Introduction
Erythromelalgia (EM) is a rare neurovascular
syndrome characterized by intermittent or continuous erythema, a
burning sensation and an elevated skin temperature in the hands,
feet and, less commonly, in the nose and ears (1). In a previous study, the incidence of EM
was shown to be 0.36 per 100,000 people per year (2). This debilitating disease can greatly
affect the patients' quality of life. Although several therapeutic
options exist, including conservative treatments such as aspirin,
serotonin reuptake inhibitors, tricyclic antidepressants and
anticonvulsants, and invasive approaches including performing a
sympathetic block or sympathectomy, no method is consistently
effective in managing the various symptoms (3). Previous investigations of EM reported
prolonged remission of symptoms with epidural infusions,
intrathecal infusion and sympathetic ganglion block with local
anesthetics (4–6). Polycythemia vera (PV) is a
myeloproliferative disease, which is occasionally accompanied by
cutaneous manifestations, including EM (7). The present study reports the case of a
72-year-old woman with EM secondary to PV who responded well to
patient-controlled epidural analgesia (PCEA) and interferon α-2b
therapy.
Case report
A 72-year-old woman presented at the Department of
Pain Management at the First Hospital of Jilin University
(Changchun, China) on 18th August 2011 with a 1-year history of
continuous, severe pricking pain with redness and swelling of the
bilateral forefeet and toes. The patient experienced high
temperature in the feet and avoided wearing socks and shoes.
Standing exacerbated the symptoms, which were relieved by elevation
or exposure of the lower extremities to cold. The face and
bilateral hands of the patient were ruddy and asymptomatic. The
patient's pertinent medical history included hypertension, heart
disease and cerebral infarction with secondary anomic aphasia 4
months prior to admission to our hospital, while her family history
was noncontributory. The patient began taking aspirin (100 mg/day)
regularly following the cerebral infarction for secondary
prevention of cerebrovascular events with no improvement of the EM
symptoms.
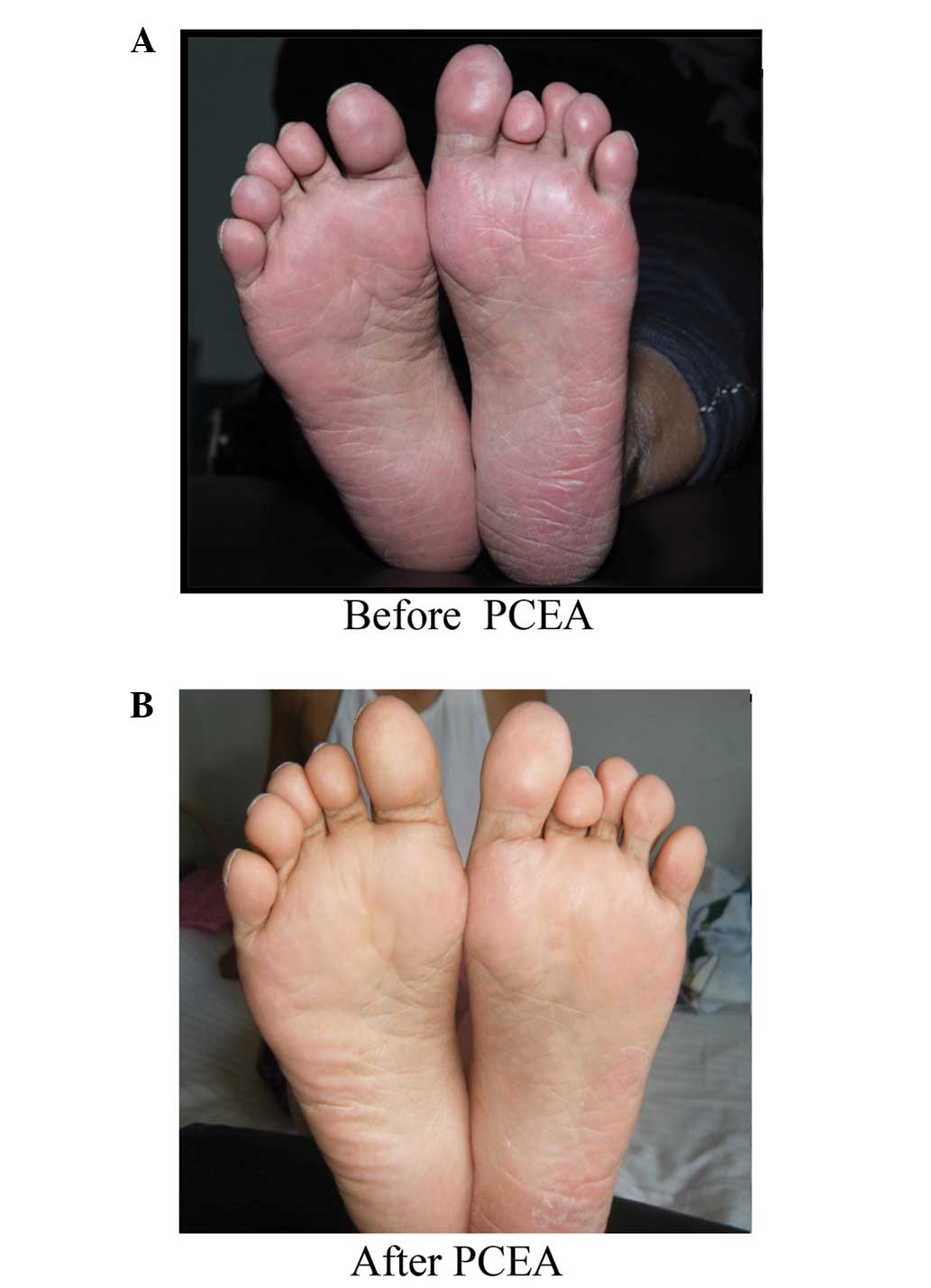
Physical examination revealed moderate bilateral
erythema, edema and an increased skin temperature of the soles of
the feet and toes (Fig. 1A).
Hyperalgesia and allodynia were present in the affected area, with
the toes being more severely affected. The visual analog scale
(VAS) score of the patient was 8 (8). Vascular Doppler investigation of the
right lower extremity and electrocardiography examination revealed
normal findings. A complete blood count (CBC) revealed elevated red
blood cell (RBC) count, hemoglobin (HGB) level and platelet (PLT)
count (Table I).
 | Table I.CBC values during the three hospital
admissions of a patient with erythromelalgia secondary to
polycythemia vera. |
Table I.
CBC values during the three hospital
admissions of a patient with erythromelalgia secondary to
polycythemia vera.
|
| First admission | Second admission | Third admission |
|
|---|
|
|
|
|
|
|
|---|
| CBC parameters | 19/08/2011 | 27/08/2011 | 15/02/2012 | 26/04/2012 | 12/05/2012 | 15/05/2012 | 17/05/2012 | Reference value |
|---|
| RBC
(1012/l) | 5.48 | 4.99 | 6.77 | 7.21 | 6.84 | 7.04 | 6.66 | 3.50–5.00 |
| HGB (g/l) | 165 | 149 | 190 | 189 | 183 | 184 | 174 | 110–150 |
| PLT
(109/l) | 396 | 424 | 315 | 534 | 304 | 268 | 256 | 100–300 |
| WBC
(109/l) | NA | NA | 10.60 | 13.24 | 5.46 | 3.84 | 3.89 |
4.00–10.00 |
A diagnosis of secondary EM was established based on
the patient's clinical manifestations, laboratory data and lack of
family history of this disease. On day 2 following admission, a
lumbar epidural catheter was inserted 4.5 cm cephalad at the L3-4
interspace. The patient was placed on PCEA with 0.2% ropivacaine,
0.2% lidocaine and dexamethasone at 100 µg/ml after a test dose.
The background infusion rate was 2 ml/h with self-administered 5-ml
boluses at intervals of ≤15 min. The patient reported remarkable
pain relief within 10 h. In addition, the abnormal physical signs
were nearly resolved. The PCEA was continued for 1 week with no
complications. After 1 week of treatment, the erythema, edema and
elevated skin temperature were completely resolved (Fig. 1B). Furthermore, the hyperalgesia and
allodynia had nearly disappeared, allowing the patient to walk
unassisted while wearing socks and shoes. After 3 days from the
discontinuation of PCEA, the patient was discharged from the
hospital on 29th August 2011 with a VAS score of 1.
The patient returned on 14th February 2012 with a
10-day history of the same symptoms and signs. A VAS score of 8 was
reported, and certain CBC parameters were found to be increased
(Table I). PCEA (0.2% ropivacaine,
0.1% lidocaine and 5 µg/ml fentanyl) was initiated and continued
for 13 days. Once again, the patient exhibited remarkable
improvement, with a VAS score of 1 at the time of discharge on 28th
February 2012. On 25th April 2012, the patient visited the
Department of Hematology at the First Hospital of Jilin University,
due to repeated abnormal CBC parameters with a constellation of
less severe EM symptoms (VAS score, 5). All the previously measured
CBC parameters were again found to be increased (Table I). A bone marrow biopsy indicated
myeloproliferative disease, and an abdominal ultrasound revealed
mild splenomegaly. Genetic testing revealed positivity for JAK2
V617F and negativity for JAK2 exon 12. The patient was diagnosed
with PV according to criteria outlined by the World Health
Organization (WHO) (9), which
included an elevated level of HGB, the presence of the JAK2V617F
mutation, and the detection of myeloproliferative disease. The
patient was placed on recombinant human interferon α-2b therapy to
treat the PV, which resulted in complete relief of the EM symptoms.
After 2 weeks, the PLT count had returned to the normal levels,
with slight decreases in the RBC count and HGB level (Table I). The patient was then discharged on
17th May 2012 with complete remission of the symptoms and signs. At
the 16-month follow-up, the patient remained asymptomatic with
continuous recombinant interferon treatment. At the 43-month
follow-up, the patient had experienced several episodes of
recurrence following discontinuation of the interferon treatment
due to side effects, including anorexia and precordial distress.
Currently, the patient is not under treatment and suffers from mild
symptoms of EM. Written informed consent was obtained from the
patient.
Discussion
EM is a rare syndrome that has not been as widely
studied as other functional vascular conditions, including
Raynaud's phenomenon and acrocyanosis (10). This syndrome is divided into the
primary EM (PEM) and secondary EM (SEM) types. Gain-of-function
mutations in SCN9A, which encodes the α-subunit of the
voltage-gated sodium channel Nav1.7, have been found to be the main
cause of PEM (11). By contrast, SEM
has been described in association with various disorders, with
essential thrombocythemia (ET) and PV as the first and second most
common causes, respectively (3). In
the patient of the present study, repeated CBC abnormalities
suggested the presence of thrombocythemia and erythrocythemia,
indicating a possible diagnosis of ET and/or PV. It was necessary
to determine which condition contributed to EM in the present case.
However, establishing the correct diagnosis was challenging, since
thrombocythemia can occur as a result of both ET and PV, and serves
as the fundamental cause of EM (7).
Due to limited knowledge of this condition and a lack of equipment
in the Department of Pain Management at our hospital, the diagnosis
of PV was confirmed by a hematologist from the Department of
Hematology, according to the criteria outlined by the WHO (9). The most appropriate diagnosis in the
present case was PV associated with thrombocythemia (PVAT).
Thrombocythemia-induced EM can be considered as a microvascular
ischemic complication of PV. In patients with PVAT, a high
hematocrit and increased whole blood viscosity aggravate the
PLT-mediated microvascular syndrome, resulting in major arterial
complications (7); this may explain
the complicated medical history of the present patient. In the
current case, interferon treatment inhibited the proliferation of
cells, particularly PLTs, more than it affected the RBC and HGB
levels, further illustrating that PLTs play a vital role in PVAT.
Low-dose aspirin (100 mg/day) resulted in no improvement,
indicating that aspirin may not be effective in patients with this
type of SEM. Interferon therapy, which targets the underlying
disease, should be considered as an alternative. In a recent study,
interferon α was associated with a significantly lower incidence of
EM than was hydroxyurea without severe hematological adverse events
in patients with JAK2 V617F-positive PV (12).
According to the shunting hypothesis, EM symptoms
are caused by tissue hypoxia, which is induced by a maldistribution
of skin microvascular blood flow with increased thermoregulatory
flow through arteriovenous shunts and an inadequate nutritive
perfusion to normal skin capillaries (13,14). If
available blood is shunted away from normal skin capillaries, the
skin will be hypoxic. The shunting hypothesis is generally
considered to be the final common pathway for PEM and SEM from a
pathophysiological perspective; however, histopathological
investigations reported that the alterations associated with PEM
were different from those associated with SEM (15–17). In
a prospective study, Kalgaard et al (15) reported histopathological findings
characterized by capillary proliferation or vascular damage in 31
of 49 specimens, mainly from patients with PEM. These nonspecific
alterations indicated the presence of skin hypoxia secondary to
increased arteriovenous shunting and insufficient capillary flow,
which is compatible with the shunting hypothesis supported by Mørk
et al (13,14).
The use of an epidural infusion with local
anesthetics and dexamethasone or fentanyl provided pronounced
symptom relief in the present patient. A possible explanation may
be sympathetic denervation, which improved the blood flow to the
skin at the capillary level, thus reversing the chronic ischemic
status secondary to increased arteriovenous shunting triggered by
hypersensitive PLT-mediated arteriolar thrombosis. This explanation
is supported by successful outcomes of other similarly aggressive
techniques, including intrathecal infusion and sympathetic ganglion
block, the therapeutic effects of which are mediated through
inhibition of the sympathetic nerves (5,6). Further
histological and physiological studies of autonomic nerve function
in patients with SEM are required to illustrate whether SEM is
sympathetically mediated.
In conclusion, the present study reported a typical
case of EM secondary to PV. The patient responded well to PCEA with
infusion of two local anesthetics plus dexamethasone or fentanyl.
No unacceptable side effects developed during the treatment.
Interferon therapy, which inhibits PLT generation, was more
effective than aspirin, which inhibits PLT aggregation. PCEA acts
on certain pathophysiological aspects of EM, while interferon
targets the protopathy; however, PCEA is able to produce long-term
effects after one treatment cycle, while interferon requires
persistent use and may result in certain side effects.
Identification of the optimal modality for the treatment of EM
secondary to PV will require further studies.
References
|
1
|
Mitchell SW: On a rare vaso-motor neurosis
of the extremities and on the maladies with which it may be
confounded. Am J Med Sci. 76:17–36. 1878. View Article : Google Scholar
|
|
2
|
Alhadad A, Wollmer P, Svensson A and
Eriksson KF: Erythromelalgia: Incidence and clinical experience in
a single centre in Sweden. Vasa. 41:43–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen JS: Erythromelalgia: New theories
and new therapies. J Am Acad Dermatol. 43:841–847. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stricker LJ and Green CR: Resolution of
refractory symptoms of secondary erythermalgia with intermittent
epidural bupivacaine. Reg Anesth Pain Med. 26:488–490. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macres S and Richeimer S: Successful
treatment of erythromelalgia with intrathecal hydromorphone and
clonidine. Clin J Pain. 16:310–313. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seishima M, Kanoh H, Izumi T, Niwa M,
Matsuzaki Y, Takasu A, Ban M and Kitajima Y: A refractory case of
secondary erythermalgia successfully treated with lumbar
sympathetic ganglion block. Br J Dermatol. 143:868–872. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Michiels JJ, Berneman Z, Schroyens W,
Koudstaal PJ, Lindemans J, Neumann HA and van Vliet HH:
Platelet-mediated erythromelalgic, cerebral, ocular and coronary
microvascular ischemic and thrombotic manifestations in patients
with essential thrombocythemia and polycythemia vera: A distinct
aspirin-responsive and coumadin-resistant arterial thrombophilia.
Platelets. 17:528–544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McCormack HM, Horne DJL and Sheather S:
Clinical applications of visual analogue scales: A critical review.
Psychol Med. 18:1007–1019. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heidrich H: Functional vascular diseases:
Raynaud's syndrome, acrocyanosis and erythromelalgia. Vasa.
39:33–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tefferi A: Polycythemia vera and essential
thrombocythemia: 2012 update on diagnosis, risk stratification, and
management. Am J Hematol. 87:285–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hisama FM, Dib-Hajj SD and Waxman SG:
SCN9A-related inherited erythromelalgia.
GeneReviews®. Pagon RA, Adam MP, Ardinger HH, et al:
University of Washington. (Seattle, WA). 1993–2015
|
|
12
|
Huang BT, Zeng QC, Zhao WH, Li BS and Chen
RL: Interferon alpha-2b gains high sustained response therapy for
advanced essential thrombocythemia and polycythemia vera with JAK2
V617F positive mutation. Leuk Res. 38:1177–1183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mørk C, Asker CL, Salerud EG and Kvernebo
K: Microvascular arteriovenous shunting is a probable pathogenetic
mechanism in erythromelalgia. J Invest Dermatol. 114:643–646. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mørk C, Kvernebo K, Asker CL and Salerud
EG: Reduced skin capillary density during attacks of
erythromelalgia implies arteriovenous shunting as pathogenetic
mechanism. J Invest Dermatol. 119:949–953. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalgaard OM, Clausen OP, Mellbye OJ, Hovig
T and Kvernebo K: Nonspecific capillary proliferation and
vasculopathy indicate skin hypoxia in erythromelalgia. Arch
Dermatol. 147:309–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Michiels JJ, Berneman Z, Schroyens W and
van Urk H: Aspirin-responsive painful red, blue, black toe, or
finger syndrome in polycythemia vera associated with
thrombocythemia. Ann Hematol. 82:153–159. 2003.PubMed/NCBI
|
|
17
|
Davis MD, Weenig RH, Genebriera J,
Wendelschafer-Crabb G, Kennedy WR and Sandroni P: Histopathologic
findings in primary erythromelalgia are nonspecific: Special
studies show a decrease in small nerve fiber density. J Am Acad
Dermatol. 55:519–522. 2006. View Article : Google Scholar : PubMed/NCBI
|