Introduction
Breast cancer (BC) is the most common form of
malignant tumor and is a leading cause of mortality in women
worldwide (1). However, the
recurrence and mortality of patients with BC has markedly decreased
as a result of improvement in comprehensive treatments, in
particular the use of adjuvant therapies including cytotoxic drugs,
endocrine therapy and more recently trastuzumab, a monoclonal
antibody against the human epidermal growth factor receptor-2
(HER-2) gene (2). The survival
benefit gained from chemotherapy in BC patients has been observed
to be equal regardless of whether chemotherapy was administered
pre- or postoperatively (3). HER-2,
also known as HER2/neu or c-erb-B2, encodes a 185-kD
transmembrane glycoprotein receptor (4). Overexpression of the HER-2 gene has
been detected in 25–30% patients with BC and is related to the
aneuploidy growth of S phase, high nuclear grade, positive axillary
node extension and low expression of hormonal receptors (5–8). In
addition, the overexpression of HER-2 may lead to the activation of
HER-2 signal pathways and uncontrolled cell differentiation
(9). Therefore, HER-2-positive
tumors have more aggressive biological behavior, and reoccur and
undergo metastasis more readily compared with HER-2-negative tumors
(4,7,8).
As a targeted drug, trastuzumab is recommended by
the Food and Drug Administration to treat patients with HER-2
positive BC (10). A number of
randomized control trials (RCTs) investigating the safety and
efficacy of trastuzumab have demonstrated that a combination of
trastuzumab and traditional drugs for chemotherapy results in a
higher survival and response rate compared with chemotherapy alone
in an adjuvant setting (11–13). In addition, trastuzumab may provide
additional clinical benefits by increasing the rates of pathologic
responses and breast-conserving therapy in a neoadjuvant setting
(14,15).
Despite this, a number of limitations still exist
regarding the administrated of trastuzumab; for example, it is not
fully understood how to optimize the treatment of combined
trastuzumab and chemotherapy. Yin et al (16) reported that concurrent administration
of trastuzumab resulted in a lower risk of mortality compared with
sequential administration. By contrast, Azim et al (17) reported that no significant difference
was observed in the overall survival (OS) rate between the two
treatment options. Currently, it is unknown how to obtain the
highest survival rates by changing the timings of trastuzumab
administration.
It has been demonstrated that the successful
administration of trastuzumab is associated with severe adverse
effects (AEs) including cardiac toxicities and brain metastases
(18–20). However, the presence of serious AEs
in a number of organs has not been studied extensively. In
addition, it is difficult to evaluate the efficacy and safety of
trastuzumab due to the limited number of studies and quantity of
data provided in previous meta-analyses (11,14,16,19).
Furthermore, odds ratios and relative risk (RR) are less
appropriate and unreliable for analyzing time-to-event outcomes, as
survivors or recovered individuals in the treatment group are
simply compared with those in a control group at a single point in
time (21). Therefore, based on the
publication of several high quality RCTs in recent years, an
updated meta-analysis was performed in the present study in order
to evaluate the prognostic effects and the magnitude of AEs caused
by trastuzumab in adjuvant and neoadjuvant settings.
Materials and methods
Search strategy and eligibility
criteria
PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Embase
(http://www.embase.com/info/helpfiles/) and Science
Direct (http://www.elsevier.com/online-tools/sciencedirect)
databases were used to identify eligible studies published between
January 1995 and March 2014. Searched keywords included the
following terms: ‘Breast cancer’, ‘trastuzumab’, ‘herceptin’,
‘HER-2’, ‘adjuvant’, ‘neoadjuvant’, ‘chemotherapy’ and ‘random’.
The reference lists of previous published meta-analyses were
manually searched and reviews were collected without language
restriction. Only RCTs that evaluated the efficacy or safety of
chemotherapy with trastuzumab in patients with HER-2 positive BC
were selected for inclusion in the present meta-analysis. Patients
included in the current study required a good performance status
(defined as a World Health Organization performance status of 0 or
1), adequate left ventricular ejection fraction (LVEF; as assessed
by multiple-gated acquisition or echocardiography scan, and was
required to be within the institutional normal range, and within
the lower limit of normal), and normal bone marrow (a blood
leukocyte count >3.0×109 cells/l; neutrophil count >1.5×109
cells/l; platelet count >100×109 cells/l; hemoglobin >10
g/dl), liver and renal function laboratory results. In the case of
obtaining multiple reports of the same trial, the report with the
longest follow-up period was selected for use. Patients who
underwent chemotherapy were only compared with patients who
received the same type of chemotherapy plus trastuzumab. Trials
testing the administration of trastuzumab in a neoadjuvant setting
were also included in this meta-analysis. The surgical modality,
chemotherapy regimens, radiotherapy and endocrine therapy were not
considered as eligibility criteria in selecting RCTs. RCTs which
evaluated biological or targeted agents other than trastuzumab were
excluded from the present study. The Preferred Reporting Items for
Systematic Reviews and Meta-Analyses (22) checklist was adhered to in the present
meta-analysis (data not shown).
Data extraction
Two reviewers independently extracted data from each
trial included in the present study. Any discrepancies were
resolved by discussing the problem with another author. The group
of patients undergoing treatment with trastuzumab and chemotherapy
were defined as the T-group, and patients only undergoing
chemotherapy were defined as the C-group. The following information
was obtained from chosen studies: Publication year, first author,
patient follow-up time, chemotherapy regimens, number of recruited
patients and the outcome events. The primary endpoint was the
number of disease-free survival (DFS) patients, which was defined
by events from the random assignment to the first documented
disease progression (local, regional, distant recurrence or
morality). Secondary endpoints included OS, tumor response and AEs.
OS was defined as events from random assignment to mortality. The
effects of treatment were assessed by the clinical response of the
patient in an adjuvant setting, which included the complete
response (CR), partial response (PR), stable disease (SD) and
progressive disease (PD). The overall response (OR) was defined as
CR plus PR. The effects of treatment in a neoadjuvant setting were
divided into pathological CR (pCR) and non-pCR. AEs were graded
according to the National Cancer Institute Common Toxicity Criteria
(NCI-CTC) version 2.0 (23). Serious
AEs were defined as grade 3–4 and fatal AEs were also included in
the present study.
Quality assessment
The quality of the included trials was evaluated by
the Cochrane Collaboration's risk of bias tool (24). This tool includes adequate sequence
generation, allocation concealment, blinding of participants and
personnel, blinding of outcome assessment, incomplete outcome data,
selective outcome reporting and other bias. Each item is recorded
as having a low, high or unclear risk of bias, and then a summary
assessment of each included trial is graded as A, minimization of
bias in all four categories: Adequate randomization, few losses to
follow up and intention-to-treat analysis, blinding of outcome
assessors, high quality outcome assessment; B, each of the criteria
in ‘A’ partially met and C, one or more of the criteria in ‘A’ not
met. Two reviewers checked the risk of bias concurrently.
Statistical analysis
Review Manager (Version 5.2; Copenhagen, Denmark:
The Nordic Cochrane Centre, The Cochrane Collaboration, 2012) was
used to perform the statistical analysis. The hazard ratio (HR) and
95% confidence interval (CI) were used to assess the survival
advantage in patients with HER-2 positive BC. If provided in the
trial, the P-value and HR from the Cox regression model was
recorded and used directly in the present meta-analysis. If it was
not available, an HR approximation was calculated indirectly using
the methods described by Tierney et al (21). The risk ratio (RR) was calculated to
estimate the relative risk of response and AEs. Statistical
heterogeneity among the included studies was evaluated using the χ2
test and quantified using I2 statistics that determined the use of
the fixed-effects (Mantel-Haenszel method) or random-effects
(DerSimonian and Laird method) model (25). The existence of homogeneity was
considered unreasonable when I2>50% and P<0.10. The
comparison of trastuzumab stratified analysis was obtained by
adjusted indirect comparison using the Bucher's method (26) and indirect treatment comparison (ITC)
software (version 1.0; Canadian Agency for Drugs and Technologies
in Health, Ottawa, Canada) that estimated the relative effects of
concurrent trastuzumab to sequential trastuzumab, weekly
trastuzumab to every 3 weeks trastuzumab through C-group, as
previously described (27,28). Finally, potential publication bias
was evaluated using funnel plots and further quantified by Begg and
Egger's tests (29,30) using STATA software (version 12.0;
College Station, TX, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of included clinical
trials
A total of 419 studies were obtained from databases
and 10 studies were obtained from references. Based on above
criteria, 13 RCTs were included in the analysis, including 9 RCTs
with adjuvant settings (31–39) and 4 with neoadjuvant settings
(40–43). One joint trial (32) which contained two similar studies
[NCCTG N9831 (18) and NSABP B-31
(20)] was included in the
meta-analysis. Longer follow-up and detailed AEs were reported by
Buzdar et al (43,44); therefore, the two studies were
combined in the analysis. The screening process that was used is
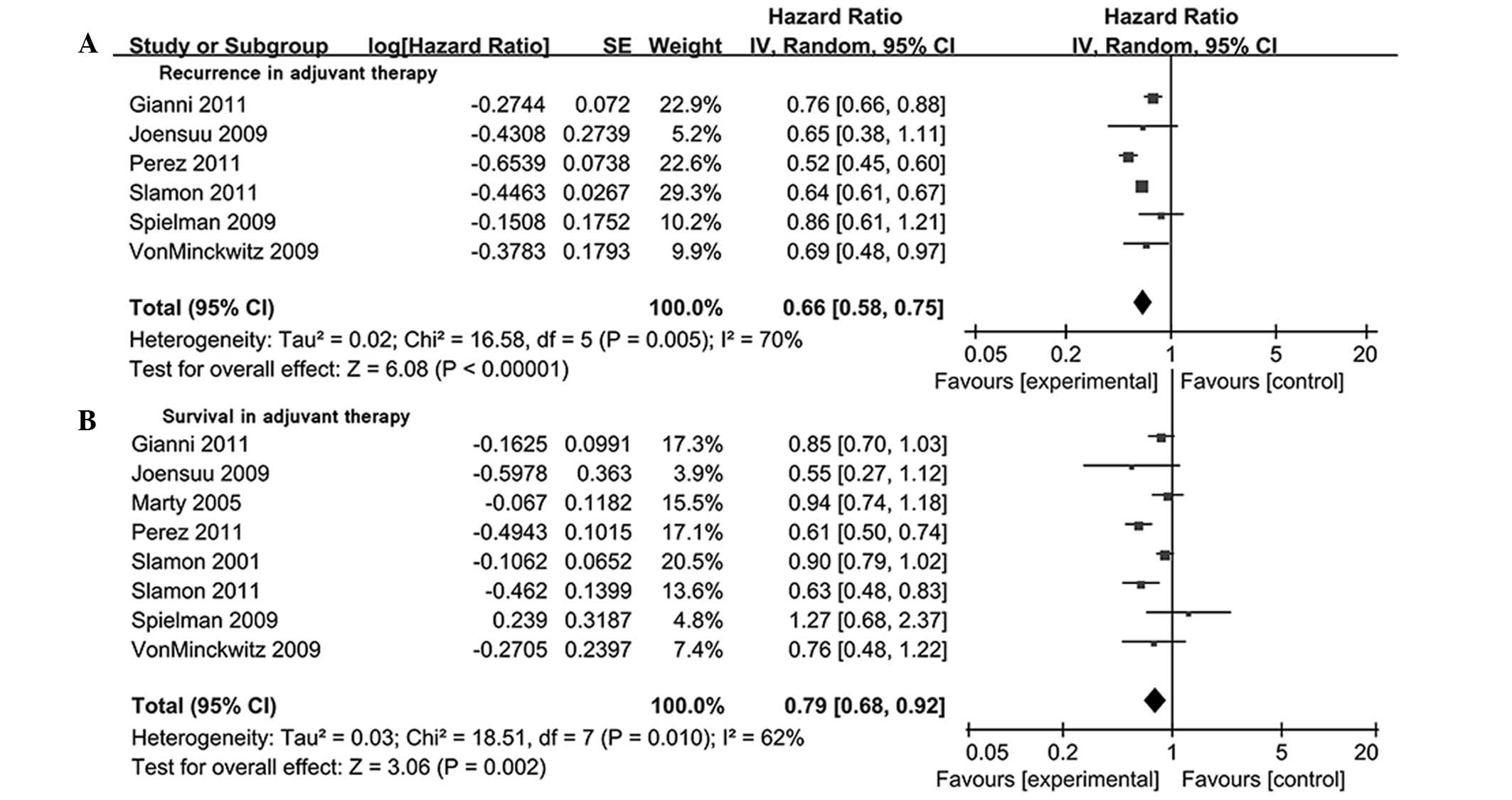
briefly described in Fig. 1.
A total of 14,546 patients were available for this
meta-analysis (31–43), of which 14,056 patients received
adjuvant therapy and 490 patients received neoadjuvant therapy. In
the adjuvant setting, 8,418 patients were assigned to the T-group
and 5,638 patients were assigned to the C-group. In the neoadjuvant
setting, 246 patients were treated with combination therapy and 244
were treated with chemotherapy alone. The HER-2 status of patients
was assessed by immunohistochemistry, and fluorescence in
situ hybridization or chromogenic in situ hybridization.
Among the trials included in the present meta-analysis, the
following trastuzumab treatment plans were used: An intravenous
initial dose of 4 mg/kg, followed by 2 mg/kg once a week, was used
in 6 trials (32,36–39,43); an
initial dose of 8 mg/kg, then 6 mg/kg every 3 weeks, was used in 5
trials (33,35,40–42); in
2 trials, trastuzumab was administered initially at 4 mg/kg,
followed by 2 mg/kg per week, and then 6 mg/kg every 3 weeks
(31) or given at 6 mg/kg every 3
weeks (34). The median follow-up
time ranged between 15.6 and 65.0 months. Baseline characteristics
of the RCTs are summarized in Table
I.
 | Table I.Baseline characteristics of the 13
randomized control trials included in the meta-analysis. |
Table I.
Baseline characteristics of the 13
randomized control trials included in the meta-analysis.
|
| Patients (n) |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Author, year | T-group | C-group | T | T-group
treatment | C-group
treatment | Follow-up
(months)a | (Refs.) |
|---|
| Adjuvant
chemotherapy |
|
|
|
|
|
|
|
| Slamon
et al, 2011 | 2,149 | 1,073 | Concurrent | (q3w)
(Dox+Cyc+Doc)+(w→q3w) T | (q3w) (Dox+Cyc+Doc)
(q3w) Doc+Car+(w→q3w) T | 65.0 | (31) |
| Perez
et al, 2011 | 2,028 | 2,017 | Concurrent | (q3w) (Dox+Cyc)+wP+
wT | (q3w)
(Dox+Cyc)+wP | 46.8 | (32) |
| Gianni
et al, 2011 | 3,397 | 1,698 | Sequential | (q3w) T for 1
year | Observation (q3w) T
for 2 years | 48.4 | (33) |
| von
Minckwitz et al, 2009 | 78 | 78 | Concurrent | Cap+(q3w) T | Cap | 15.6 | (34) |
|
Spielman et al,
2009 | 260 | 268 | Sequential |
(q3w)T+(F+E+Cyc)/(E+Doc) |
(F+E+Cyc)/(E+Doc) | 47.0 | (35) |
| Joensuu
et al, 2009 | 116 | 116 | Concurrent | Doc+F+E+Cyc+T
V+F+E+Cyc+T | Doc+F+E+Cyc
V+F+E+Cyc | 62.0 | (36) |
|
Gasparini et al,
2007 | 63 | 60 | Sequential | wP+wT | wP | 16.6 | (37) |
| Marty
et al, 2005 | 92 | 94 | Concurrent | (q3w) Doc+wT | (q3w) Doc |
40.9/35.9b | (38) |
| Slamon
et al, 2001 | 235 | 234 | Concurrent (q3w)
P+T | (q3w) (A+Cyc)+wT
(q3w) P | (q3w) (A+Cyc) | 35.0 | (39) |
| Neoadjuvant
chemotherapy |
|
|
|
|
|
|
|
| Steger
et al, 2014 | 44 | 49 | Concurrent | (q3w)
(E+Doc±Cap)+T | (q3w)
(E+Doc±Cap) | NR | (40) |
| Pierga
et al, 2010 | 62 | 58 | Concurrent | (q3w)
(E+Cyc+Doc)+(q3w) T | (q3w)
(E+Cyc+Doc) | NR | (41) |
| Gianni
et al, 2010 | 117 | 118 | Concurrent
(Cyc+M+F)+(q3w) T | (q3w)
(Dox+P)+(q4w) | (q3w) (Dox+P)+(q4w)
(Cyc+M+F) | 38.4 | (42) |
| Buzdar
et al, 2007 | 23 | 19 | Concurrent | P+F+E+Cyc+wT | P+F+E+Cyc | 36.1 | (43) |
Methodological quality assessment
Each trial included a statement regarding
randomization and a detailed description was included in 7 trials.
One study was randomized using a code envelope (37), two by computer program (40,41), and
four via block method (34,36,38,43). Two
trials utilized the blind method (38,42). All
trials published as full text articles were judged to be grade
B.
Efficacy and overall analysis of
trastuzumab on DFS and OS in an adjuvant setting
In an adjuvant setting, 6 trials (31–36) with
10,503 patients reported disease recurrence. The random effects
model (I2=70%) indicated that the disease recurrence risk in C
group (26.1%; 1,370/5,246) was higher compared that in the T group
[18.8%; 987/5,257; HR=0.66; 95% CI (0.58–0.75), P<0.00001;
Fig. 2A]. There were 8 studies
(30–35,37,38) with
complete information on OS. In comparison with the C-group, there
was a significant relative reduction in the risk of mortality in
the T-group [21%; HR=0.79; 95% CI (0.68–0.92); P=0.002; I2=62%;
Fig. 2B].
Subgroup analysis of trastuzumab on
DFS and OS in an adjuvant setting
To further analyze the effects of different time
schedules of trastuzumab administration on the survival rate,
patients were divided into concurrent and sequential groups, then
into patients treated weekly and every 3 weeks. With regards to
DFS, the effect of each factor was consistent with the overall
result (Fig. 3A). ITC demonstrated
that there was no statistical difference between the concurrent and
sequential groups (RR=0.779; P=0.06059), but that the risk of
recurrence following a weekly treatment plan was lower than the
risk of patients on a 3 weekly treatment plan (RR=0.697;
P=0.01128). However, the effect of trastuzumab on OS differed
between the T-group and C-group; concurrent (RR=0.75; P=0.003) and
weekly (RR=0.78; P=0.04) use of trastuzumab significantly lowered
the risk of mortality in comparison with the C-groups. The
differences between the sequential and C-group (P=0.18), and the 3
weekly treatment and C-group (P=0.1) were not significantly
different with regards to OS (Fig.
3B).
Overall analysis of trastuzumab on DFS
and OS in a neoadjuvant setting
Only 2 RCTs contained survival analysis data in a
neoadjuvant setting. The NOAH trial (42) demonstrated that the addition of
trastuzumab could reduce the risk of relapse and mortality in
comparison with the C-group. Buzdar et al observed that the
DFS at 1 and 3 years was 100% in the T-group (P=0.041) (43). The data required to evaluate DFS and
OS in neoadjuvant treatment groups was not available.
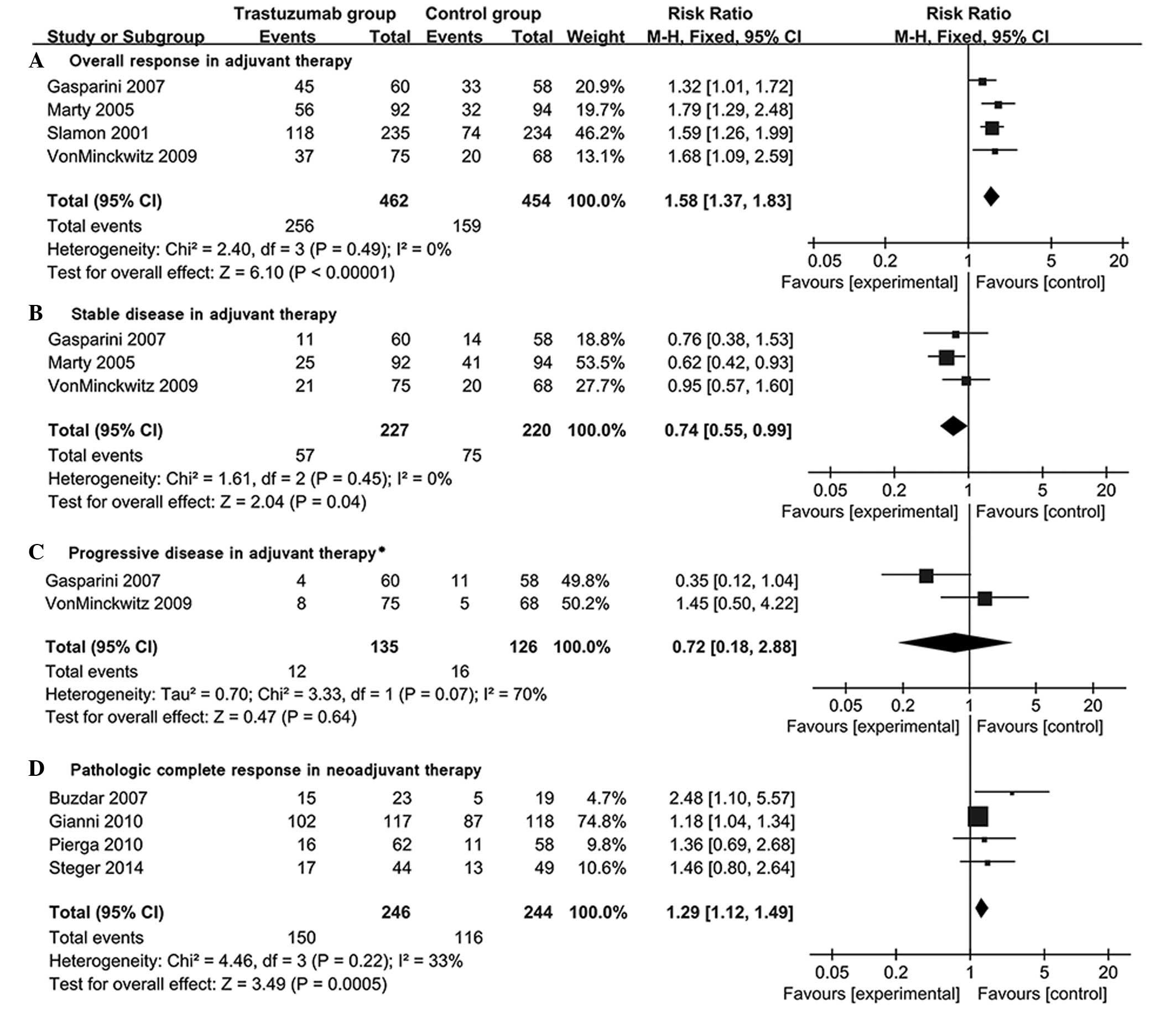
Response rates in adjuvant and
neoadjuvant settings
Tumor response data was available in 4 adjuvant and
4 neoadjuvant trials. Among these, 3 trials (37–39)
adopted the World Health Organization criteria (45) for the response measurement, 2 trials
(34,42) used the response evaluation criteria
in solid tumors and 1 (41) adopted
chevalier criteria. The criteria in a further 2 trials were unclear
(40,43). These studies (34,37–43) were
incorporated in the response analysis for relatively high
concordance among these criteria (46). The OR, SD and PD was analyzed in 4, 3
and 2 adjuvant setting trials, respectively. The rate of OR was
significantly higher in the T-group in comparison with the C-group
[RR=1.58; 95% CI (1.37–1.83); P<0.00001; Fig. 4A). However, the rate of SD in the
T-group was significantly lower than that in the C-group [RR=0.74,
95% CI (0.55–0.99), P=0.04; Fig.
4B]. There was no statistical difference between the rate of PD
between the two groups [RR=0.72; 95% CI (0.18–2.88); P=0.64;
Fig. 4C]. The estimated pCR in 4
neoadjuvant trials demonstrated that the pCR in the T-group (61%;
150/246) was significantly higher compared with the C-group [48%;
116/244; RR=1.29; 95% CI (1.12–1.49); P=0.0005; Fig. 4D].
Safety
According to NCI-CTC 2.0, serious AEs reported in ≥2
trials or with an incidence of >5% were classified into
different systems for analysis. Based on these criteria, serious
AEs in hematological and lymphatic systems, the digestive tract,
the circulatory system, respiratory tract, nervous system,
musculoskeletal system and others were studied in the present
meta-analysis.
Serious AEs in an adjuvant
setting
In comparison with the C-group, patients in the
T-group were more likely to suffer from neutropenia (61.9 vs.
54.2%, P<0.0001), leukopenia (57.1 vs. 48.8%, P<0.0001),
diarrhea (2.9 vs. 1.6%, P=0.002), a decrease in LVEF (8.6 vs. 4.4%,
P=0.007), congestive heart failure (CHF) (2.4 vs. 0.4%,
P<0.00001) and skin/nail changes, including alopecia, rashes,
hand-foot syndrome and erythema (3.2 vs. 2.0%, P=0.02). There was
no statistical difference between other serious AEs (Table II). With respect to fatal AEs, there
was no significant difference between fatal AEs in patients in the
T- (0.4%, 17/4,036) and C-groups (0.2%, 10/4,060, P=0.19).
 | Table II.Incidence of specific serious
AEs. |
Table II.
Incidence of specific serious
AEs.
|
|
|
| AE patients/Total
patients |
|
| Heterogeneity |
|---|
|
|
|
|
|
|
|
|
|---|
| System organ | Specific AEs | Trials (n) | T-group | C-group | RR (95% CI) | P-value | P-value | I2
(%) |
|---|
| Adjuvant
chemotherapy setting |
|
|
|
|
|
|
|
|
|
Hematologic and lymphatic
system | Neutropenia | 4 | 805/1,300 | 693/1,278 | 1.14
(1.08–1.21) |
<0.0001 | 0.64 | 0 |
|
| Febrile
neutropenia | 4 | 140/1,300 | 115/1,278 | 1.20
(0.95–1.52) | 0.12 | 0.79 | 0 |
|
| Anemia | 3 |
34/1,237 |
28/1,218 | 1.19
(0.73–1.94) | 0.49 | 0.47 | 0 |
|
| Leukopenia | 2 | 662/1,160 | 558/1,144 | 1.17
(1.08–1.26) |
<0.0001 | 0.71 | 0 |
|
|
Thrombocytopenia | 2 |
22/1,145 |
18/1,124 | 1.20
(0.64–2.23) | 0.57 | 0.40 | 0 |
|
Digestive tract system | Vomiting | 3 |
76/1,237 |
70/1,218 | 1.07
(0.78–1.46) | 0.68 | 0.53 | 0 |
|
| Diarrhea | 5 | 85/2,982 |
49/2,997 | 1.69
(1.21–2.38) |
0.002 | 0.17 | 38 |
|
|
Nausea/anorexia | 2 | 63/1,160 |
63/1,144 | 0.98
(0.70–1.38) | 0.93 | 0.54 | 0 |
|
|
Stomatitis/mucositis | 3 | 34/1,237 |
43/1,218 | 0.78
(0.50–1.21) | 0.27 | 0.80 | 0 |
|
Circulatory system | LVEF decrease | 7 | 331/3,832 | 175/3,935 | 2.00
(1.21–3.29) |
0.007 | 0.0006 | 75 |
|
| CHF | 7 | 112/4,682 |
18/4,749 | 6.01
(3.71–9.74) | <0.00001 | 0.13 | 40 |
|
Respiratory tract system | Dyspnea | 2 |
4/140 |
6/134 | 0.64
(0.18–2.22) | 0.48 | 0.27 | 16 |
| Nervous
system | Sensory
neuropathy | 3 |
5/232 | 13/228 | 0.39
(0.15–1.03) | 0.06 | 0.89 | 0 |
|
Musculoskeletal system | Arthralgia | 3 |
47/2,842 |
39/2,863 | 1.19
(0.79–1.81) | 0.41 | 0.26 | 25 |
|
| Myalgia | 2 |
59/1,160 |
58/1,144 | 1.00
(0.70–1.43) | 0.99 | 0.98 | 0 |
|
Other | Edema | 3 |
2/232 |
4/228 | 0.55
(0.12–2.54) | 0.44 | 0.87 | 0 |
|
| Skin/nail
changea | 4 |
62/1,914 |
39/1,947 | 1.55
(1.08–2.21) | 0.02 | 0.98 | 0 |
|
| Fever | 2 |
2/169 |
1/168 | 1.66
(0.22–12.50) | 0.63 | 0.63 | 0 |
|
| Infection | 4 | 137/2,890 | 124/2,903 | 1.09
(0.86–1.36) | 0.48 | 0.14 | 45 |
|
|
Asthenia/fatigue | 4 |
92/1,300 |
85/1,278 | 1.06
(0.80–1.41) | 0.68 | 0.77 | 0 |
|
| Painb | 2 |
15/1,774 |
8/1,813 | 1.92
(0.82–4.50) | 0.14 | 0.29 | 11 |
|
| Fatal AEs | 4 |
17/4,036 |
10/4,060 | 1.66
(0.78–3.51) | 0.19 | 0.47 | 0 |
| Neoadjuvant
chemotherapy setting |
|
|
|
|
|
|
|
|
|
Other | Neutropenia | 3 | 93/262 | 82/248 | 1.13
(0.74–1.74) | 0.56 | 0.08 | 60 |
|
| Febrile
neutropenia | 3 | 26/200 | 20/190 | 1.17
(0.71–1.93) | 0.54 | 0.53 | 0 |
|
|
Stomatitis/mucositis | 2 | 3/177 | 8/171 | 0.36
(0.10–1.33) | 0.12 | 0.64 | 0 |
|
| LVEF decrease | 2 | 35/138 | 24/132 | 1.38
(0.87–2.19) | 0.17 | 0.69 | 0 |
Serious AEs in a neoadjuvant
setting
There was no statistical difference in the incidence
of neutropenia, febrile neutropenia, stomatitis/microsites and LVEF
reduction between the T- and C-groups. No mortalities were recorded
as a result of treatment-related toxicities in the two groups
(Table II).
Subgroup analysis of cardiac toxicity
in an adjuvant setting
To further evaluate the cardiac toxicities of
trastuzumab in an adjuvant setting, subgroup analysis was
performed. Trastuzumab was assigned to concurrent chemotherapy in 5
trials (n=3,838) (31,32,34,37,38),
while 2 trials (33,35) assigned sequential therapy (n=3,929).
In the concurrent trials, the incidence of an LVEF decrease
(RR=1.54) and CHF (RR=4.79) in the T-group was significantly higher
than in the C-group (P<0.00001). Similar results were observed
in the sequential trials (LVEF, RR=4.66, P<0.00001; CHF,
RR=12.63, P<0.0001). ITC demonstrated that the incidence of a
reduction in LVEF in the concurrent trials was significantly lower
than that in the sequential trials (RR=0.33, P=0.00704). No
significant difference was detected between the incidence of CHF in
the concurrent and sequential trials (RR=0.379, P=0.36255).
In trials where trastuzumab was administered weekly,
the incidence of LVEF decrease (RR=1.16) and CHF (RR=3.34) was not
significantly different between the T- and C-groups (P=0.47 and
P=0.19, respectively). However, the RR of LVEF decrease (4.62) and
CHF (11.2) was significantly higher in the C-group in comparison
with the T-group when trastuzumab was administered every 3 weeks
(P<0.00001, P<0.0001, respectively; Table III).
 | Table III.Relative risk of cardiac toxicity in
patients with human epidermal growth factor receptor-2-positive
breast cancer treated with adjuvant trastuzumab, stratified by
timing. |
Table III.
Relative risk of cardiac toxicity in
patients with human epidermal growth factor receptor-2-positive
breast cancer treated with adjuvant trastuzumab, stratified by
timing.
|
|
| T-group (n) | C-group (n) |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Treatment | Trials (n) | Cardiac events | Total | Cardiac events | Total | RR (95% CI) | P-value |
|---|
| LVEF reduction |
|
|
|
|
|
|
|
|
Concurrent | 5 | 240 | 1,890 | 155 | 1,948 | 1.54
(1.27–1.86) | <0.00001 |
|
Sequential | 2 | 91 | 1,942 | 20 | 1,987 | 4.66
(2.89–7.53) | <0.00001 |
|
Weekly | 3 | 45 | 771 | 41 | 856 | 1.16
(0.77–1.76) | 0.47 |
| Every 3
weeks | 3 | 92 | 2,019 | 20 | 2,061 | 4.62
(2.88–7.41) | <0.00001 |
| CHF |
|
|
|
|
|
|
|
|
Concurrent | 5 | 75 | 2,740 | 15 | 2,762 | 4.79
(2.80–8.17) | <0.00001 |
|
Sequential | 2 | 37 | 1,942 | 3 | 1,987 | 12.63
(3.90–40.92) | <0.0001 |
|
Weekly | 3 | 53 | 1621 | 8 | 1670 | 3.34
(0.55–20.44) | 0.19 |
| Every 3
weeks | 3 | 38 | 2,019 | 3 | 2,061 | 11.2
(3.76–33.35) | <0.0001 |
Publication bias
The sensitivity analysis revealed that there was no
heterogeneity of recurrence and mortality once the joint study had
been excluded from the analysis (32). This may have been caused by the
slight design inconformity in individual trials. For example,
patients with high-risk, node-negative BC were eligible to be
included in the N9831, but not the B-31, trial, and the
intention-to-treat analysis was not used in either of the 2 trials.
No individual study affected the reduction in LVEF as the omission
of any of the studies did not have an impact on the analysis.
Funnel plots of DFS, OS, OR, LVEF reduction and CHF in an adjuvant
setting, and pCR in a neoadjuvant setting, revealed no evident
publication bias. The results were further confirmed by the Begg's
and Egger's tests (Table IV).
 | Table IV.Publication bias accessed by Begg and
Egger tests. |
Table IV.
Publication bias accessed by Begg and
Egger tests.
| Items | Begg's test | Egger's test |
|---|
| Disease free
survival | 1.000 | 0.904 |
| Overall
survival | 0.711 | 0.682 |
| OR in adjuvant
setting | 0.734 | 0.627 |
| pCR in neoadjuvant
setting | 0.308 | 0.150 |
| LVEF reduction | 0.764 | 0.651 |
| Congestive heart
failure | 0.548 | 0.562 |
Discussion
Trastuzumab serves a crucial function in the
treatment of patients that are HER-2 positive. To evaluate the
prognostic effects of trastuzumab, the AEs and administration
timings of trastuzumab in a number of clinical trials in adjuvant
and neoadjuvant settings for patients with HER-2 positive BC were
collected, and high quality RCTs published in recent years were
included and analyzed in the present meta-analysis. In comparison
with published meta-analyses which have reviewed trastuzumab
treatment in patients with BC (Table
V), a number of improvements were achieved in the current
study. The present meta-analysis contained 13 RCTs with a total of
14,546 patients with HER-2 positive BC; to the best of our
knowledge this is the largest number of samples included in a
meta-analysis of this topic. In addition, an increased number of
trials with longer follow-up periods were studied in the present
meta-analysis in comparison with previous analyses.
 | Table V.Meta-analyses on efficacy and safety
of trastuzumab with chemotherapy for HER-2 positive breast
cancer. |
Table V.
Meta-analyses on efficacy and safety
of trastuzumab with chemotherapy for HER-2 positive breast
cancer.
| Author, year | Object | Studies | Patients | Outcomes | Effect | Major findings | (Refs.) |
|---|
| Valachis et
al, 2011a | BC | 5 | 515 | pCR rate, BCS rate,
neutropenia, neutropenic fever, cardiac AEs | RR | The probability to
achieve pCR was higher for trastuzumab group. No significant
difference in terms of BCS rate. The addition of trastuzumab did
not increase the incidence of neutropenia, neutropenic fever, and
cardiac AEs. | (14) |
| Yin et al,
2011 | Early stage BC | 6 | 12,877 | DFS, OS, recurrence
of locoregional, distant, contralateral BC and CNS | OR | Patients in
trastuzumab group derived benefit in DFS, OS, locoregional and
distant recurrence, but did worse in CNS recurrence. Concomitant
use of trastuzumab lowered the risk of death but bore a higher CNS
recurrence risk, OS and CNS recurrence had no difference between
the sequential and observation groups. | (16) |
| Bria et al,
2008 | Early stage BC | 5 | 1,186 | DFS & OS | RR | DFS and OS were
significantly prolonged in trastuzumab group with a higher
incidence of CHF, LFEV reduction and BM. | (19) |
|
|
|
|
| Cardio toxicity
& BM | RR |
|
|
| Olson et al,
2013 | BC | 4 | 9,020 | CNS recurrence | RR | Adjuvant
trastuzumab was associated with a significant increased risk of CNS
metastases as the site of first recurrence in BC patients. | (47) |
| Brollo et
al, 2013 | BC ≥60 years | 5 | 1,084 | DFS | HR | Compared with
chemotherapy alone, 47% relative risk reduction was observed in
trastuzumab group. Proportion of cardiac events in older patients
treated with trastuzumab was 5%. | (48) |
|
|
|
|
| Cardio
toxicity | RR |
|
|
| Petrelli et
al, 2012b | Early stage BC | 6 | 13,331 | DFS, OS | HR | In concomitant
analysis, DFS and OS were longer, but risk of severe cardiac events
had no increased in the trastuzumab group; In sequential analysis,
DFS was longer but not OS, risk of severe cardiac events was
increased in trastuzumab group. | (49) |
|
|
|
|
| Cardiac events | RR |
|
|
| Liao et al,
2011 | Advanced stage
BC | 4 | 815 | OS | HR | Addition of
trastuzumab provided substantial benefit in terms of ORR, CR, and
OS. The incidence of neutropenia, grade 3–4 neutropenia, overall
anemia and cardiac toxicity were significantly higher in
trastuzumab group. | (50) |
|
|
|
|
| ORR & CR | RR |
|
|
|
|
|
|
| Cardio toxicity,
neutropenia, anemia | OR |
|
|
| Dahabreh et
al, 2008 | Early stage BC | 5 | 13,493 | DFS, mortality,
locoregional recurrence, distant recurrence | RR | Superiority was
observed for patients receiving trastuzumab with respect to DFS,
mortality, locoregional recurrence, and distant recurrence.
Patients in trastuzumab group had a higher risk for CHF, LVEF, and
CNS metastasis. | (51) |
|
|
|
|
| Cardio toxicity,
CNS recurrence | RR |
|
|
| Zhu et al,
2013c | Metastatic BC | 5 | 1,043 | OS, TTP | HR | The addition of
trastuzumab to chemotherapy improved OS, while to hormone therapy
did not. All trastuzumab-containing regimens increased cardiac
toxicity and grade 3–4 AEs. | (11) |
|
|
|
|
| ORR, clinical
benefit ratio | RR |
|
|
|
|
|
|
| Cardiac toxicity,
grade 3–4 AEs | RR |
|
|
| Moja et al,
2012 | Early stage BC | 8 | 11,991 | OS, DFS | HR | Adjuvant
trastuzumab improved survival with increased risk of cardiac
toxicity. Also, studies with concurrent administration showed
similar efficacy and toxicity results to sequential studies. | (52) |
|
|
|
|
| Cardiac
toxicity | RR |
|
|
The results from the present study demonstrated that
the administration timings of trastuzumab can influence patient
survival and cardiac toxicity. Aside from the most commonly studied
cardiac toxicity, AEs of a number of systems and organs were
analyzed in adjuvant and neoadjuvant settings in the present
meta-analysis. DFS and OS may be regarded as the golden criteria to
evaluate the long-term effects of trastuzumab, and the results from
the current study demonstrated that the addition of trastuzumab in
chemotherapy is able to increase DFS and OS in patients with HER-2
positive BC more effectively than in patients treated with
chemotherapy alone, and similar results have been reported in
previous meta-analyses (47–52). Notably, a number of subtle
differences in the benefits of DFS and OS emerged when analyzing
studies with extended follow-up periods. A joint analysis included
in the present meta-analysis indicated that DFS and OS at 3 years
follow-up were 87.1 and 94.3% in the T-group, and 75.4 and 91.7% in
C-group, respectively (32);
however, at 4 years, DFS and OS were 85.3 and 86.6% in the T-group,
and 67.1 and 91.4% in the C-group, respectively. Compared with the
C-group, the benefit of DFS at 4-years in T-group still displayed
statistical significance in the HERA trial, however, the OS benefit
was no longer statistically significant by intention-to-treat
analysis (33,53). A previous meta-analysis demonstrated
that the benefits of certain chemotherapies may improve after a 10-
and 15-year follow up period (2). In
summary, it was observed in the present study that long-term
observation is required to determine the extent of the benefits of
trastuzumab treatment in patients with HER-2 positive BC.
The subgroup analysis results from the present study
determined that the optimum method of administering trastuzumab is
to follow specific timings of administration in order to improve
the DFS in patients with BC. In comparison with the C-group, the
rate of OS improved in trials concurrently administering
trastuzumab; however, this improvement was not observed in studies
that administered trastuzumab sequentially. Notably, the present
meta-analysis demonstrated that there is a significant improvement
in DFS and OS when trastuzumab is administered weekly. By contrast,
the administration of trastuzumab every 3 weeks improved DFS but
not OS. In addition, ITC concluded that weekly trastuzumab
administration improved the rate of DFS and OS compared with
administration every 3 weeks. Survival analysis was not performed
in the neoadjuvant setting due to the absence of mature data;
therefore, it was not possible to arbitrarily conclude that the use
of trastuzumab is beneficial for the survival outcomes of patients
with HER-2 positive BC.
In an adjuvant setting, the OR was higher in the
T-group in comparison with the C-group; however, the rates of SD
and PD in the C-group were higher compared with those in the
T-group. This may be a result of the small sample size and the
movement of patients in the C-groups of two studies (37,38), who
changed from receiving just chemotherapy to receiving chemotherapy
plus trastuzumab prior to pre-specified endpoints. One of the two
aforementioned studies (38)
involved 56.4% (53/94) patients in C-group who crossed over to
receive trastuzumab, which may have diluted some of the survival
benefit conferred by trastuzumab. In addition, a number of patients
in the T-group who responded to treatment were not followed-up
after receiving the initial efficacy results.
With the exception of efficacy, AEs are important
factors that should be taken into consideration when using
trastuzumab. In an adjuvant setting, the present meta-analysis
demonstrated that there are higher risks of grade 3–4 neutropenia,
leukopenia, diarrhea, skin/nail change, LVEF reduction and CHF in
T-groups in comparison with C-groups; however, there was no
difference in the presence of fatal AEs between the two groups.
Fortunately, trastuzumab-associated AEs were manageable and
reversible by discontinuing treatment (54–56). In
the neoadjuvant setting, there was no statistical difference in the
incidence of AEs between the two groups, and no mortalities
resulting from trastuzumab-related toxicities were recorded. The
present meta-analysis therefore indicates that AEs resulting from
trastuzumab are manageable in adjuvant and neoadjuvant
settings.
Cardiac toxicity is a common adverse reaction to
trastuzumab and limits its use in patients with BC (20). Therefore, a detailed subgroup
analysis of cardiac AEs resulting from trastuzumab administration
was performed in an adjuvant setting. This analysis demonstrated
that weekly administration of trastuzumab does not increase the
incidence of LVEF reduction and CHF, while the other three
time-intervals (every 3 weeks, concurrent and sequential) may
result in an increased risk of cardiac toxicity. ITC demonstrated
that concurrent and weekly trastuzumab administration may result in
fewer cardiac AEs in comparison with trastuzumab administered
sequentially and every 3 weeks, and this was a similar result to
that obtained by direct comparison. Although the results of ITC had
not been seriously affected by the characteristics of patients, the
quality, statistical methods and the intervention measures across
trials, which are used as the evaluation standard of ITC analysis
validity, differed between trials. In particular, the length of the
follow-up period and the time frame of the RCTs may create bias
within the results. Therefore, the results from the present study
should be interpreted with caution.
There were a number of inconsistencies within the
trials analyzed in this meta-analysis. The LVEF baseline recordings
were obtained at different time points; for example, in one trial
the baseline reading was recorded following completion of the
primary treatment (which included surgery, radiotherapy and
neoadjuvant or adjuvant chemotherapy, or both) (33), while in another trial the baseline
reading was recorded prior to chemotherapy (36). The reported data regarding reduction
in LVEF also had a number of inconsistencies; a decline of >10%
in LVEF was observed in 3 trials (31,33,34),
>15% in 3 trials (32,35,38) and
>20% in one trial (36). In
addition, patients were considered ineligible if ventricular
hypertrophy was detected on echocardiography in the NSABP B-31
trial, and poorly controlled hypertension and clinically
significant pericardial effusion were exclusion criteria in the
N9831 trial (57). In view of these
inconsistencies, the results from the present study may have been
different if the same criteria were applied to all RCTs
analyzed.
A number limitations existed in the present study;
although the Begg's and Egger's tests revealed no publication bias
in the meta-analysis, the studies included in the analysis were
conducted by different investigators from different institutions.
Thus, potential publication bias remains a possibility. In
addition, inconsistent methods of accessing the HER-2 status and
different treatment schedules (weekly, every 3 weeks, concurrent
and sequential) of patients may influence the accuracy of results.
Furthermore, patients in the C-group changed to receive trastuzumab
during the study; this may have reduced the survival advantage and
treatment efficacy to an extent. Despite the limitations mentioned
above, the prediction of the efficacy and safety of trastuzumab may
be regarded as accurate and dependable in the present study.
In conclusion, the present meta-analysis supports
the clinical practice of treating patients with HER-2 positive BC
with a combination of trastuzumab and chemotherapy. AEs resulting
from trastuzumab treatment were acceptable and manageable both in
adjuvant and neoadjuvant settings. Furthermore, with regards to
survival and cardiac toxicity, concurrent and weekly administration
of trastuzumab has been demonstrated to be more effective than
treatment with trastuzumab sequentially and every 3 weeks. Further
research is required to confirm the findings of the present study
and support the use of trastuzumab in clinical practice.
Acknowledgements
The present study was supported by the Project of
the National Natural Science Foundation of China (grant no.
81572874)
Glossary
Abbreviations
Abbreviations:
|
BC
|
breast cancer
|
|
HER-2
|
human epidermal growth factor
receptor-2
|
|
RCTs
|
randomized control trials
|
|
AEs
|
adverse events
|
|
LVEF
|
left ventricular ejection fraction
|
|
CHF
|
congestive heart failure
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
OR
|
overall response
|
|
HR
|
hazard ratio
|
|
RR
|
risk ratio
|
|
CI
|
confidence interval
|
|
T-group
|
trastuzumab plus chemotherapy
group
|
|
C-group
|
chemotherapy group
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mauri D, Pavlidis N and Ioannidis JP:
Neoadjuvant versus adjuvant systemic treatment in breast cancer: A
meta-analysis. J Natl Cancer Inst. 3:188–194. 2005. View Article : Google Scholar
|
|
4
|
Reese DM and Slamon DJ: HER-2/neu signal
transduction in human breast and ovarian cancer. Stem Cells.
15:1–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ross JS, Slodkowska EA, Symmans WF,
Pusztai L, Ravdin PM and Hortobagyi GN: The HER-2 receptor and
breast cancer: Ten years of targeted anti-HER-2 therapy and
personalized medicine. Oncologist. 14:320–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nabholtz JM and Gligorov J:
Docetaxel/trastuzumab combination therapy for the treatment of
breast cancer. Expert Opin Pharmacother. 6:1555–1564. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Slamon DJ, Godolphin W, Jones LA, Holt JA,
Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J and Ullrich A:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moasser MM: The oncogene HER2: Its
signaling and transforming functions and its role in human cancer
pathogenesis. Oncogene. 26:6469–6487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oostra DR and Macrae ER: Role of
trastuzumab emtansine in the treatment of HER2-positive breast
cancer. Breast Cancer (Dove Med Press). 6:103–113. 2014.PubMed/NCBI
|
|
11
|
Zhu ZL, Zhang J, Chen ML and Li K:
Efficacy and safety of Trastuzumab added to standard treatments for
HER2-positive metastatic breast cancer patients. Asian Pac J Cancer
Prev. 14:7111–7116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jelovac D and Wolff AC: The adjuvant
treatment of HER2-positive breast cancer. Curr Treat Options Oncol.
13:230–239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Osoba D, Slamon DJ, Burchmore M and Murphy
M: Effects on quality of life of combined trastuzumab and
chemotherapy in women with metastatic breast cancer. J Clin Oncol.
20:3106–3113. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valachis A, Mauri D, Polyzos NP,
Chlouverakis G, Mavroudis D and Georgoulias V: Trastuzumab combined
to neoadjuvant chemotherapy in patients with HER2-positive breast
cancer: A systematic review and meta-analysis. Breast. 20:485–490.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rastogi P, Anderson SJ, Bear HD, Geyer CE,
Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil
SR, et al: Preoperative chemotherapy: Updates of national surgical
adjuvant breast and Bowel project protocols B-18 and B-27. J Clin
Oncol. 26:778–785. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yin W, Jiang Y, Shen Z, Shao Z and Lu J:
Trastuzumab in the adjuvant treatment of HER2-positive early breast
cancer patients: A meta-analysis of published randomized controlled
trials. PLoS One. 6:e210302011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Azim HA Jr, de Azambuja E, Paesmans M and
Piccart-Gebhart MJ: Sequential or concurrent administration of
trastuzumab in early breast cancer? Too early to judge. J Clin
Oncol. 28:e353–e354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perez EA, Suman VJ, Davidson NE, Sledge
GW, Kaufman PA, Hudis CA, Martino S, Gralow JR, Dakhil SR, Ingle
JN, et al: Cardiac safety analysis of doxorubicin and
cyclophosphamide followed by paclitaxel with or without trastuzumab
in the north central cancer treatment group N9831 adjuvant breast
cancer trial. J Clin Oncol. 26:1231–1238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bria E, Cuppone F, Fornier M, Nisticò C,
Carlini P, Milella M, Sperduti I, Terzoli E, Cognetti F and
Giannarelli D: Cardiotoxicity and incidence of brain metastases
after adjuvant trastuzumab for early breast cancer: The dark side
of the moon? A meta-analysis of the randomized trials. Breast
Cancer Res Treat. 109:231–239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan-Chiu E, Yothers G, Romond E, Geyer CE
Jr, Ewer M, Keefe D, Shannon RP, Swain SM, Brown A, Fehrenbacher L,
et al: Assessment of cardiac dysfunction in a randomized trial
comparing doxorubicin and cyclophosphamide followed by paclitaxel,
with or without trastuzumab as adjuvant therapy in node-positive,
human epidermal growth factor receptor 2-overexpressing breast
cancer: NSABP B-31. J Clin Oncol. 23:7811–7819. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. BMJ. 339:b25352009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Holmes DR: Intraoperative radiotherapy in
breast conserving surgery. J Surg Oncol. 110:68–74. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L and Sterne JA:
Cochrane Bias Methods Group; Cochrane Statistical Methods Group:
The Cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bucher HC, Guyatt GH, Griffith LE and
Walter SD: The results of direct and indirect treatment comparisons
in meta-analysis of randomized controlled trials. J Clin Epidemiol.
50:683–691. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Loke YK, Pradhan S, Yeong JK and Kwok CS:
Comparative coronary risks of apixaban, rivaroxaban and dabigatran:
A meta-analysis and adjusted indirect comparison. Br J Clin
Pharmacol. 78:707–717. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang N and Sobieraj DM: Indirect treatment
comparison of new oral anticoagulants for the treatment of acute
venous thromboembolism. Thromb Res. 133:1145–1151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Slamon D, Eiermann W, Robert N, Pienkowski
T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, et
al: Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J
Med. 365:1273–1283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perez EA, Romond EH, Suman VJ, Jeong JH,
Davidson NE, Geyer CE Jr, Martino S, Mamounas EP, Kaufman PA and
Wolmark N: Four-year follow-up of trastuzumab plus adjuvant
chemotherapy for operable human epidermal growth factor receptor
2-positive breast cancer: Joint analysis of data from NCCTG N9831
and NSABP B-31. J Clin Oncol. 29:3366–3373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gianni L, Dafni U, Gelber RD, Azambuja E,
Muehlbauer S, Goldhirsch A, Untch M, Smith I, Baselga J, Jackisch
C, et al: Treatment with trastuzumab for 1 year after adjuvant
chemotherapy in patients with HER2-positive early breast cancer: A
4-year follow-up of a randomised controlled trial. Lancet Oncol.
12:236–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
von Minckwitz G, du Bois A, Schmidt M,
Maass N, Cufer T, de Jongh FE, Maartense E, Zielinski C, Kaufmann
M, Bauer W, et al: Trastuzumab beyond progression in human
epidermal growth factor receptor 2-positive advanced breast cancer:
A german breast group 26/breast international group 03–05 study. J
Clin Oncol. 27:1999–2006. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Spielmann M, Roché H, Delozier T, Canon
JL, Romieu G, Bourgeois H, Extra JM, Serin D, Kerbrat P, Machiels
JP, et al: Trastuzumab for patients with axillary-node-positive
breast cancer: Results of the FNCLCC-PACS 04 trial. J Clin Oncol.
27:6129–6134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Joensuu H, Bono P, Kataja V, Alanko T,
Kokko R, Asola R, Utriainen T, Turpeenniemi-Hujanen T, Jyrkkiö S,
Möykkynen K, et al: Fluorouracil, epirubicin and cyclophosphamide
with either docetaxel or vinorelbine, with or without trastuzumab,
as adjuvant treatments of breast cancer: Final results of the
FinHer Trial. J Clin Oncol. 27:5685–5692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gasparini G, Gion M, Mariani L, Papaldo P,
Crivellari D, Filippelli G, Morabito A, Silingardi V, Torino F,
Spada A, et al: Randomized Phase II Trial of weekly paclitaxel
alone versus trastuzumab plus weekly paclitaxel as first-line
therapy of patients with Her-2 positive advanced breast cancer.
Breast Cancer Res Treat. 101:355–365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marty M, Cognetti F, Maraninchi D, Snyder
R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Antón A, Lluch A,
et al: Randomized phase II trial of the efficacy and safety of
trastuzumab combined with docetaxel in patients with human
epidermal growth factor receptor 2-positive metastatic breast
cancer administered as first-line treatment: The M77001 study
group. J Clin Oncol. 23:4265–4274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Steger GG, Greil R, Lang A, Rudas M,
Fitzal F, Mlineritsch B, Hartmann BL, Bartsch R, Melbinger E,
Hubalek M, et al: Epirubicin and docetaxel with or without
capecitabine as neoadjuvant treatment for early breast cancer:
Final results of a randomized phase III study (ABCSG-24). Ann
Oncol. 25:366–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pierga JY, Delaloge S, Espié M, Brain E,
Sigal-Zafrani B, Mathieu MC, Bertheau P, Guinebretière JM,
Spielmann M, Savignoni A and Marty M: A multicenter randomized
phase II study of sequential epirubicin/cyclophosphamide followed
by docetaxel with or without celecoxib or trastuzumab according to
HER2 status, as primary chemotherapy for localized invasive breast
cancer patients. Breast Cancer Res Treat. 122:429–437. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gianni L, Eiermann W, Semiglazov V,
Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M,
Lichinitser M, et al: Neoadjuvant chemotherapy with trastuzumab
followed by adjuvant trastuzumab versus neoadjuvant chemotherapy
alone, in patients with HER2-positive locally advanced breast
cancer (the NOAH trial): A randomised controlled superiority trial
with a parallel HER2-negative cohort. Lancet. 375:377–384. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Buzdar AU, Valero V, Ibrahim NK, Francis
D, Broglio KR, Theriault RL, Pusztai L, Green MC, Singletary SE,
Hunt KK, et al: Neoadjuvant therapy with paclitaxel followed by
5-fluorouracil, epirubicin and cyclophosphamide chemotherapy and
concurrent trastuzumab in human epidermal growth factor receptor
2-positive operable breast cancer: An update of the initial
randomized study population and data of additional patients treated
with the same regimen. Clin Cancer Res. 13:228–233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Buzdar AU, Ibrahim NK, Francis D, Booser
DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano
SH, et al: Significantly higher pathologic complete remission rate
after neoadjuvant therapy with trastuzumab, paclitaxel and
epirubicin chemotherapy: Results of a randomized trial in human
epidermal growth factor receptor 2-positive operable breast cancer.
J Clin Oncol. 23:3676–3685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
World Health Organisation: WHO handbook
for reporting results of cancer treatment. WHO offset publication
no 48. World Health Organisation. (Geneva, Switzerland). 1979.
|
|
46
|
Khokher S, Qureshi MU and Chaudhry NA:
Comparison of WHO and RECIST criteria for evaluation of clinical
response to chemotherapy in patients with advanced breast cancer.
Asian Pac J Cancer Prev. 13:3213–3218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Olson EM, Abdel-Rasoul M, Maly J, Wu CS,
Lin NU and Shapiro CL: Incidence and risk of central nervous system
metastases as site of first recurrence in patients with
HER2-positive breast cancer treated with adjuvant trastuzumab. Ann
Oncol. 24:1526–1533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Brollo J, Curigliano G, Disalvatore D,
Marrone BF, Criscitiello C, Bagnardi V, Kneubil MC, Fumagalli L,
Locatelli M, Manunta S and Goldhirsch A: Adjuvant trastuzumab in
elderly with HER-2 positive breast cancer: A systematic review of
randomized controlled trials. Cancer Treat Rev. 39:44–50. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Petrelli F and Barni S: Meta-analysis of
concomitant compared to sequential adjuvant trastuzumab in breast
cancer: The sooner the better. Med Oncol. 29:503–510. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liao C, Yin F, Huang P, Cao Y and Gao F: A
meta-analysis of randomized controlled trials comparing
chemotherapy plus trastuzumab with chemotherapy alone in
HER-2-positive advanced breast cancer. Breast J. 17:109–111. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dahabreh IJ, Linardou H, Siannis F,
Fountzilas G and Murray S: Trastuzumab in the adjuvant treatment of
early-stage breast cancer: A systematic review and meta-analysis of
randomized controlled trials. Oncologist. 13:620–630. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Moja L, Tagliabue L, Balduzzi S, Parmelli
E, Pistotti V, Guarneri V and D'Amico R: Trastuzumab containing
regimens for early breast cancer. Cochrane Database Syst Rev.
4:CD0062432012.PubMed/NCBI
|
|
53
|
Piccart-Gebhart MJ, Procter M,
Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga
J, Bell R, Jackisch C, et al: Trastuzumab after adjuvant
chemotherapy in HER2-positive breast cancer. N Engl J Med.
353:1659–1672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Russell SD, Blackwell KL, Lawrence J,
Pippen JE Jr, Roe MT, Wood F, Paton V, Holmgren E and Mahaffey KW:
Independent adjudication of symptomatic heart failure with the use
of doxorubicin and cyclophosphamide followed by trastuzumab
adjuvant therapy: A combined review of cardiac data from the
national surgical adjuvant breast and Bowel project B-31 and the
north central cancer treatment group N9831 clinical trials. J Clin
Oncol. 28:3416–3421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Procter M, Suter TM, de Azambuja E, Dafni
U, van Dooren V, Muehlbauer S, Climent MA, Rechberger E, Liu WT,
Toi M, et al: Longer-term assessment of trastuzumab-related cardiac
adverse events in the Herceptin adjuvant (HERA) trial. J Clin
Oncol. 28:3422–3428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
de Azambuja E, Bedard PL, Suter T and
Piccart-Gebhart M: Cardiac toxicity with anti-HER-2 therapies: What
have we learned so far? Target Oncol. 4:77–88. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Romond EH, Perez EA, Bryant J, Suman VJ,
Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman
PA, et al: Trastuzumab plus adjuvant chemotherapy for operable
HER2-positive breast cancer. N Engl J Med. 353:1673–1684. 2005.
View Article : Google Scholar : PubMed/NCBI
|