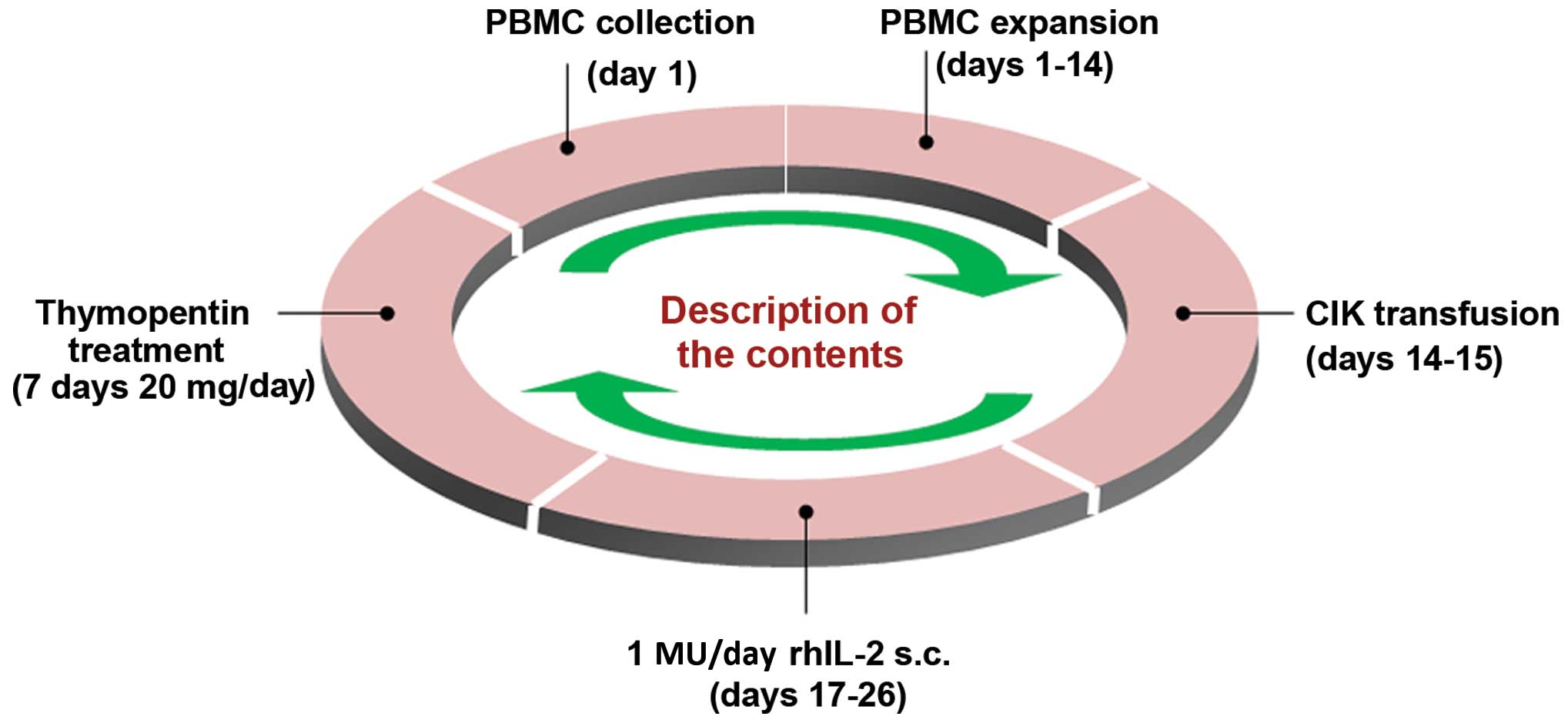
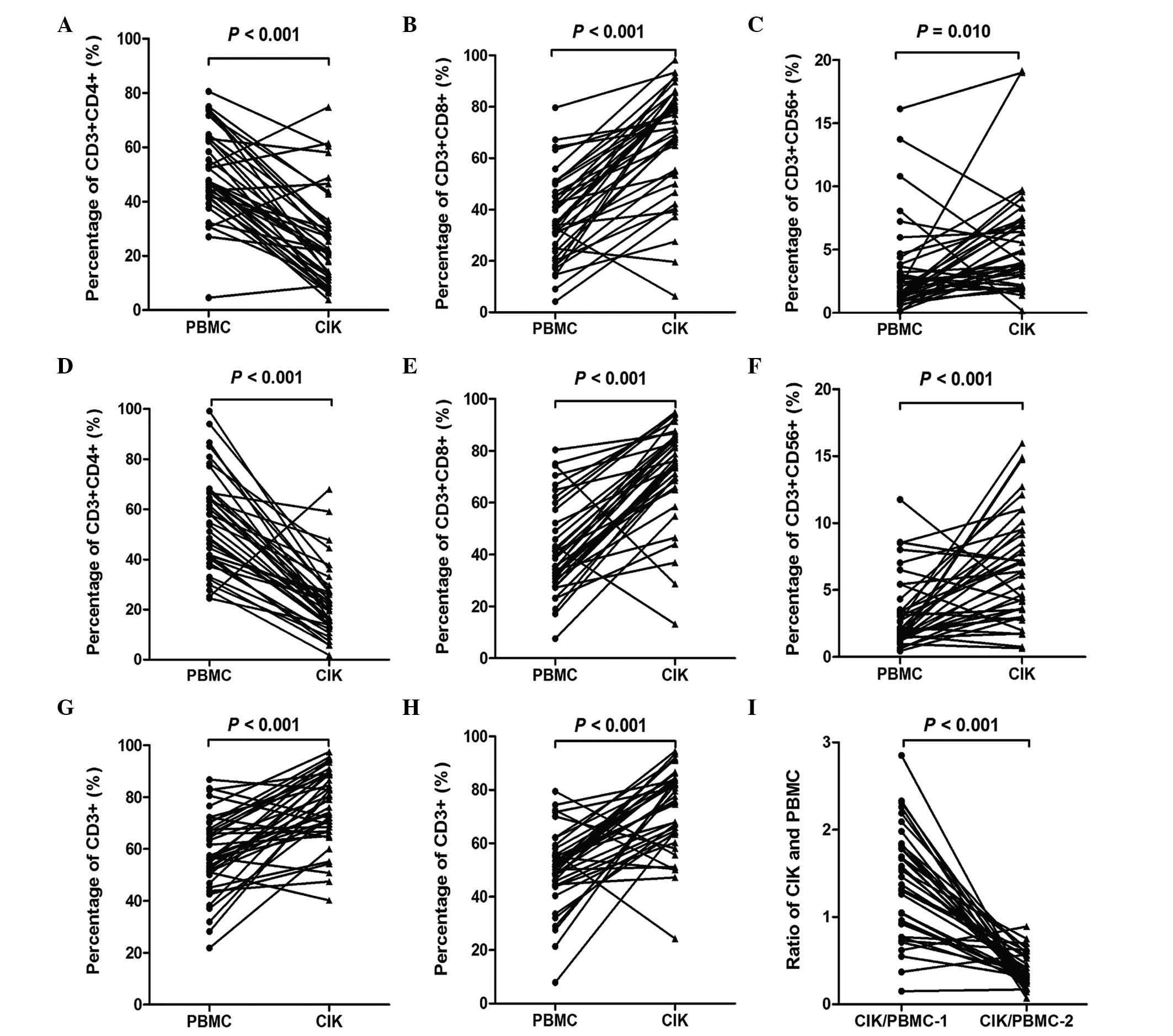
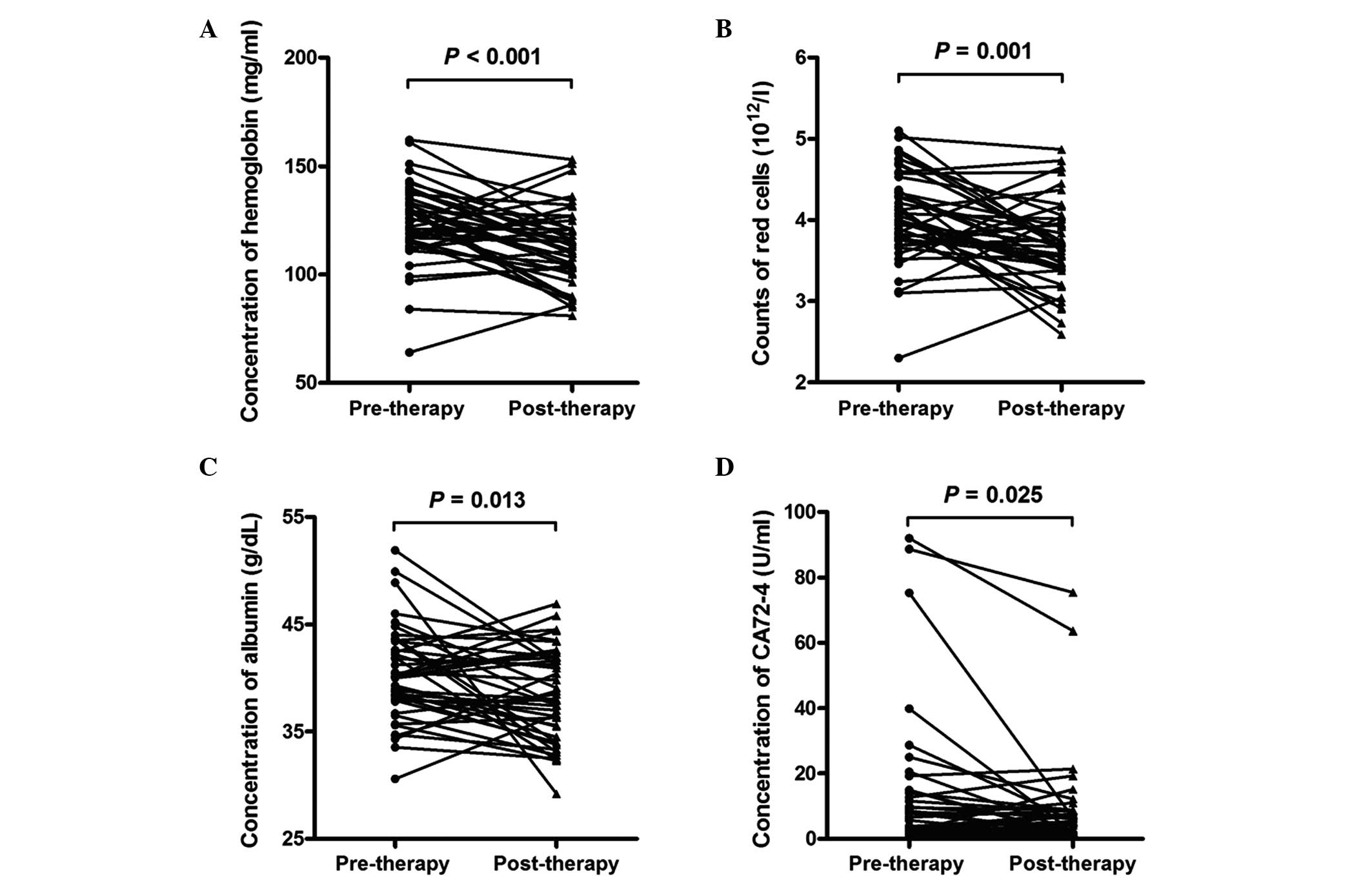
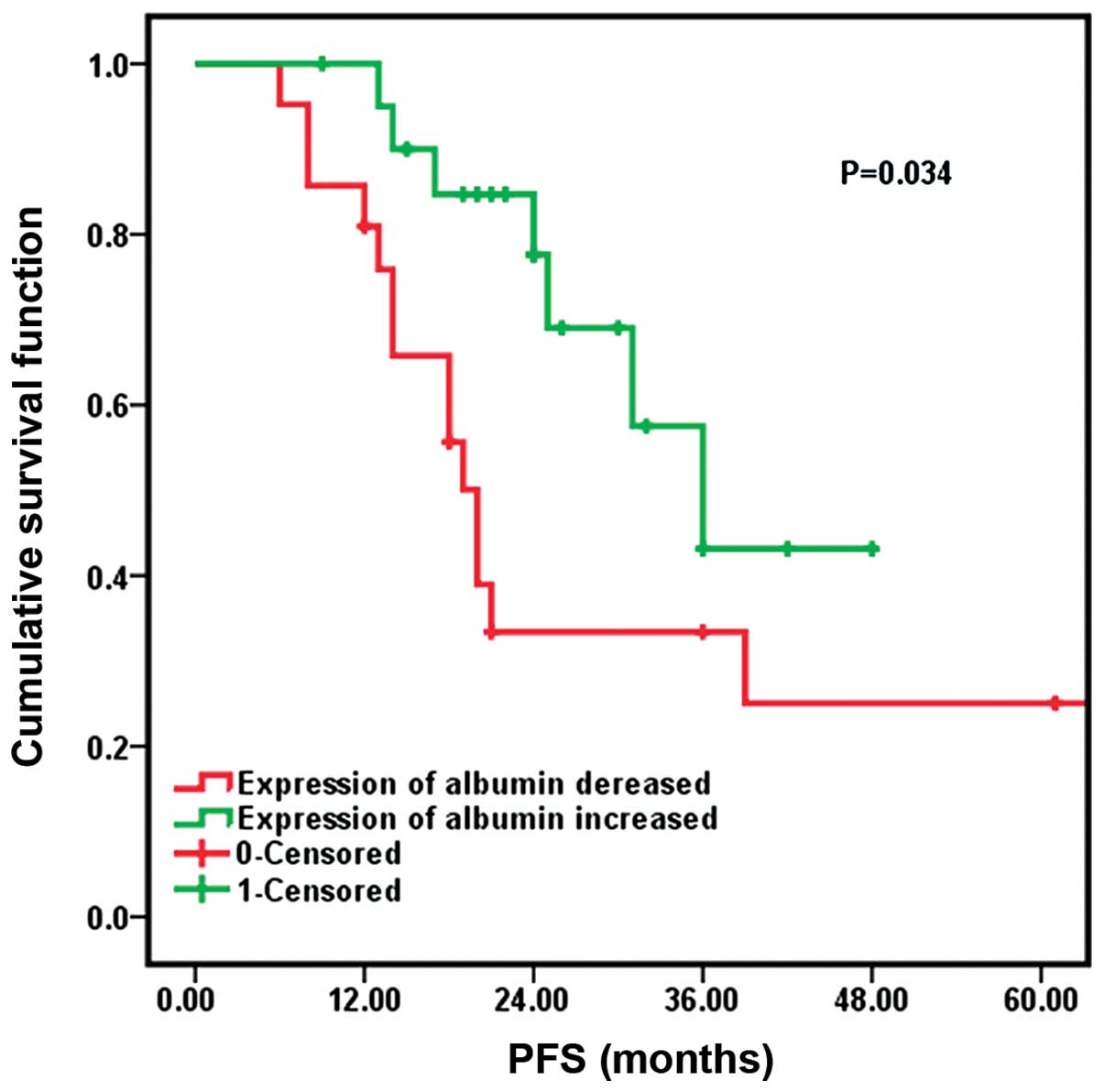
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
She J, Yang P, Hong Q and Bai C: Lung
cancer in China: Challenges and interventions. Chest.
143:1117–1126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spiro SG, Tanner NT, Silvestri GA, Janes
SM, Lim E, Vansteenkiste JF and Pirker R: Lung cancer: Progress in
diagnosis, staging and therapy. Respirology. 15:44–50. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pallis AG, Serfass L, Dziadziusko R, van
Meerbeeck JP, Fennell D, Lacombe D, Welch J and Gridelli C:
Targeted therapies in the treatment of advanced/metastatic NSCLC.
Eur J Cancer. 45:2473–2487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fridman WH, Pagès F, Sautès-Fridman C and
Galon J: The immune contexture in human tumours: Impact on clinical
outcome. Nat Rev Cancer. 12:298–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fischbach MA, Bluestone JA and Lim WA:
Cell-based therapeutics: The next pillar of medicine. Sci Transl
Med. 5:179ps72013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sangiolo D: Cytokine induced killer cells
as promising immunotherapy for solid tumors. J Cancer. 2:363–368.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu PH and Negrin RS: A novel population of
expanded human CD3+CD56+ cells derived from T
cells with potent in vivo antitumor activity in mice with severe
combined immunodeficiency. J Immunol. 153:1687–1696.
1994.PubMed/NCBI
|
|
10
|
Jiang J, Wu C and Lu B: Cytokine-induced
killer cells promote antitumor immunity. J Transl Med. 11:832013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang FS, Liu MX, Zhang B, Shi M, Lei ZY,
Sun WB, Du QY and Chen JM: Antitumor activities of human autologous
cytokine-induced killer (CIK) cells against hepatocellular
carcinoma cells in vitro and in vivo. World J Gastroenterol.
8:464–468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang L, Ren B, Li H, Yu J, Cao S, Hao X
and Ren X: Enhanced antitumor effects of DC-activated CIKs to
chemotherapy treatment in a single cohort of advanced
non-small-cell lung cancer patients. Cancer Immunol Immunother.
62:65–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gammaitoni L, Giraudo L, Leuci V,
Todorovic M, Mesiano G, Picciotto F, Pisacane A, Zaccagna A, Volpe
MG, Gallo S, et al: Effective activity of cytokine-induced killer
cells against autologous metastatic melanoma including cells with
stemness features. Clin Cancer Res. 19:4347–4358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jakel CE, Vogt A, Gonzalez-Carmona MA and
Schmidt-Wolf IG: Clinical studies applying cytokine-induced killer
cells for the treatment of gastrointestinal tumors. J Immunol Res.
2014:8972142014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Wang J, Wang Y, Lu XC, Fan H, Liu
Y, Zhang Y, Feng KC, Zhang WY, Chen MX, et al: Autologous CIK cell
immunotherapy in patients with renal cell carcinoma after radical
nephrectomy. Clin Dev Immunol. 2013:1956912013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JS, Chung IS, Lim SH, Park Y, Park MJ,
Kim JY, Kim YG, Hong JT, Kim Y and Han SB: Preclinical and clinical
studies on cytokine-induced killer cells for the treatment of renal
cell carcinoma. Arch Pharm Res. 37:559–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu C, Jiang J, Shi L and Xu N: Prospective
study of chemotherapy in combination with cytokine-induced killer
cells in patients suffering from advanced non-small cell lung
cancer. Anticancer Res. 28:3997–4002. 2008.PubMed/NCBI
|
|
18
|
Shi SB, Ma TH, Li CH and Tang XY: Effect
of maintenance therapy with dendritic cells: Cytokine-induced
killer cells in patients with advanced non-small cell lung cancer.
Tumori. 98:314–319. 2012.PubMed/NCBI
|
|
19
|
Han RX, Liu X, Pan P, Jia YJ and Yu JC:
Effectiveness and safety of chemotherapy combined with dendritic
cells co-cultured with cytokine-induced killer cells in the
treatment of advanced non-small-cell lung cancer: A systematic
review and meta-analysis. PLoS One. 9:e1089582014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Y, Lin G, Guo ZQ, Zhou ZF, He ZY and
Ye YB: Effects of MICA expression on the prognosis of advanced
non-small cell lung cancer and the efficacy of CIK therapy. PloS
One. 8:e690442013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
González-Cao M: Immunotherapy for lung
cancer. Transl Lung Cancer Res. 4:675–677. 2015.PubMed/NCBI
|
|
23
|
Mahoney KM and Atkins MB: Prognostic and
predictive markers for the new immunotherapies. Oncology 28 Suppl.
3:39–48. 2014.
|
|
24
|
Wang Y, Dai H, Li H, Lv H, Wang T, Fu X
and Han W: Growth of human colorectal cancer SW1116 cells is
inhibited by cytokine-induced killer cells. Clin Dev Immunol.
2011:6214142011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gehrmann U, Hiltbrunner S, Georgoudaki AM,
Karlsson MC, Näslund TI and Gabrielsson S: Synergistic induction of
adaptive antitumor immunity by codelivery of antigen with
α-galactosylceramide on exosomes. Cancer Res. 73:3865–3876. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Belardelli F and Ferrantini M: Cytokines
as a link between innate and adaptive antitumor immunity. Trends
Immunol. 23:201–208. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vivier E, Raulet DH, Moretta A, Caligiuri
MA, Zitvogel L, Lanier LL, Yokoyama WM and Ugolini S: Innate or
adaptive immunity? The example of natural killer cells. Science.
331:44–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu F, Wang ZB, Lu P, Xu ZL, Chen WZ, Zhu H
and Jin CB: Activated anti-tumor immunity in cancer patients after
high intensity focused ultrasound ablation. Ultrasound Med Biol.
30:1217–1222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ciernik IF, Berzofsky JA and Carbone DP:
Induction of cytotoxic T lymphocytes and antitumor immunity with
DNA vaccines expressing single T cell epitopes. J Immunol.
156:2369–2375. 1996.PubMed/NCBI
|
|
30
|
Kirkwood JM, Butterfield LH, Tarhini AA,
Zarour H, Kalinski P and Ferrone S: Immunotherapy of cancer in
2012. CA Cancer J Clin. 62:309–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li R, Wang C, Liu L, Du C, Cao S, Yu J,
Wang SE, Hao X, Ren X and Li H: Autologous cytokine-induced killer
cell immunotherapy in lung cancer: A phase II clinical study.
Cancer Immunol Immunother. 61:2125–2133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li DP, Li W, Feng J, Chen K and Tao M:
Adjuvant chemotherapy with sequential cytokine-induced killer (CIK)
cells in stage IB non-small cell lung cancer. Oncol Res. 22:67–74.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhong R, Han B and Zhong H: A prospective
study of the efficacy of a combination of autologous dendritic
cells, cytokine-induced killer cells, and chemotherapy in advanced
non-small cell lung cancer patients. Tumour Biol. 35:987–994. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhong R, Teng J, Han B and Zhong H:
Dendritic cells combining with cytokine-induced killer cells
synergize chemotherapy in patients with late-stage non-small cell
lung cancer. Cancer Immunol Immunother. 60:1497–1502. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Huang SL, Wu YF, Wei J, Bao R and
Zhou DH: Expansion of CIK/NK cells from cord blood by using
different combinations of stem cell factor, FLT3 ligand and
interleukin 2, 7, 15 in vitro. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
12:350–354. 2004.(In Chinese). PubMed/NCBI
|
|
36
|
Li Y, Schmidt-Wolf IG, Wu YF, Huang SL,
Wei J, Fang J, Huang K and Zhou DH: Optimized protocols for
generation of cord blood-derived cytokine-induced killer/natural
killer cells. Anticancer Res. 30:3493–3499. 2010.PubMed/NCBI
|
|
37
|
Ritchie RF, Palomaki GE, Neveux LM,
Navolotskaia O, Ledue TB and Craig WY: Reference distributions for
the negative acute-phase serum protein–s, albumin, transferrin and
transthyretin: A practical, simple and clinically relevant approach
in a large cohort. J Clin Lab Anal. 13:273–279. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Don BR and Kaysen G: Serum albumin:
Relationship to inflammation and nutrition. Semin Dial. 17:432–437.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
von Meyenfeldt M: Cancer-associated
malnutrition: An introduction. Eur J Oncol Nurs. 9(Suppl 2):
S35–S38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gupta D and Lis CG: Pretreatment serum
albumin as a predictor of cancer survival: A systematic review of
the epidemiological literature. Nutr J. 9:692010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yeun JY and Kaysen GA: Factors influencing
serum albumin in dialysis patients. Am J Kidney Dis. 32(Suppl 4):
S118–S125. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Siddiqui A, Heinzerling J, Livingston EH
and Huerta S: Predictors of early mortality in veteran patients
with pancreatic cancer. Am J Surg. 194:362–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Onate-Ocana LF, Aiello-Crocifoglio V,
Gallardo-Rincón D, Herrera-Goepfert R, Brom-Valladares R, Carrillo
JF, Cervera E and Mohar-Betancourt A: Serum albumin as a
significant prognostic factor for patients with gastric carcinoma.
Ann Surg Oncol. 14:381–389. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lis CG, Grutsch JF, Vashi PG and
Lammersfeld CA: Is serum albumin an independent predictor of
survival in patients with breast cancer? JPEN J Parenter Enteral
Nutr. 27:10–15. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Seve P, Ray-Coquard I, Trillet-Lenoir V,
Sawyer M, Hanson J, Broussolle C, Negrier S, Dumontet C and Mackey
JR: Low serum albumin levels and liver metastasis are powerful
prognostic markers for survival in patients with carcinomas of
unknown primary site. Cancer. 107:2698–2705. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Burkholder B, Huang RY, Burgess R, Luo S,
Jones VS, Zhang W, Lv ZQ, Gao CY, Wang BL, Zhang YM and Huang RP:
Tumor-induced perturbations of cytokines and immune cell networks.
Biochim Biophys Acta. 1845:182–201. 2014.PubMed/NCBI
|
|
47
|
Souza SL, Da Montalli Assumpção LV and
Ward LS: Impact of previous thyroid autoimmune diseases on
prognosis of patients with well-differentiated thyroid cancer.
Thyroid. 13:491–495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Krauze MT, Tarhini A, Gogas H and Kirkwood
JM: Prognostic significance of autoimmunity during treatment of
melanoma with interferon. Semin Immunopathol. 33:385–391. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Weber J: Ipilimumab: Controversies in its
development, utility and autoimmune adverse events. Cancer Immunol
Immunother. 58:823–830. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Braumuller H, Wieder T, Brenner E, Aßmann
S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M,
Griessinger C, et al: T-helper-1-cell cytokines drive cancer into
senescence. Nature. 494:361–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Salih HR, Rammensee HG and Steinle A:
Cutting edge: Down-regulation of MICA on human tumors by
proteolytic shedding. J Immunol. 169:4098–4102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ascierto PA, Capone M, Urba WJ, Bifulco
CB, Botti G, Lugli A, Marincola FM, Ciliberto G, Galon J and Fox
BA: The additional facet of immunoscore: Immunoprofiling as a
possible predictive tool for cancer treatment. J Transl Med.
11:542013. View Article : Google Scholar : PubMed/NCBI
|