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kaji H, Kanatani M, Sugimoto T and Chihara
K: Statins modulate the levels of osteoprotegerin/receptor
activator of NFkappaB ligand mRNA in mouse bone-cell cultures. Horm
Metab Res. 37:589–592. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ahn KS, Sethi G and Aggarwal BB: Reversal
of chemoresistance and enhancement of apoptosis by statins through
down-regulation of the NF-kappaB pathway. Biochem Pharmacol.
75:907–913. 2008. View Article : Google Scholar : PubMed/NCBI
|