Introduction
Atherosclerotic (ATS) plaques consists of two main
components: (i) Soft, lipid-rich atheromatous mass and (ii) hard,
collagen-rich sclerotic tissue (1).
The sclerotic component, which is fibrous tissue, is the more
voluminous component of the plaque which appears to stabilize the
plaques and protect against disruption. In contrast, the less
voluminous atheromatous component is less stable as the soft
atheromatous gruel destabilizes the plaque, thus leaving it
vulnerable to rupture. Upon plaque rupture, the highly thrombogenic
gruel is exposed to the blood, leading to thrombosis, which is a
characteristic feature of unstable plaques (1).
The majority of disrupted plaques have been shown to
have a large necrotic core with a thin overlying fibrous cap, with
substantial inflammation and limited calcification (2). It has been hypothesized that they do
not cause critical narrowing of the carotid lumen, due to outward
(expansive or positive) remodeling of the arterial segment
(3). In addition, it has been
suggested that lesions with similar histomorphological
characteristics, but intact fibrous caps, have a high risk of
rupture (2).
The aim of the present study was to characterize a
number of carotid ATS plaques, which were obtained by
endarterectomy, for the purpose of determining the importance of
various pathological characteristics, including fibrous cap
thickness and plaque, necrotic core, macrophage and calcification
areas. The study included stable plaques [SP; including
fibroatheromas (FAs) and fibrocalcified plaques (FCs)], vulnerable
plaques [VP; including thin-cap fibroatheromas (TCFAs)], and
unstable plaques [USP; featuring plaque rupture (PR), plaque
erosion (PE) and calcified nodules (CNs)], as previously defined by
pathologists (4).
The present study elucidated the associations
between plaque characteristics and the risk factors for the
development of CVD including age, smoking, arterial hypertension,
hyperlipidemia and diabetes mellitus. We hypothesized that the
present study may contribute to the improved understanding of acute
coronary events and underlying plaque composition and its
association with coronary risk factors, in order to improve the
timely identification of vulnerable plaques.
Materials and methods
Patients
A total of 26 patients that underwent carotid artery
endarterectomy to treat high-grade internal carotid artery stenosis
at the ‘Prof. Dr. George I.M. Georgescu’ Institute of
Cardiovascular Diseases (Iasi, Romania) between January and
December 2013 participated in the present study. Excised carotid
ATS plaques were obtained from 20 male and 6 female patients, aged
35–80 years, who presented with transient ischemic attack symptoms
upon diagnostic consultation. Ethical approval for the present
study was granted by the Institutional Board at Prof. Dr. George
I.M. Georgescu’ Institute of Cardiovascular Diseases. Written
informed consent was obtained from the patients.
Histological examination
Carotid endarterectomy was performed under
anesthesia using fentanyl (5–15 mcg/kg; Gedeon Richter Plc.,
Budapest, Hungary), midazolam (0.05–0.1 mg/kg; Hofman-La Roche
Ltd., Basel, Switzerland), and rocuronium (0.6 mg/kg; Fresenius
Kabi Austria GmbH, Graz, Austria) for induction and maintained with
isoflurane (Rompharm Company, Otopeni, Romania). The entire intimal
carotid plaques (length, ~1 cm) were removed from the carotid
arteries. Excessively fragmented plaques (>2 pieces) were not
subjected to further analysis. The carotid artery atherosclerotic
plaques were subsequently sectioned at 3–4-mm intervals and
processed for histological examination. All sections were stained
with hematoxylin and eosin, elastic Van Gieson, Masson's trichrome
and movat pentachrome (all Bio Optica Milano SpA, Milan, Italy).
Hematoxylin stains the nuclei and calcified material in cells blue,
whereas eosin stains eosinophilic structures in various shades of
red. Elastic Van Gieson is a histological stain for elastic and
collagen fibers and Masson's trichrome and movat pentachrome stain
muscle fibers, collagen and nuclei. Masson's trichome stain is used
to detect connective tissue (green) and muscle tissue (red)
characterized by fibrotic and degenerative changes; whereas movat
pentachrome stain differentiates between the various ages and types
of collagen and connective tissue matrix in the ATS plaques.
Microscopic sections were analyzed with the observer blinded to the
clinical status of the patients whose plaques were being
examined.
Classification
ATS plaques were classified as either VP, SP or USP,
as previously described (5). Lesions
displaying a thin fibrous cap with infiltration of macrophages and
a large necrotic core containing numerous cholesterol clefts were
defined TCFA, a type of VP. In carotid TCFAs, the area of the
necrotic core is ≤3 mm2, and the cap thickness of a
vulnerable lesion is ≤165 µm. With regard to SPs, those with a
large lipid-necrotic core containing extracellular lipid,
cholesterol crystals and necrotic debris covered by a thick fibrous
cap were considered to be FAs, whereas plaques with a small or
absent lipid-laden necrotic core and a thick fibrous cap overlying
extensive accumulations of calcium in the intima close to the media
were considered FC plaques. In USP thrombotic plaques, thrombi
occur as a consequence of one of the three following events: PR, PE
or, less frequently, CNs. PR was defined as an area of fibrous cap
disruption in which the overlying thrombus was in continuity with
the underlying necrotic core. PE was identified when a thrombus
covers a fibrous cap with no defect. Typically, the endothelium is
absent from the erosion site. CNs are lesions with fibrous cap
disruption and thrombi associated with dense nodules exhibiting
calcification.
Histomorphometric analysis
Histological assessment was performed by an
experienced pathologist using an optical microscope (CX41; Olympus
Corporation, Tokyo, Japan). The measurements were visualized using
color image analysis software (QuickPHOTO MICRO 3.0, PROMICRA,
S.r.o., Prague, Czech Republic). Quantitative morphometry included
measurement of the plaque, lipid necrotic core, inflammatory and
calcified areas and fibrous cap thickness.
Risk factor assessment
The following potential risk factors (RFs) for
atherosclerosis were also assessed: Age, gender, diabetes mellitus
(DM), arterial hypertension, history of cigarette smoking,
cerebrovascular diseases and hyperlipidemia. All RF data was
obtained from the patients' files.
Statistical analysis
The association among the pathological
characteristics of the three defined plaque types, as well as among
plaque types and ATS RFs were evaluated. Data are expressed as mean
values and percentages, calculated using Excel software (Microsoft
Corporation, Redmond, WA, USA).
Results
Plaque types
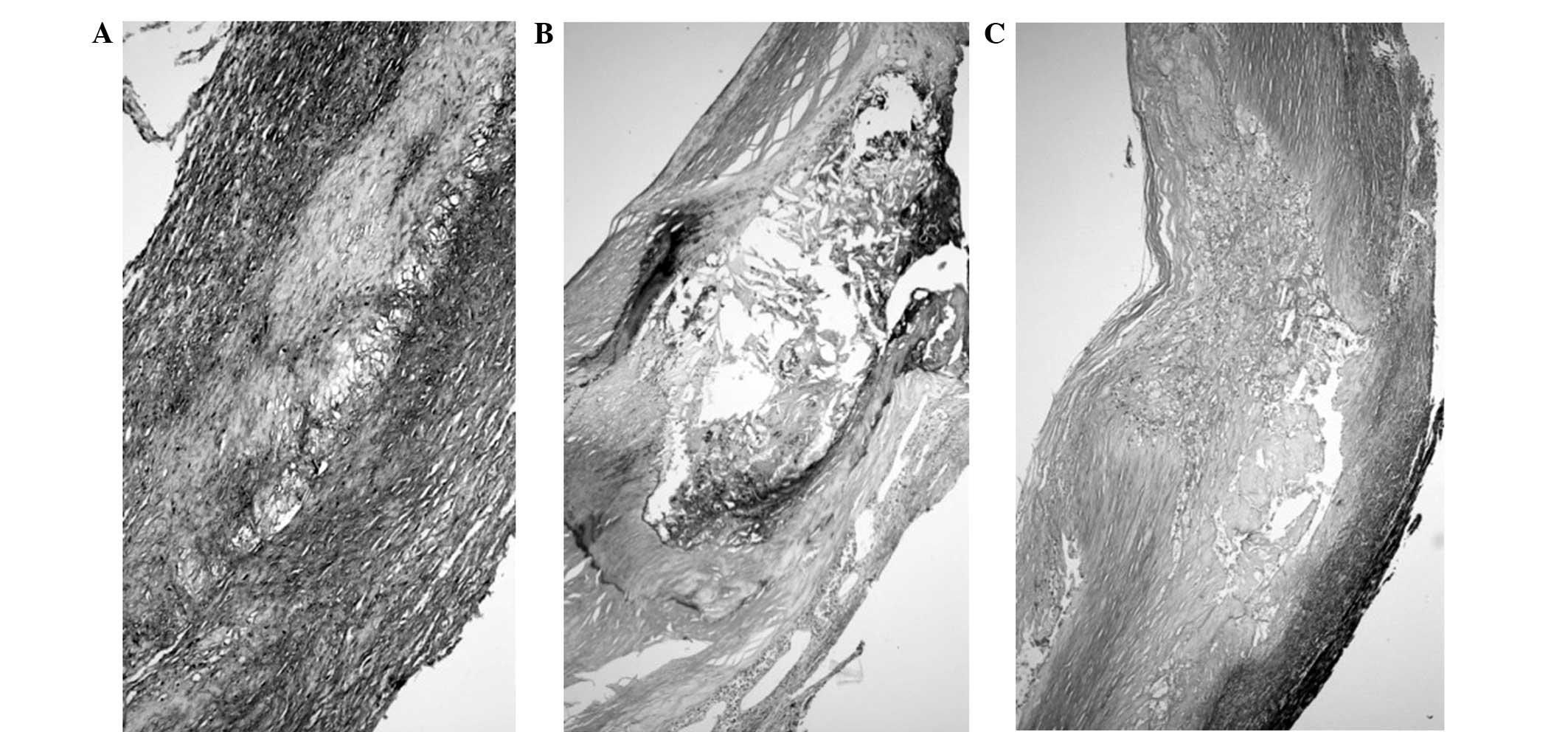
A total of 26 carotid ATS plaques were
histologically analyzed and classified as follows (Table I): i) 6 cases of SP (23%), including
4 fibroatheroma (Fig. 1A) and 2
fibrocalcified plaques (Fig. 1B);
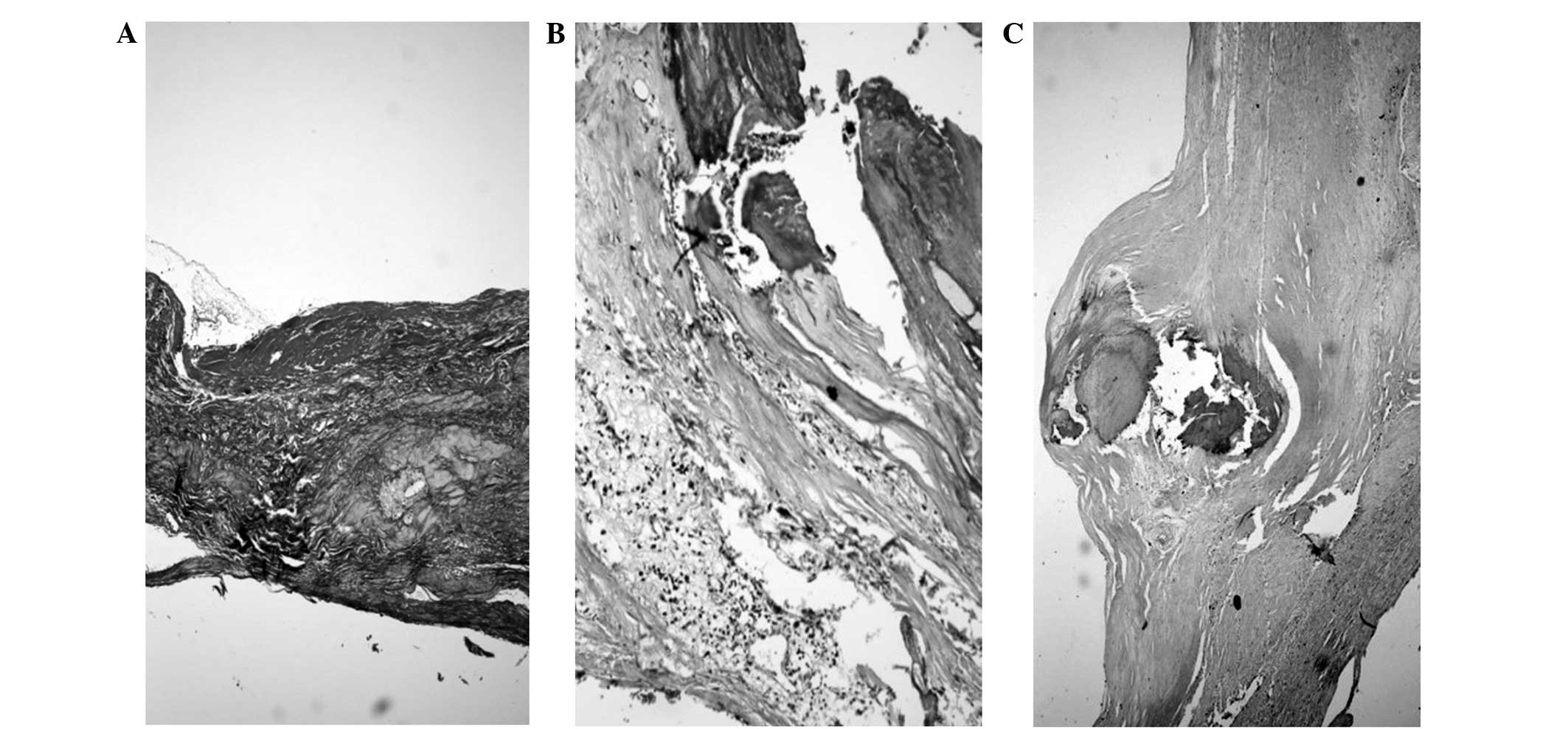
ii) 14 cases of VP (54%), indicated by TCFA (Fig. 1C); and iii) 6 USPs (23%), including 2
cases each of PE (Fig. 2A), PR
(Fig. 2B) and CNs (Fig. 2C).
 | Table I.Carotid atherosclerotic plaques. |
Table I.
Carotid atherosclerotic plaques.
|
| Patients (n=26) |
|---|
|
|
|
|---|
| Plaque type | n | % |
|---|
| Stable | 6 | 23 |
|
Fibroatheroma | 4 |
|
|
Fibro-calcified | 2 |
|
| Vulnerable | 14 | 54 |
| Thin-cap
fibroatheroma | 14 |
|
| Unstable | 6 | 23 |
| Plaque
erosion | 2 |
|
| Plaque
rupture | 2 |
|
| Calcified
nodules | 2 |
|
Histomorphometric analysis
Morphometric analysis was performed to evaluate the
fibrous cap thickness and necrotic core size, in addition to the
inflammatory and calcified plaque areas of the ATS plaques
(Table II).
 | Table II.Carotid atherosclerotic plaque
measurements. |
Table II.
Carotid atherosclerotic plaque
measurements.
| Plaque type | Fibrous cap, µm | Necrotic core, % | Inflammatory
infiltrate, % | Calcification, % |
|---|
| FA | 351 (173–654) | 56.99
(32.24–87.19) | Minimum | – |
| FC | 270 (170–371) | 46.86 (37–56.72) | – | 6.23 (8.11–4.36) |
| TCFA | 21.91 (5–44) | 25.90
(3.04–60.22) | 8.41 (0–25.87) | – |
| PR | 11.66 (6–20) | 22.03
(2.64–32.50) | 3.04
(2.64–32.50) | – |
| PE | 13.13 (10–16) | 70.29
(51.97–87.74) | 6.69 (5–8.35) | 17.85
(14.11–21.46) |
| CN | 20.33 (11–35) | – | Minimum | 136.599 |
USPs exhibited the smallest fibrous cap thickness
and a large lipid core area in the total plaque area, and exhibited
inflammatory infiltrate. VPs were found to have a fibrous cap
thickness of <164 µm and lipid core areas of varying sizes, and
showed considerable inflammatory infiltrate. Plaque instability was
found to be highly associated with fibrous cap thickness, lipid
core area and plaque size. The incidence of the various RFs is
summarized in Table III.
 | Table III.Incidence rates of risk factors in
relation to ATS lesions. |
Table III.
Incidence rates of risk factors in
relation to ATS lesions.
|
| Risk factors (n) |
|---|
|
|
|
|---|
| ATS lesion | Age >50 years | Smoking | AHT | HL | DM | CVD |
|---|
| SP | 2 | 1 | 5 | 2 | 1 | 0 |
| VP | 10 | 4 | 14 | 8 | 4 | 10 |
| USP | 5 | 2 | 6 | 3 | 4 | 5 |
| Total patients
(%) | 17 (65.38) | 7 (26.92) | 25 (96.15) | 13 (50.00) | 9 (34.61) | 15 (57.69) |
The age of the patients, as well as the prevalence
of the RFs for ATS differed between the SP and USP groups (data not
shown). Arterial hypertension was found to be coexistent with the
development of ATS plaques in 96.15% of all cases. Furthermore, age
appeared to be an associated factor in ATS development.
Hyperlipidemia was detected in 50% of all cases. DM was present in
34.61% of cases and was one of the investigated RFs involved in ATS
progression in the vessels. Smoking was involved in 26.92% of all
cases, suggesting that it is an important risk factor in early and
late thrombosis. Involvement of other peripheral vessels in ATS
process was identified in 57.69% of all cases.
Risk factors
The age of the patients and the prevalence of the
RFs for ATS in the SP group differed from the USP group: In order
of decreasing frequency, the RFs were higher in VPs compared with
the USPs and SPs (data not shown). Table IV shows the cumulative RFs in each
patient.
 | Table IV.Incidence of the cumulative
cardiovascular risk factors in each patient. |
Table IV.
Incidence of the cumulative
cardiovascular risk factors in each patient.
|
| Cardiovascular risk
factors (n) |
|---|
|
|
|
|---|
| ATS lesion | 6 | 5 | 4 | 3 | 2 | 1 |
|---|
| SP | 0 | 0 | 2 | 0 | 0 | 0 |
| VP | 3 | 4 | 10 | 1 | 0 | 0 |
| USP | 2 | 1 | 3 | 0 | 0 | 0 |
| Total patients
(%) | 5 (19.23) | 5 (19.23) | 15 (57.69) | 1 (3.84) | 0 (0.00) | 0 (0.00) |
Taking into consideration the number of the RFs
involved in the pathogenesis of ATS in each patient, the following
frequencies were observed (Table
IV): One patient (3.84%) had three RFs, 15 patients (57.69%)
had 4 RFs, 5 patients (19.23%) had 5 RFs, and 5 patients (19.23%)
had 6 RFs. No patient was found to have 1 or 2 RFs. The most
frequent cumulative factors were identified in patients with VPs,
followed by patients with USPs.
Discussion
Atherosclerosis is a major cause of morbidity and
mortality worldwide, and despite the advances in the understanding
of its pathogenesis, the factors that determine atheromatous plaque
instability remain largely unknown. The prediction of plaque
vulnerability to rupture and subsequent thrombosis would be useful
in the development of diagnostic and therapeutic approaches
(2). By correlating
histomorphological examinations and imaging results, the present
study aimed to develop an improved criteria for the diagnostic
processing of ATS plaques.
Cap thickness has been identified as a crucial
characteristic for the distinction between TCFAs and FAs, as TCFAs
are known to have a thinner cap (4).
However, only 1 case of plaque rupture (2.66%) and 3 cases of TCFAs
(12.5%) had a cap thickness >15 µm. Moreno (2), suggested that monitoring cap thickness
may be an option for predicting future behavior of an ATS lesion
(6).
Further separation of plaque ruptures and TCFAs from
FAs is indicated by the simultaneous presence of plaque
inflammation and necrotic core area (3). Regarding FAs, definitory issues were
cap thickness ≥165 µm and necrotic core area ≥3.5 mm2
(4).
Excluding fibrous cap thickness from the analysis,
plaque inflammation allowed for the separation of plaque rupture
and TCFAs from FAs with a proportion of ≥3 times greater in VPs and
RPs compared with FAs (6). Hirayama
et al (7) found a
discriminatory level of inflammation consisting in >0.2
mm2 macrophage area/high-power microscopic field.
In a previous study, the mean area of macrophages
per plaque area, infiltrating the region of the fibrous cap and the
shoulder was significantly greater in the non-calcified plaque
sections compared with the calcified sections (7). Although the present study had certain
limitations, including the low sample size and lack of statistical
analysis, the results indicated that a larger macrophage-rich area
is present in the fibrous cap and the shoulder region of
noncalcified plaques. Furthermore, noncalcified carotid plaques
commonly exhibit a higher degree of fibrous cap inflammation, a key
process in fibrous cap disruption.
Calcification in ATS lesions is relatively common
and has been implicated as a risk factor for increased
cardiovascular morbidity and mortality (8,9), while
Bayturan et al (10) and
Hashimoto et al (11)
demonstrated that carotid ATS plaque calcification is a structural
marker for carotid plaque stability.
In the present study, 14 plaques were defined as
TCFAs. These plaques were not ruptured but were considered of high
risk; thus, detection of such plaques is the key aim of
diagnosis.
PRs and fissures usually occur in the fibrous cap
and shoulder regions. Rupture of the ATS plaques is responsible for
the majority of acute coronary events, and such lesions have been
shown to exhibit distinct histopathological features (12,13). The
present results indicated that fibrous cap inflammation and
susceptibility to disruption are more likely to occur in
noncalcified compared with calcified plaques. We speculate that the
quantitative assessment of carotid plaque calcification using
imaging modalities may help identify patients with asymptomatic
vulnerable carotid plaques who are at risk of cerebrovascular
ischemic events and would benefit from carotid interventions.
Intimal thickness and lipid core area have been
associated with RFs for coronary ATS disease (14), and may be valid markers of early
carotid atherosclerosis from pathology. In the present study, the
prevalence of hypertension, hyperlipidemia, and current history of
smoking was higher in symptomatic than compared with asymptomatic
cases (data not shown). Therefore, the identification of RFs for
carotid atherosclerosis among patients with coronary heart disease
may provide an evidential basis for prevention.
Currently, among the most promising fields in the
study of atherosclerosis is the development of imaging techniques
that facilitate gross, microscopic and molecular characterization
of in vivo ATS plaques (2).
Modern visualization techniques are able to demonstrate the
presence of inflammation, macrophage infiltration, angiogenesis,
apoptosis, and other cellular and molecular features of plaques
that may be involved in plaque destabilization and subsequent
clinical events even in living patients (15). In addition, a recent computed
tomography coronary angiography study by Versteylen (3) demonstrated that large plaques with
calcified micronodules are the most prone to rupture. Such imaging
techniques may facilitate the more specific characterization of ATS
plaques and the identification of characteristics that are
associated with and directly responsible for plaque rupture. All
these approaches; however, should only complement histopathological
investigations, which more specifically confirm the identity of
lesions.
Atheromatous plaques may become unstable due to
increases in size, increased intra- and extracellular lipid
accumulation, as well as intraplaque hemorrhage (16). Based on these results, diagnostic
modalities that detect plaque size, and hemorrhage, and/or lipid
content are most likely to be useful in the prediction of unstable
plaques. Furthermore, atheromatous plaques may become vulnerable
due to fibrous cap thinning, which is associated with cap
inflammation, a key process in fibrous cap disruption (17). Therefore, diagnostic methods that
detect plaque size and hemorrhage, lipid content and the thinnest
fibrous cap can prove considerably useful in determining USPs and
VPs.
The most common RFs that were found to be associated
with stable, vulnerable and unstable ATS plaques were age and
hypertension. Hypertension in atherosclerosis indicates disease
progression and may become life-threatening (1,16);
however, associations between specific risk factors and the
composition of plaque are yet to be elucidated.
References
|
1
|
Falk E, Shah PK and Fuster V: Coronary
plaque disruption. Circulation. 92:657–671. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moreno PR: Vulnerable plaque: Definition,
diagnosis and treatment. Cardiol Clin. 28:1–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Versteylen MO: Additive value of
semi-automated quantification of coronary artery disease using
cardiac computed tomographic angiography to predict for future
acute coronary syndrome. J Am Coll Cardiol. 61:2296–2305. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Furie KL, Kasner SE, Adams RJ, Albers GW,
Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN,
et al: American Heart Association Stroke Council, Council on
Cardiovascular Nursing, Council on Clinical Cardiology, and
Interdisciplinary Council on Quality of Care and Outcomes Research:
Guidelines for the prevention of stroke in patients with stroke or
transient ischemic attack: A guideline for healthcare professionals
from the american heart association/american stroke association.
Stroke. 42:227–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Virmani R, Kolodgie FD, Burke AP, Farb A
and Schwartz SM: Comprehensive Morphological Classification of
Atherosclerotic Lesions. Arterioscler Thromb Vasc Biol.
20:1262–1275. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fishbein MC: The vulnerable and unstable
atherosclerotic plaque. Cardiovasc Pathol. 19:6–11. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hirayama A, Saito S, Ueda Y, Takayama T,
Honye J, Komatsu S, Yamaguchi O, Li Y, Yajima J, Nanto S, et al:
Qualitative and quantitative changes in coronary plaque associated
with atorvastatin therapy. Circ J. 73:718–725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brajovic MD, Marković N, Loncar G,
Sekularac N, Kordić D, Despotović N, Erceg P, Donfrid B, Stefanović
Z, Bajcetić M, et al: The influence of various morphologic and
hemodynamic carotid plaque characteristics on neurological events
onset and deaths. ScientificWorldJournal. 9:509–521. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SH, Hong MK, Park DW, Lee SW, Kim YH,
Lee CW, Kim JJ, Park SW and Park SJ: Impact of plaque
characteristics analyzed by intravascular ultrasound on long-term
clinical outcomes. Am J Cardiol. 103:1221–1226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bayturan O, Tuzcu EM, Nicholls SJ, Balog
C, Lavoie A, Uno K, Crowe TD, Magyar WA, Wolski K and Kapadia S:
Attenuated plaque at nonculprit lesions in patients enrolled in
intravascular ultrasound atherosclerosis progression trials. JACC
Cardiovasc Interv. 2:672–678. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hashimoto H, Tagaya M, Niki H and Etani H:
Computer-assisted analysis of heterogeneity on B-mode imaging
predicts instability of asymptomatic carotid plaque. Cerebrovasc
Dis. 28:357–364. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mortaz Hejri S, Mostafazadeh D and
Sahraian M: Carotid endarterectomy for carotid stenosis in patients
selected for coronary artery bypass graft surgery. Cochrane
Database Syst Rev. CD0060742009.PubMed/NCBI
|
|
13
|
Finn AV, Kramer MC, Vorpahl M, Kolodgie FD
and Virmani R: Pharmacotherapy of coronary atherosclerosis. Expert
Opin Pharmacother. 10:1587–1603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
WRITING GROUP MEMBERS. Lloyd-Jones D,
Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB,
Ford E, Furie K, Gillespie C, et al: American Heart Association
Statistics Committee and Stroke Statistics Subcommittee: Heart
disease and stroke statistics-2010 update: A report from the
American Heart Association. Circulation. 121:e46–e215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Motoyama S, Sarai M, Harigaya H, Anno H,
Inoue K, Hara T, Naruse H, Ishii J, Hishida H and Wong ND: Computed
tomographic angiography characteristics of atherosclerotic plaques
subsequently resulting in acute coronary syndrome. J Am Coll
Cardiol. 54:49–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Van der Wal AC and Becker AE:
Atherosclerotic plaque rupture - pathologic basis of plaque
stability and instability. Cardiovasc Res. 41:334–344. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kolodgie FD, Virmani R, Burke AP, Farb A,
Weber DK, Kutys R, Finn AV and Gold HK: Pathologic assessment of
the vulnerable human coronary plaque. Heart. 90:1385–1391. 2004.
View Article : Google Scholar : PubMed/NCBI
|