Introduction
Acute myeloid leukemia (AML) is a heterogeneous
hematologic malignancy, which is characterized by the clonal
expansion of myeloblasts in the peripheral blood, bone marrow and
other types of tissue (1,2). AML is a complex disease and various
chemotherapeutic strategies may be useful for its treatment,
including CAG [a combination of cytarabine (Ara-C), aclarubicin
(Acl) and granulocyte colony-stimulating factor (G-CSF)], for
relapsed and refractory AML (3); HAG
[a combination of homoharringtonine (HHT), Ara-C and G-CSF], for
relapsed or refractory AML and geriatric AML (4); HA (a mixture of HHT and Ara-C) for
elderly patients with AML with relatively low toxicity and
reasonable response rate (5); HAA
(HHT, Ara-C and Acl) for young AML patients (6); IA [a combination of idarubicin (IDA)
and Ara-C], for newly diagnosed AML (7); and all-trans retinoic
acid/arsenic trioxide (ATRA/ATO) for acute promyelocytic leukemia
(8).
AML pathogenesis is complex, involving an
interaction between genetic and epigenetic aberrations (9–12). AML
is caused by various factors, including the accumulated damaging
effects of genetic mutations and aberrant epigenetic modifications
(13). Aberrant promoter methylation
is frequently found in human malignancies including AML (14–16). The
Cancer Genome Atlas has determined that 44% of patients with AML
exhibit gene mutations that regulate genomic DNA methylation
(17). Although the molecular risk
stratification of AML is largely based on genetic markers, DNA
methylation may also have prognostic value (18).
Promoter hypermethylation of tumor suppressor genes
has been recognized as a cause of oncogenesis (19). Identification of specific epigenetic
modifications may explain the complexity and genomic instability of
neoplastic diseases, and provide a basis for targeted therapy
(20). Among these genes, the
hypermethylation of cyclin-dependent kinase inhibitor 2B
(CDKN2B) has been found to be associated with an increased
risk of leukemia (P=0.001; odds ratio = 9.67; 95% confidence
interval = 2.48–37.75) (13). Solute
carrier family 19 member 3 (SLC19A3) has been observed to be
epigenetically downregulated in gastric cancer (21). Furthermore, deleted in lung and
esophageal cancer 1 (DLEC1), as a tumor suppressor gene, may
contribute to tumorigenesis (22).
The present study examined whether chemotherapy
induced alterations in the methylation of CDKN2B,
SLC19A3 and DLEC1 genes, and whether there was a
correlation between the methylation changes and the prognosis of
patients with AML.
Materials and methods
Patients
Bone marrow genomic DNA was obtained from 15
patients with AML recruited from Yuyao People's Hospital (Yuyao,
China) between November 2012 and June 2013. There were 7 male and 8
female patients with a mean age of 51.8±15.8 years (range, 19–76
years), including two M1, seven M2, five M3, and one M4 AML
subtypes. The 2 patients with subtype M1 AML were treated with HAA
and CAG regimens, respectively. The regimens of the 7 patients with
subtype M2 AML included CAG, IA, HAA, AA (Ara-C plus Acla) and DA
[daunorubicin (DNR) plus Ara-C]. Among the 5 patients with subtype
M3 AML three were treated with ATRA accompanied by ATO, DNR, HA or
AD (Ara-C plut dexamethasone), and the regimens of the other two
were IA and HA, respectively. The regimen of the 1 patient with M4
subtype AML comprised a combination of IA, CAG and HHT. The
clinical parameters of the patients with AML are summarized in
Table I.
 | Table I.Clinical parameters of the patients
with AML. |
Table I.
Clinical parameters of the patients
with AML.
| Patient number | Gender | Age (years) | AML subtype | Treatment
regimen |
|---|
| 1 | Male | 23 | M3 | ATRA/ATO |
| 2 | Male | 40 | M3 | DNR + ATRA |
| 3 | Male | 67 | M4b | IA + CAG + HHT |
| 4 | Male | 59 | M3 | HA |
| 5 | Male | 55 | M1 | HAA |
| 6 | Male | 76 | M2a | CAG |
| 7 | Male | 66 | M2 | IA |
| 8 | Female | 48 | M2a | HAA |
| 9 | Female | 66 | M2a | CAG |
| 10 | Female | 56 | M2 | CAG + AA + DA |
| 11 | Female | 50 | M2a | IA |
| 12 | Female | 19 | M2a | HAA |
| 13 | Female | 51 | M3 | ATRA/ATO + HA +
AD |
| 14 | Female | 40 | M3 | IA |
| 15 | Female | 59 | M1 | CAG |
The patients were classified for AML subtype
according to World Health Organization guidelines (23), and were reevaluated in order to
fulfill the diagnostic criteria published by Fasan et al
(24). Specifically, the patients
were checked for clinical parameters, cytogenetic abnormalities,
molecular markers and abnormal hematopoiesis. The prognosis of the
patients was determined according to the National Comprehensive
Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for
acute myeloid leukemia (version 2.2013).
Patients were identified as being in complete
remission (CR) if they were did not require transfusions, and had
normal cytogenetics, absolute neutrophil count >1,000/µl, marrow
blasts <5%, and no extramedullary disease. Patients were
considered to be in partial remission (PR) if they had normal blood
counts and a reduction in bone marrow blasts to 5–25% (≥50%
reduction). Worse prognosis was defined when patients after
chemotherapy showed none of the aforementioned remission symptoms,
or had worse symptoms including worse cytogenetics, increased
accumulation of myeloblasts, immature cells in bone marrow,
extramedullary leukemic cell infiltration, or mortality.
Clinical pathological data and chemotherapy regimens
were obtained from the patients' medical records and pathology
files. The study protocol was approved by the Ethics Committee of
Yuyao People's Hospital. All patients who participated in the study
signed written informed consent forms.
DNA extraction and bisulphite DNA
modification
DNA was extracted from bone marrow nucleated cells
using a nucleic acid extraction analyzer (Lab-Aid 820; Xiamen
Zeesan Biotech Co., Ltd., Xiamen, China). DNA concentrations were
measured using a NanoDrop 1000 spectrophotometer (Thermo Fisher
Scientific Inc., Waltham, MA, USA). Methylation of the DNA samples
was then analyzed by the classic sodium bisulfite method (25), using an EZ DNA Methylation-Gold kit™
(Zymo Research Corporation, Irvine, CA, USA).
Methylation-specific polymerase chain
reaction (MSP)
The methylation status of the three genes was
determined by conventional MSP (26). Polymerase chain reaction (PCR) was
conducted in a final volume of 20 µl containing 1.5 µl modified
DNA, 0.5 µl forward and reverse primers, 10 µl Zymo Taq™ Premix
(Zymo Research Corporation) and 7.5 µl DNAase/RNAase-free water.
DNA amplification was performed on Veriti® PCR machine
(Applied Biosystems; Thermo Fisher Scientific) under the following
conditions: 10 min of denaturation at 95°C followed by 30 or 35
cycles of 30 sec at 94°C, 45 sec at the annealing (or melting)
temperature (Table II), 1 min at
72°C, and 72°C for 7 min, prior to storage at 4°C. PCR products
were subjected to analysis using a Qsep100 automated nucleic acid
analysis system (BiOptic, Inc., La Cañada Flintridge, CA, USA).
Samples were determined to be methylated or unmethylated on the
basis of the visible peaks generated by the Q-Analyzer. The
methylated and unmethylated primer sequences of CDKN2B
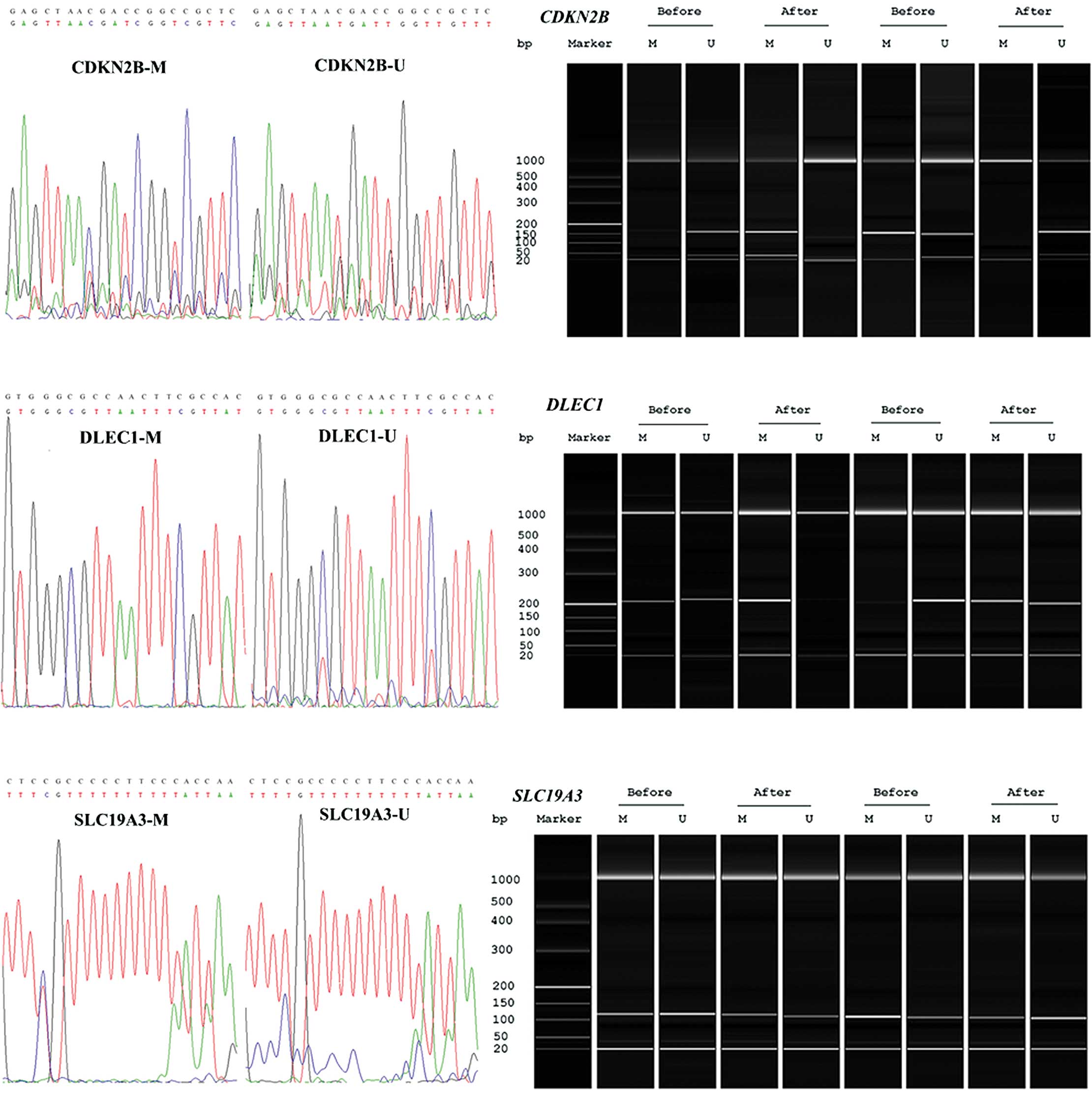
(27), SLC19A3 (28) and DLEC1 (29) genes are presented in Table II. Some of the DNA samples were
sequenced using an ABI 3730 DNA Analyzer (Applied Biosystems;
Thermo Fisher Scientific, Inc.), and the results indicated a
successful bisulfite conversion and amplification (Fig. 1).
 | Table II.MSP primers and reaction conditions
in the PCR amplification. |
Table II.
MSP primers and reaction conditions
in the PCR amplification.
| Gene | Primer | Sequence (5′ to
3′) | Product length
(bp) | Tm (°C)/cycles |
|---|
| CDKN2B | MF |
GCGTTCGTATTTTGCGGTT | 148 | 55/30 |
|
| MR |
CGTACAATAACCGAACGACCGA |
|
|
|
| UF |
TGTGATGTGTTTGTATTTTGTGGTT | 154 | 57/30 |
|
| UR |
CCATACATAACCAAACAACCAA |
|
|
| SLC19A3 | MF |
GTTTGGACGTTCGGATTC | 114 | 57/30 |
|
| MR |
CGCGACTATCGAATAAATCC |
|
|
|
| UF |
AAGGTTTGGATGTTTGGATTT | 114 | 55/30 |
|
| UR |
ACCCACAACTATCAAATAAATCC |
|
|
|
| DLEC1 | MF |
GATTATAGCGATGACGGGATTC | 193 | 57/35 |
|
| MR |
ACCCGACTAATAACGAAATTAACG |
|
|
|
| UF |
TGATTATAGTGATGATGGGATTTGA | 193 | 55/30 |
|
| UR |
CCCAACTAATAACAAAATTAACACC |
Statistical analysis
Comparisons between CDKN2B, SLC19A3
and DLEC1 promoter methylation were performed using the
correction formula of a χ2 test. Statistical analysis
was performed using the SPSS statistical package version 16.0
(SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5.0 software
(GraphPad Software, Inc., La Jolla, CA, USA).
Results
Chemotherapy regimens of patients with
AML
MSP was conducted on the pre- and
post-chemotherapeutic tissue samples obtained from 15 patients with
AML to determine whether chemotherapy induced promoter methylation
changes in CDKN2B, SLC19A3 and DLEC1 genes. A
total of 10 regimens were applied, including ATRA/ATO, DNR and
ATRA, IA, CAG, HHT, HA, HAA, AA, DA, AD. These patients with AML
consisted of two M1, seven M2, five M3, and one M4 subtypes
(Table I). Among them, 4 patients
(numbers 1, 4, 5 and 7) achieved remission and 2 patients (numbers
6 and 15) had a worse prognosis following chemotherapy, which was
accompanied by changes in methylation (Table I).
Methylation and unmethylation primer sets were used
to differentiate the methylation into full methylation (M/M),
partial methylation (M/U) and unmethylation (U/U) statuses. In
Table III, the methylation changes
in different genes following chemotherapy, according to gender, are
shown. For CDKN2B, 1 patient with subtype M2 AML (age 76
years, CAG regimen) changed from unmethylation to partial
methylation; 1 patient with subtype M3 AML (age 23 years, treated
with ATRA/ATO) changed from unmethylation to full methylation; 1
patient with subtype M1 AML (age 55 years, HAA regimen) and 1
patient with subtype M2 AML (age 66 years, IA regimen) changed from
partial methylation to full methylation; and 1 patient with subtype
M3 AML (age 59 years, HA regimen) changed from partial methylation
to unmethylation. For DLEC1, one M1 patient (age 59 years,
CAG regimen) changed from partial to full methylation.
 | Table III.Alterations in the methylation status
of various genes following chemotherapy by gender. |
Table III.
Alterations in the methylation status
of various genes following chemotherapy by gender.
|
|
| Prior to primary
chemotherapy | Following primary
chemotherapy |
|---|
|
|
|
|
|
|---|
| Gene | Gender | M | U | M% | M | U | M% |
|---|
| CDKN2B | Females (n=8) | 8 | 0 | 100 | 8 | 0 | 100 |
|
| Males (n=7) | 5 | 2 | 71.43 | 6 | 1 | 85.71 |
| SLC19A3 | Females (n=8) | 8 | 0 | 100 | 8 | 0 | 100 |
|
| Males (n=7) | 7 | 0 | 100 | 7 | 0 | 100 |
| DLEC1 | Females (n=8) | 8 | 0 | 100 | 8 | 0 | 100 |
|
| Males (n=7) | 5 | 2 | 71.43 | 5 | 2 | 71.43 |
Methylation changes of CDKN2B
following chemotherapeutic treatments
Chemotherapy-induced changes in CDKN2B
methylation status were only observed in males (Table IV). The patients with
chemotherapy-induced CDKN2B methylation changes comprised
two M3, two M2 and one M1 male cases. As shown in Table IV, the correlation between increased
CDKN2B methylation and prognostic effect was inconsistent.
Improved prognosis along with reduced CDKN2B methylation was
observed in one M3 case (age 59 years, HA regimen). Conversely,
improved prognostic effects along with increased CDKN2B
methylation were also observed in one M3 patient (age 23 years,
ATRA/ATO treatment regimen), one M2 patient (age, 66 years, IA
treatment regimen) and one M1 patient (age 55 years, HAA treatment
regimen). These results suggested a male-associated
chemotherapy-induced hypermethylation of CDKN2B.
 | Table IV.Association between gene
hypermethylation and prognosis in patients with AML. |
Table IV.
Association between gene
hypermethylation and prognosis in patients with AML.
| Gene | Hypermethylation
relieves AML | Hypermethylation
accelerates AML or hypomethylation relieves AML | No association
(n) |
|---|
| CDKN2B |
|
|
|
| Females
(n=8) | 0 | 0 | 8 |
| Males
(n=7) | No. 1: 23 years;
M3; ATRA/ATO | No. 4: 59 years;
M3; HA |
|
|
| No. 5: 55 years;
M1; HAA | No. 6: 76 years;
M2a; CAG | 2 |
|
| No. 7: 66 years;
M2; IA |
|
|
| SLC19A3 |
|
|
|
| Females
(n=8) | 0 | 0 | 8 |
| Males
(n=7) | 0 | 0 | 7 |
| DLEC1 |
|
|
|
| Females
(n=8) | 0 | No. 15: 59 years;
M1; CAG | 7 |
| Males
(n=7) | 0 | 0 | 7 |
Methylation changes of SLC19A3 and
DLEC1 following chemotherapeutic treatments
In addition, the present study identified a single
female patient who presented adverse prognostic effects in addition
to exhibiting DLEC1 hypermethylation (M1 patient, age 59
years, CAG treatment regimen; Table
IV). Furthermore, the results demonstrated that the methylation
status of SLC19A3 did not change in any patient following
chemotherapy (Table IV).
Gene methylation changes and prognosis
in AML patients
The present investigation demonstrated that
CDKN2B hypermethylation may be specific to male patients
with AML (Table III), and that
DLEC1 hypermethylation in females with AML may result in a
worse prognosis following primary chemotherapy (Table IV).
Discussion
The aim of the present study was to identify
methylation biomarkers in order to guide individualized
chemotherapy. The results of the present study revealed gender
dimorphism in the chemotherapy-induced hypermethylation of
CDKN2B and DLEC1. The chemotherapy-induced
hypermethylation of DLEC1 may have resulted in the poor
prognosis of AML in one of the female patients. Male-specific
chemotherapy-induced hypermethylation of CDKN2B was also
identified.
Resistance to drugs is one of the most pertinent
aspects of treatment failure in cancers. Accumulating evidence
suggests that aberrant DNA methylation is involved in the drug
resistance of tumor cells and influences the prognosis of patients
with AML (30,31). AML is complex and has numerous
subtypes and differences among individuals, which makes it
difficult to predict the therapeutic outcomes of treatments.
In the present study, three cancer-associated genes
were selected in order to investigate the association of their
methylation changes with treatment outcomes. These genes were
CDKN2B, SLC19A3 and DLEC1. CDKN2B is a
cyclin-dependent kinase inhibitor, located in a region that is
frequently mutated or aberrantly methylated in a wide variety of
tumors including leukemia (32). A
previous study demonstrated that the expression of CDKN2B,
which had previously been silenced by hypermethylation, was
increased following treatment with decitabine in myelodysplastic
syndromes (33). CDKN2B
methylation decreased significantly in patients who achieved CR
following a DAA (decitabine, aclacinomycin and Ara-C) treatment
regimen, thereby demonstrating that decitabine may have a
demethylation effect (34).
SLC19A3 encodes the thiamine transporter expressed at the
apical surface of polarized cells (35). SLC19A3 mRNA expression has
been shown to be downregulated by DNA methylation in colon cancer
cell lines (36). DLEC1 has
been demonstrated to act as a tumor suppressor gene in the
tumorigenesis and progression of numerous types of carcinoma, such
as multiple lymphomagenesis, and thus it may serve as a
non-invasive tumor marker (37).
DLEC1 methylation has also shown the potential to serve as
an independent marker of poor survival in squamous cell carcinoma
lung cancer (38).
In conclusion, the results of the present study
suggest that male-specific chemotherapy-induced hypermethylation
occurs in the CDKN2B promoter. Female-specific
chemotherapy-induced hypermethylation of the DLEC1 promoter
may correlate with a worse prognostic outcome. These results also
showed there were no methylation alterations in SLC19A3
following chemotherapy. Due to the complexity of AML and the
variety of treatment regimens, that may be used further studies are
required in a larger sample set in order to verify these
preliminary results.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 31100919
and 81371469), the Zhejiang Provincial Natural Science Foundation
(grant no. LR13H020003), Ningbo City Medical Science and Technology
Projects (grant no. 2014A20), and the K.C. Wong Magna Fund of
Ningbo University.
References
|
1
|
Estey EH: Acute myeloid leukemia: 2013
update on risk-stratification and management. Am J Hematol.
88:318–327. 2013.PubMed/NCBI
|
|
2
|
Davis AS, Viera AJ and Mead MD: Leukemia:
An overview for primary care. Am Fam Physician. 89:731–738.
2014.PubMed/NCBI
|
|
3
|
Liu L, Zhang Y, Jin Z, Zhang X, Zhao G, Si
Y, Lin G, Ma A, Sun Y, Wang L and Wu D: Increasing the dose of
aclarubicin in low-dose cytarabine and aclarubicin in combination
with granulocyte colony-stimulating factor (CAG regimen) can safely
and effectively treat relapsed or refractory acute myeloid
leukemia. Int J Hematol. 99:603–608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carlotti ME, Carpignano R, Gasco MR and
Trotta M: Optimization of emulsions. Int J Cosmet Sci. 13:209–219.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J, Lü S, Yang J, Song X, Chen L,
Huang C, Hou J and Zhang W: A homoharringtonine-based induction
regimen for the treatment of elderly patients with acute myeloid
leukemia: A single center experience from China. J Hematol Oncol.
2:322009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin J, Jiang DZ, Mai WY, Meng HT, Qian WB,
Tong HY, Huang J, Mao LP, Tong Y, Wang L, et al: Homoharringtonine
in combination with cytarabine and aclarubicin resulted in high
complete remission rate after the first induction therapy in
patients with de novo acute myeloid leukemia. Leukemia.
20:1361–1367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee YJ, Moon JH, Kim JG, Chae YS, Kang BW,
Lee SJ, Choi JY, Shin HC, Seo JW and Sohn SK: Prospective
randomization trial of G-CSF-primed induction regimen versus
standard regimen in patients with AML. Chonnam Med J. 47:80–84.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang H, Qin Y, Xu R, You X, Teng R, Yang
L, Xu M and Liu H: Combination therapy with arsenic trioxide,
all-trans retinoic acid and chemotherapy in acute promyelocytic
leukemia patients with various relapse risks. Leuk Res. 36:841–845.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lo-Coco F and Hasan SK: Understanding the
molecular pathogenesis of acute promyelocytic leukemia. Best Pract
Res Clin Haematol. 27:3–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stirewalt DL, Pogosova-Agadjanyan EL,
Tsuchiya K, Joaquin J and Meshinchi S: Copy-neutral loss of
heterozygosity is prevalent and a late event in the pathogenesis of
FLT3/ITD AML. Blood Cancer J. 4:e2082014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tao YF, Xu LX, Lu J, Cao L, Li ZH, Hu SY,
Wang NN, Du XJ, Sun LC, Zhao WL, et al: Metallothionein III (MT3)
is a putative tumor suppressor gene that is frequently inactivated
in pediatric acute myeloid leukemia by promoter hypermethylation. J
Transl Med. 12:1822014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Conway O'Brien E, Prideaux S and Chevassut
T: The epigenetic landscape of acute myeloid leukemia. Adv Hematol.
2014:1031752014.PubMed/NCBI
|
|
13
|
Jiang D, Hong Q, Shen Y, Xu Y, Zhu H, Li
Y, Xu C, Ouyang G and Duan S: The diagnostic value of DNA
methylation in leukemia: A systematic review and meta-analysis.
PLoS One. 9:e968222014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sonnet M, Claus R, Becker N, Zucknick M,
Petersen J, Lipka DB, Oakes CC, Andrulis M, Lier A, Milsom MD, et
al: Early aberrant DNA methylation events in a mouse model of acute
myeloid leukemia. Genome Med. 6:342014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pluta A, Nyman U, Joseph B, Robak T,
Zhivotovsky B and Smolewski P: The role of p73 in hematological
malignancies. Leukemia. 20:757–766. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Esteller M: Profiling aberrant DNA
methylation in hematologic neoplasms: A view from the tip of the
iceberg. Clin Immunol. 109:80–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cancer Genome Atlas Research Network:
Genomic and epigenomic landscapes of adult de novo acute myeloid
leukemia. N Engl J Med. 368:2059–2074. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tao YF, Ni J, Lu J, Wang N, Xiao PF, Zhao
WL, Wu D, Pang L, Wang J, Feng X and Pan J: The promoter of miR-663
is hypermethylated in Chinese pediatric acute myeloid leukemia
(AML). BMC Med Genet. 14:742013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baylin SB and Jones PA: A decade of
exploring the cancer epigenome-biological and translational
implications. Nat Rev Cancer. 11:726–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leone G, Voso MT, Teofili L and Lübbert M:
Inhibitors of DNA methylation in the treatment of hematological
malignancies and MDS. Clin Immunol. 109:89–102. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu X, Lam EK, Wang X, Zhang J, Cheng YY,
Lam YW, Ng EK, Yu J, Chan FK, Jin H and Sung JJ: Promoter
hypermethylation mediates downregulation of thiamine receptor
SLC19A3 in gastric cancer. Tumour Biol. 30:242–248. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kwong J, Lee JY, Wong KK, Zhou X, Wong DT,
Lo KW, Welch WR, Berkowitz RS and Mok SC: Candidate
tumor-suppressor gene DLEC1 is frequently downregulated by promoter
hypermethylation and histone hypoacetylation in human epithelial
ovarian cancer. Neoplasia. 8:268–278. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Walter RB, Othus M, Burnett AK, Löwenberg
B, Kantarjian HM, Ossenkoppele GJ, Hills RK, van Montfort KG,
Ravandi F, et al: Significance of FAB subclassification of ‘acute
myeloid leukemia, NOS’ in the 2008 WHO classification: Analysis of
5848 newly diagnosed patients. Blood. 121:2424–2431. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fasan A, Alpermann T, Haferlach C,
Grossmann V, Roller A, Kohlmann A, Eder C, Kern W, Haferlach T and
Schnittger S: Frequency and prognostic impact of CEBPA proximal,
distal and core promoter methylation in normal karyotype AML: A
study on 623 cases. PLoS One. 8:e543652013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marzese DM, Huang SK and Hoon DS: In situ
sodium bisulfite modification of genomic DNA from microdissected
melanoma paraffin-embedded archival tissues. Methods Mol Biol. Dec
13–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen C, Wang L, Liao Q, Huang Y, Ye H,
Chen F, Xu L, Ye M and Duan S: Hypermethylation of EDNRB promoter
contributes to the risk of colorectal cancer. Diagn Pathol.
8:1992013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vidal DO, Paixão VA, Brait M, Souto EX,
Caballero OL, Lopes LF and Vettore AL: Aberrant methylation in
pediatric myelodysplastic syndrome. Leuk Res. 31:175–181. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Shi J, Xu L, Shi B, Hou P and Ji
M: Aberrant DNA methylation of drug metabolism and transport genes
in nodular goiter. Thyroid Res. 4:152011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qu X, Jiang N, Xu F, Shao L, Tang G,
Wilkinson B and Liu W: Cloning, sequencing and characterization of
the biosynthetic gene cluster of sanglifehrin A, a potent
cyclophilin inhibitor. Molecular Biosyst. 7:852–861. 2011.
View Article : Google Scholar
|
|
30
|
Moreira MA, Bagni C, de Pinho MB,
Mac-Cormick TM, dos Santos Mota M, Pinto-Silva FE, Daflon-Yunes N
and Rumjanek VM: Changes in gene expression profile in two
multidrug resistant cell lines derived from a same drug sensitive
cell line. Leuk Res. 38:983–987. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Si XX and Sun YJ: Aberrant DNA methylation
and drug resistance of tumor cells. Yi Chuan. 36:411–419. 2014.(In
Chinese). PubMed/NCBI
|
|
32
|
Li J, Bi L, Lin Y, Lu Z and Hou G:
Clinicopathological significance and potential drug target of
p15INK4B in multiple myeloma. Drug Des Devel Ther. 8:2129–2136.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Santos FP, Kantarjian H, Garcia-Manero G,
Issa JP and Ravandi F: Decitabine in the treatment of
myelodysplastic syndromes. Expert Rev Anticancer Ther. 10:9–22.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song LX, Xu L and Li X, Chang CK, Zhang Y,
Wu LY, He Q, Zhang QX and Li X: Clinical outcome of treatment with
a combined regimen of decitabine and aclacinomycin/cytarabine for
patients with refractory acute myeloid leukemia. Ann Hematol.
91:1879–1886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Subramanian VS, Marchant JS and Said HM:
Biotin-responsive basal ganglia disease-linked mutations inhibit
thiamine transport via hTHTR2: Biotin is not a substrate for
hTHTR2. Am J Physiol Cell Physiol. 291:C851–C859. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ikehata M, Ueda K and Iwakawa S: Different
involvement of DNA methylation and histone deacetylation in the
expression of solute-carrier transporters in 4 colon cancer cell
lines. Biol Pharm Bull. 35:301–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Z, Li L, Su X, Gao Z, Srivastava G,
Murray PG, Ambinder R and Tao Q: Epigenetic silencing of the 3p22
tumor suppressor DLEC1 by promoter CpG methylation in non-Hodgkin
and Hodgkin lymphomas. J Transl Med. 10:2092012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Seng TJ, Currey N, Cooper WA, Lee CS, Chan
C, Horvath L, Sutherland RL, Kennedy C, McCaughan B and
Kohonen-Corish MR: DLEC1 and MLH1 promoter methylation are
associated with poor prognosis in non-small cell lung carcinoma. Br
J Cancer. 2:375–82. 2008. View Article : Google Scholar
|