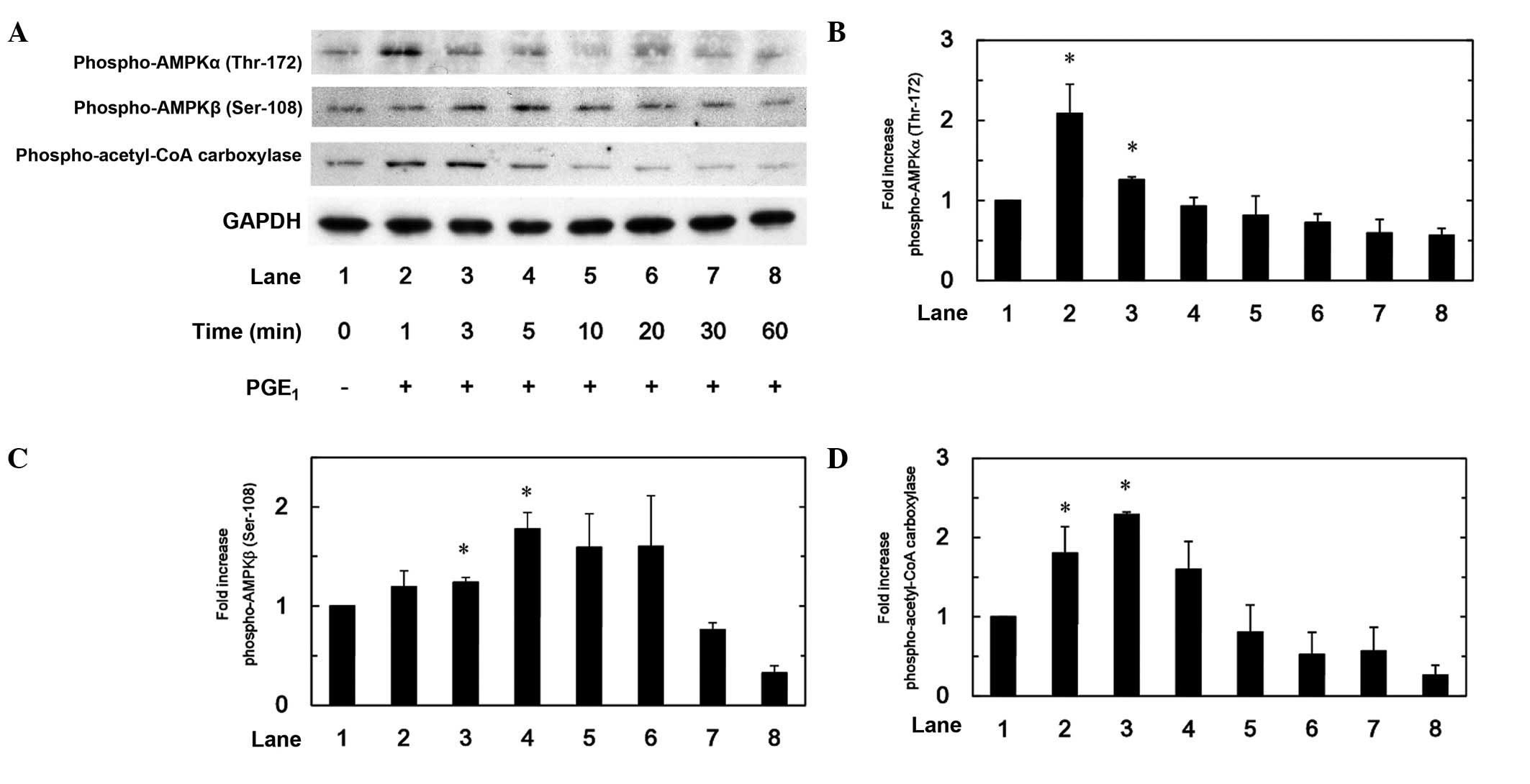
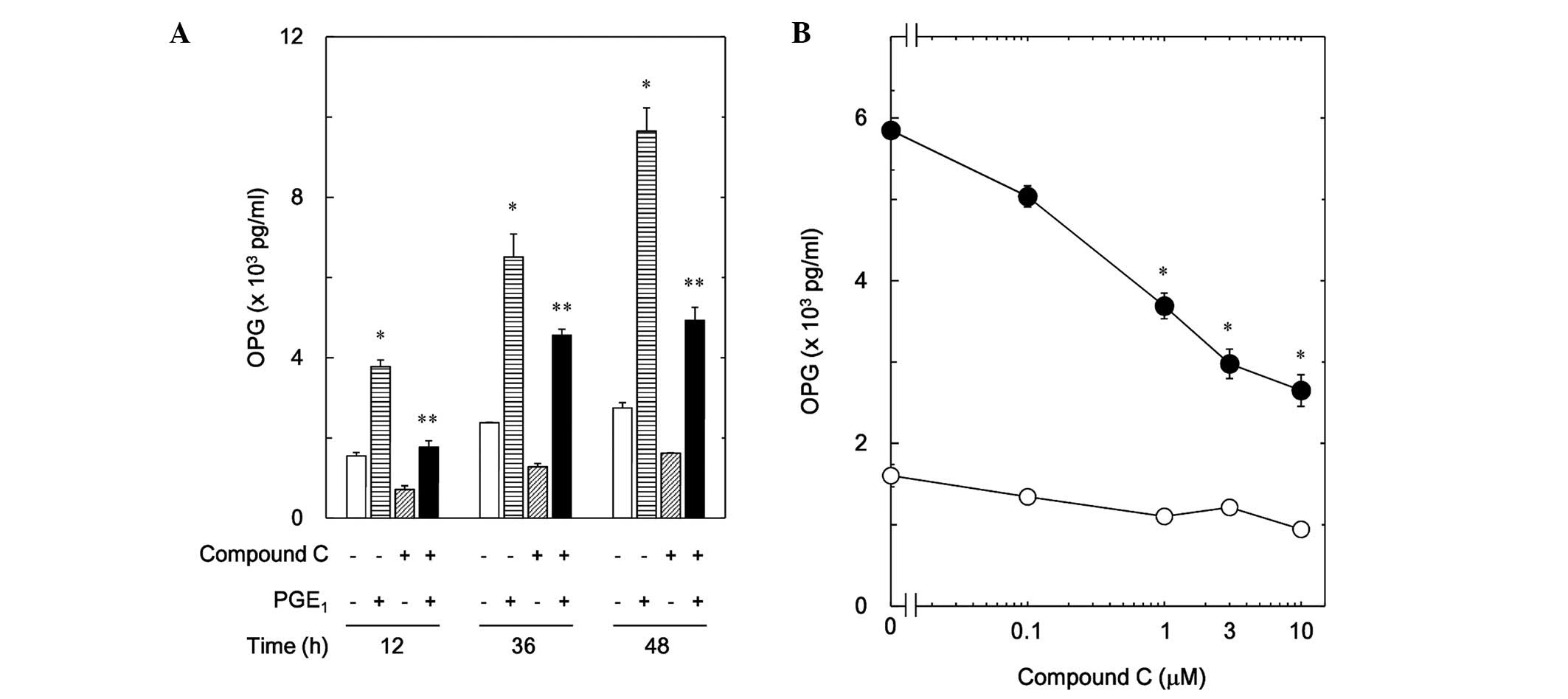
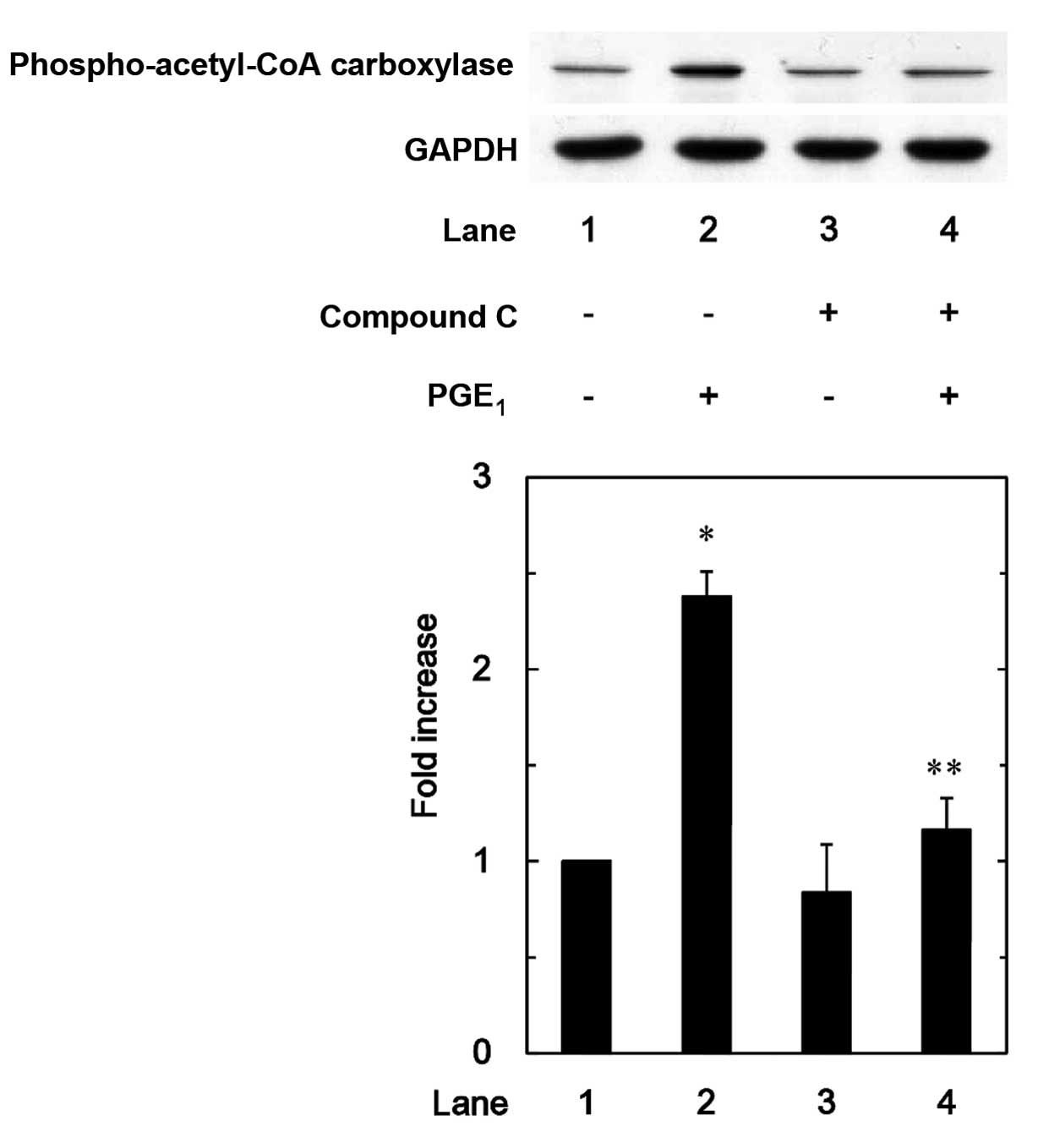
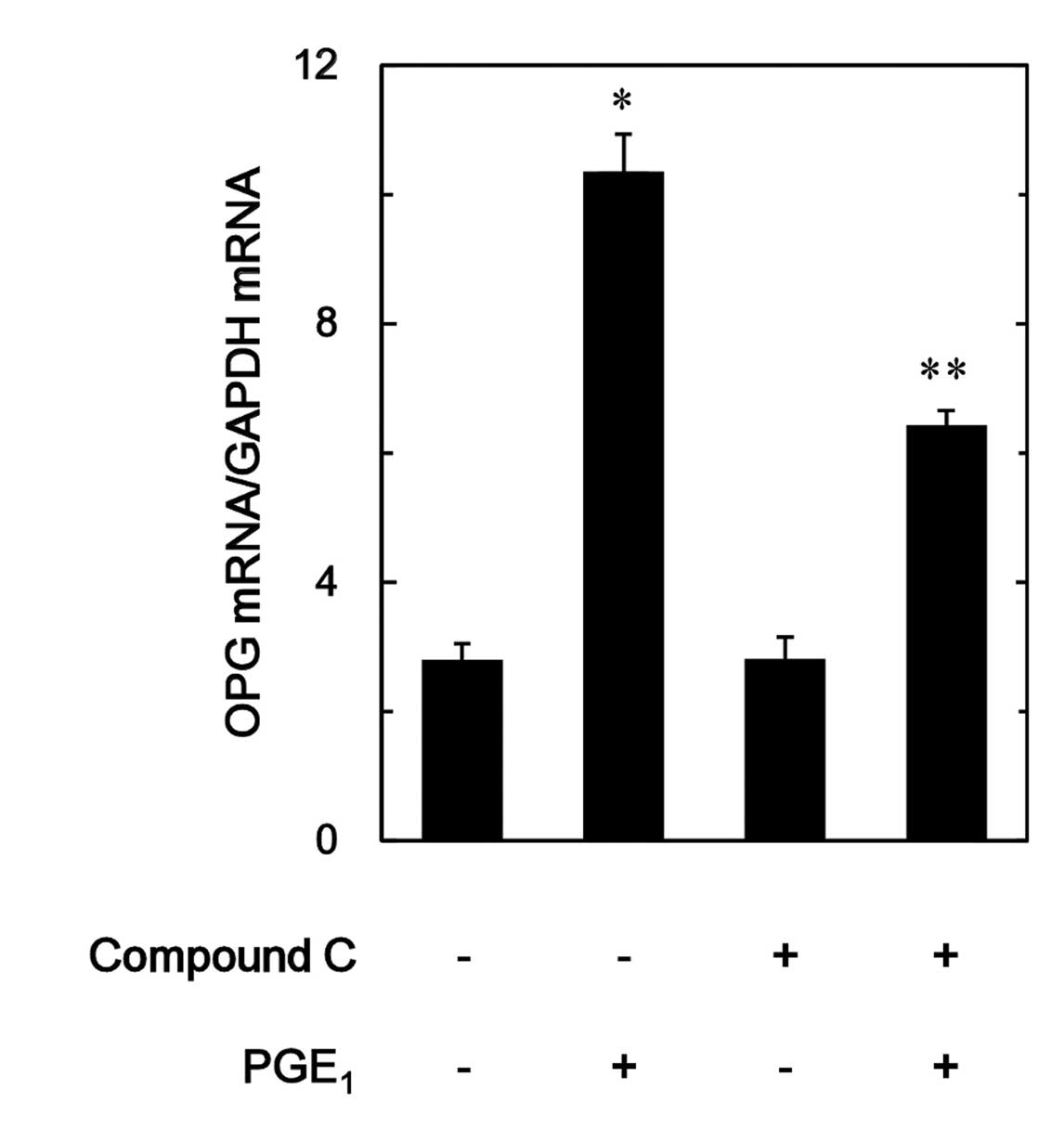
|
1
|
Karsenty G and Wagner EF: Reaching a
genetic and molecular understanding of skeletal development. Dev
Cell. 2:389–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fogarty S and Hardie DG: Development of
protein kinase activators: AMPK as a target in metabolic disorders
and cancer. Biochim Biophys Acta. 1804:581–591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth, autophagy and
metabolism. Nat Cell Biol. 13:1016–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rutter GA and Leclerc I: The AMP-regulated
kinase family: Enigmatic targets for diabetes therapy. Mol Cell
Endocrinol. 297:41–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shah M, Kola B, Bataveljic A, Arnett TR,
Viollet B, Saxon L, Korbonits M and Chenu C: AMP-activated protein
kinase (AMPK) activation regulates in vitro bone formation and bone
mass. Bone. 47:309–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kato K, Tokuda H, Adachi S,
Matsushima-Nishiwaki R, Natsume H, Yamakawa K, Gu Y, Otsuka T and
Kozawa O: AMP-activated protein kinase positively regulates
FGF-2-stimulated VEGF synthesis in osteoblasts. Biochem Biophys Res
Commun. 400:123–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kato K, Tokuda H, Matsushima-Nishiwaki R,
Natsume H, Kondo A, Ito Y, Kozawa O and Otsuka T: AMPK limits
IL-1-stimulated IL-6 synthesis in osteoblasts: Involvement of
IκB/NF-κB pathway. Cell Signal. 24:1706–1712. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hikiji H, Takato T, Shimizu T and Ishii S:
The roles of prostanoids, leukotrienes, and platelet-activating
factor in bone metabolism and disease. Prog Lipid Res. 47:107–126.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu Z, Fu C, Li X, Song Y, Li C, Zou M,
Guan Y and Zhu Y: Prostaglandin E2 promotes endothelial
differentiation from bone marrow-derived cells through AMPK
activation. PLoS One. 6:e235542011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et
al: Osteoprotegerin: A novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kong YY, Yoshida H, Sarosi I, Tan HL,
Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G,
Itie A, et al: OPGL is a key regulator of osteoclastogenesis,
lymphocyte development and lymph-node organogenesis. Nature.
397:315–323. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Theoleyre S, Wittrant Y, Tat SK, Fortun Y,
Redini F and Heymann D: The molecular triad OPG/RANK/RANKL:
Involvement in the orchestration of pathophysiological bone
remodeling. Cytokine Growth Factor Rev. 15:457–475. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamamoto N, Otsuka T, Kuroyanagi G, Kondo
A, Kainuma S, Nakakami A, Matsushima-Nishiwaki R, Kozawa O and
Tokuda H: Resveratrol reduces prostaglandin E1-stimulated
osteoprotegerin synthesis in osteoblasts: Suppression of
stress-activated protein kinase/c-Jun N-terminal kinase.
Prostaglandins Other Lipid M Mediat. 116(117): 57–63. 2015.
View Article : Google Scholar
|
|
15
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kozawa O, Tokuda H, Miwa M, Kotoyori J and
Oiso Y: Cross-talk regulation between cyclic AMP production and
phosphoinositide hydrolysis induced by prostaglandin E2 in
osteoblast-like cells. Exp Cell Res. 198:130–134. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kato K, Ito H, Hasegawa K, Inaguma Y,
Kozawa O and Asano T: Modulation of the stress-induced synthesis of
hsp27 and alpha B-crystallin by cyclic AMP in C6 rat glioma cells.
J Neurochem. 66:946–950. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hawley SA, Davison M, Woods A, Davies SP,
Beri RK, Carling D and Hardie DG: Characterization of the
AMP-activated protein kinase kinase from rat liver and
identification of threonine 172 as the major site at which it
phosphorylates AMP-activated protein kinase. J Biol Chem.
271:27879–27887. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou G, Myers R, Li Y, Chen Y, Shen X,
Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al: Role of
AMP-activated protein kinase in mechanism of metformin action. J
Clin Invest. 108:1167–1174. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tokuda H, Kozawa O, Miwa M and Uematsu T:
p38 mitogen-activated protein (MAP) kinase but not p44/p42 MAP
kinase is involved in prostaglandin E1-induced vascular endothelial
growth factor synthesis in osteoblasts. J Endocrinol. 170:629–638.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanno Y, Tokuda H, Nakajima K, Ishisaki A,
Shibata T, Numata O and Kozawa O: Involvement of SAPK/JNK in
prostaglandin E(1)-induced VEGF synthesis in osteoblast-like cells.
Mol Cell Endocrinol. 220:89–95. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kyriakis JM and Avruch J: Mammalian
mitogen-activated protein kinase signal transduction pathways
activated by stress and inflammation. Physiol Rev. 81:807–869.
2001.PubMed/NCBI
|
|
24
|
Widmann C, Gibson S, Jarpe MB and Johnson
GL: Mitogen-activated protein kinase: Conservation of a
three-kinase module from yeast to human. Physiol Rev. 79:143–180.
1999.PubMed/NCBI
|
|
25
|
Kanazawa I, Yamaguchi T, Yano S, Yamauchi
M and Sugimoto T: Metformin enhances the differentiation and
mineralization of osteoblastic MC3T3-E1 cells via AMP kinase
activation as well as eNOS and BMP-2 expression. Biochem Biophys
Res Commun. 375:414–419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jang WG, Kim EJ, Lee KN, Son HJ and Koh
JT: AMP-activated protein kinase (AMPK) positively regulates
osteoblast differentiation via induction of Dlx5-dependent Runx2
expression in MC3T3E1 cells. Biochem Biophys Res Commun.
404:1004–1009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim JE, Ahn MW, Baek SH, Lee IK, Kim YW,
Kim JY, Dan JM and Park SY: AMPK activator, AICAR, inhibits
palmitate-induced apoptosis in osteoblast. Bone. 43:394–404. 2008.
View Article : Google Scholar : PubMed/NCBI
|