Introduction
Acute kidney injury (AKI) is a complex clinical
problem associated with significant short-term morbidity and
mortality (up to 50%) (1), and
lacking effective pharmacologic interventions. Patients with AKI
experience longer-term risks for progressive end stage renal
disease, which diminishes the health-related quality of life of a
patient and creates a burden on the healthcare system (2). Renal ischemia-reperfusion injury (IRI)
is one of the leading causes of AKI in both native and transplanted
kidneys (3,4).
The damaging effects of renal IRI comprise a complex
interrelated sequence of events, eventually resulting in apoptosis
and necrosis of the renal cells (5).
One of these events is the endothelial stress as described by
Brodsky and Goligorsky (6) where the
concept of ‘no-reflow’ is adopted in addition to the consequence of
cellular cross-talk dialog between the epithelium and endothelium
in response to renal ischemia. Reactive oxygen species burst is
another underlying mechanism of AKI during the reperfusion of
ischemic tissues, in which reactive oxygen species stimulate the
generation of inflammatory cytokines, pro-apoptotic mediators and
further oxidative stress (7). The
high morbidity and mortality rates associated with IRI/AKI and
unsatisfactory results from currently available clinical
therapeutic approaches have prompted further research (8). This has included the investigation of
stem cells, which are undifferentiated cells able to renew and
differentiate into one or more cell types that have the potential
to ameliorate IRI (8). In
particular, mesenchymal stem cells (MSCs), which are multi-potent
adult stem cells that have multi-lineage differentiation potential
and immunosuppressive properties are suggested to be an ideal
candidate cell-type for immunomodulation and regenerative medicine
(9). Furthermore, adipose-derived
stem cells (ADSCs) are an emerging cellular therapy (10). The plasticity of ADSCs and their
ability to differentiate into cells of mesodermal origin, such as
adipocyte, osteocyte, chondrocyte and myocyte lineages have been
demonstrated (11,12).
The aim of the present study was to evaluate the
effect of ADSCs on renal IRI. In addition, renal histopathological
changes were assessed using a new histopathological scoring system
that evaluates features associated with both injury and
regeneration.
Materials and methods
Ethical approval
The experimental protocol was approved by the Local
Ethical Committee or the Faculty of Medicine, Mansoura University
(Mansoura, Egypt).
Isolation and characterization of
ADSCs
Isolation
The isolation of MSCs from adipose tissue was
conducted as previously described by Bunnell et al (13). The cells were maintained in an
incubator supplied with a humidified atmosphere of 5%
CO2 at 37°C. Cell cultivation was maintained up to the
third passage, and cells were then characterized by
immunophenotypic and differentiation capability.
Characterization by flow cytometric analysis
Cells were characterized using cell surface markers
by fluorescence-activated cell sorting (FACS) analyses. The cells
were stained with different fluorescently labeled monoclonal
antibodies, including fluorescein isothiocyanate-conjugated
anti-rat CD45 (11–0461),
phycoerythrin (PE)-conjugated anti-rat CD29 (12–0291) and PE/Cy5-conjugated CD90
(15–0900) (all: eBioscience, Inc.,
San Diego, CA, USA). The fluorescence intensity of the cells was
evaluated by flow cytometry using an EPICS-XL instrument (Beckman
Coulter, Miami, FL, USA).
Differentiation capability
i) Osteogenic differentiation. ADSCs at the third
passage were seeded in a 6-well plate (0.03 million cells/well),
and when they were 80% confluent, osteogenesis differentiation
medium was added to 4 of the wells, comprising: Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS), 0.1 µM dexamethasone, 50 µM ascorbic acid and 10 mM
β-glycerol phosphate (all Sigma-Aldrich, St. Louis, MO, USA).
Complete culture medium, consisting of DMEM and FBS, was added to
the other 2 wells as a negative control. The medium was changed
twice per week for 2–3 weeks. The differentiation potential for
osteogenesis was assessed by staining with 40 mM Alizarin Red (pH
4.1; Sigma-Aldrich) following fixation in 10% neutral-buffered
formalin (Sigma-Aldrich).
ii) Adipogenic differentiation. ADSCs at the third
passage were seeded in a 6-well plate (0.2 million cells/well), and
when they were 100% confluent, adipogenesis differentiation medium
was added to 4 of the wells, comprising: DMEM supplemented with 10%
FBS, 1 µM dexamethasone, 500 µM isobutylmethylxanthine (IBMX), 5
µg/ml insulin and 200 µM indomethacin (Sigma-Aldrich); and complete
culture medium was added to the other 2 wells as a negative
control. The medium was changed twice per week for 2 weeks. The
differentiation potential for adipogenesis and the formation of
intracellular lipid droplets were assessed by staining with Oil Red
O (Sigma-Aldrich) following fixation in 10% neutral buffered
formalin.
Animal grouping and renal ischemia/reperfusion
model
This study was conducted using male Sprague-Dawley
rats (n=72; Charles River Laboratories, Wilmington, MA, USA)
weighing 250–300 g. The model of IRI was created as described by
Jablonski et al (14) with
modifications, as described below. The rats were anesthetized by
the administration of diazepam [15 mg/kg, intraperitoneally (i.p.)]
and ketamine (150 mg/kg, i.p.; both Sigma-Aldrich). The inhalation
of halothane (Sigma-Aldrich) was used to prolong the duration of
anesthesia when necessary. Through a midline incision, the
abdominal cavity was exposed and the left renal pedicle was
isolated and clamped with a non-traumatic arterial clamp for 45
min. Immediately prior to removal of the clamp, the right kidney
was exposed extracorporeally, its pedicle was ligated with a 4/0
silk suture, and the kidney was removed from the abdominal cavity.
The occlusion and reperfusion of the left kidney were verified
visually by the observation of changes in color. Afterwards, 1 ml
saline at 37°C was injected into the abdomen and the incision was
sutured in two layers. Using a warming light, the temperature was
adjusted to ~37°C.
The rats were randomly assigned to three groups, as
follows (n=24/group): i) The sham-operated control group, in which
the rats were maintained under anesthesia for 45 min and underwent
surgery without the occlusion of the left renal pedicle; ii) the
positive control group, in which the rats were subjected to IRI
modeling and were administered culture media following 4 h of IRI;
and iii) the ADSC group, in which the rats were administered
1×106 ADSCs via the tail vein following 4 h of IRI
modeling. In addition, each group was further divided into four
subgroups according to the time post-IRI (n=6/subgroup): Subgroup A
rats were sacrificed 24 h post-renal IRI injury; subgroup B rats
were sacrificed 3 days post-renal IRI injury; subgroup C rats were
sacrificed 7 days post-renal IRI; and subgroup D rats were
sacrificed 14 days post-IRI. Prior to sacrifice, a 24 h-urine
sample was collected using a metabolic cage. Rats were sacrificed
by the administration of an overdose of thiopental. Blood samples
were collected from the heart and immediately centrifuged using the
Hettich Universal 32R centrifuge (DJB Labcare Ltd.,
Buckinghamshire, UK) at ~3,000 × g for 5 min at room temperature,
then taken for biochemical measurements. The left kidney was
removed for histological evaluation.
Biochemical measurements
Renal function tests (serum creatinine and
creatinine clearance) were conducted using a commercially available
kit (CREATININE liquicolor; HUMAN Gesellschaft für Biochemica und
Diagnostica mbH, Wiesbaden, Germany). Creatinine clearance was
calculated using the following formula: Creatinine clearance
(ml/min)= U × V/P. Where U (mg/dl) is the concentration of
creatinine in urine, P (mg/dl) is the concentration of creatinine
in plasma and V is the volume of urine collected in a duration of
time (min).
Malondialdehyde (MDA) levels in the serum (nmol/ml)
and renal tissue MDA (nmol/g) were determined
spectrophotometrically in all groups. Briefly, blood samples were
left for 20 min to allow clotting, after which they were
centrifuged at 1,500 × g for 5 min at room temperature to obtain
serum, which was stored at −80°C until further analysis. Prior to
dissection, renal tissues were perfused with phosphate-buffered
saline (pH 7.4) containing 0.16 mg/ml heparin (Sigma-Aldrich) to
remove blood cells or clots. Subsequently, the tissues were
homogenized in cold potassium phosphate buffer (50 mM;
Sigma-Aldrich) and then centrifuged at 1,792 × g for 15 min. The
supernatant was separated and maintained at −80°C until further
analysis. MDA levels were determined using the MDA Assay kit
(NWK-MDA01; Northwest Life Science Specialties, LLC, Portland, WA,
USA) in which MDA reacts with thiobarbituric acid to form a
colorimetric product that was measured at 532 nm using the Jenway
7305 spectrophotometer (Bibby Scientific Ltd., Staffordshire,
UK).
Renal histopathology
Histopathological analysis
Perfusion fixation of the whole animal was conducted
and one half of the left kidney was processed for light microscopic
observation, according to standard procedures. The kidneys were
preserved in 10% neutral buffered formalin, after which the kidneys
were embedded in paraffin wax, cut into 4-µm sections, and stained
with hematoxylin and eosin (H&E). Histopathological changes
were analyzed in the different regions of the kidney, specifically,
in the cortex, outer stripe of the outer medulla (OSOM), inner
stripe of the outer medulla (ISOM) and inner medulla. In each
layer, scores were calculated using a scoring system that
considered active injury changes, regenerative changes and chronic
changes:
Active injury scoring
Changes associated with active injury included
necrotic tubules and interstitial infiltration by inflammatory
cells. The degree of tubular injury was quantified according to the
number of necrotic tubules counted per high power field (HPF).
Necrotic tubules were given a score of 1, 2, 3 or 4, corresponding
to 1–3, 4–5, 6–10 and >10 per HPF, respectively.
Scoring of regenerative changes
Mitotic figures were counted as the number/10 HPFs
and given a score of 1, 2 or 3, corresponding to 1–2, 3–5 and >5
per 10 HPFs. Solid interstitial sheets of cells were given a score
of 1, 2 or 3, corresponding to 1–2, 3–5 and >5 per HPF. Solid
tubules were scored as 1, 2 or 3 corresponding to 1–2, 3–5 and
>5 per HPF. Tubules with large vesicular nuclei and tubules
lined by cells having hyperchromatic prominent nuclei and little
cytoplasm were scored as present or absent.
Scoring of chronic changes
Interstitial fibrosis was scored as 1, 2, 3 or 4
corresponding to a fibrosed interstitium content of <25, 25–50,
50–75 and >75%, respectively. Tubular atrophy was scored as 1, 2
or 3 corresponding to 1–5, 6–10 and 7–10 per HPF, respectively.
Statistical analysis
Data was analyzed using SPSS software, version 16
(SPSS, Inc., Chicago, IL, USA). Variables were tested for normality
distribution using Kolmogrov-Smirnov test. Normally distributed
variables were presented as mean ± standard deviation (SD). Two-way
analysis of variance was used for comparisons among the three
groups and within the groups followed by Bonferroni's post hoc
multiple comparisons. Paired t-test was used for paired comparisons
within the same group. P≤0.05 was considered statistically
significant. Non-parametric variables (pathology scores) were
presented as median (minimum-maximum). The Mann-Whitney U test was
used for intergroup comparisons and the χ2 test was used
for comparisons within the same group.
Results
Immunophenotypic characterization
Cultures of ADSCs were analyzed for the expression
of cell-surface markers. The ADSCs were negative for the
hematopoietic lineage marker CD45 (1.63±0.18%), but were positive
for CD29 and CD90 (98.7±2.95 and 92±1.7%, respectively).
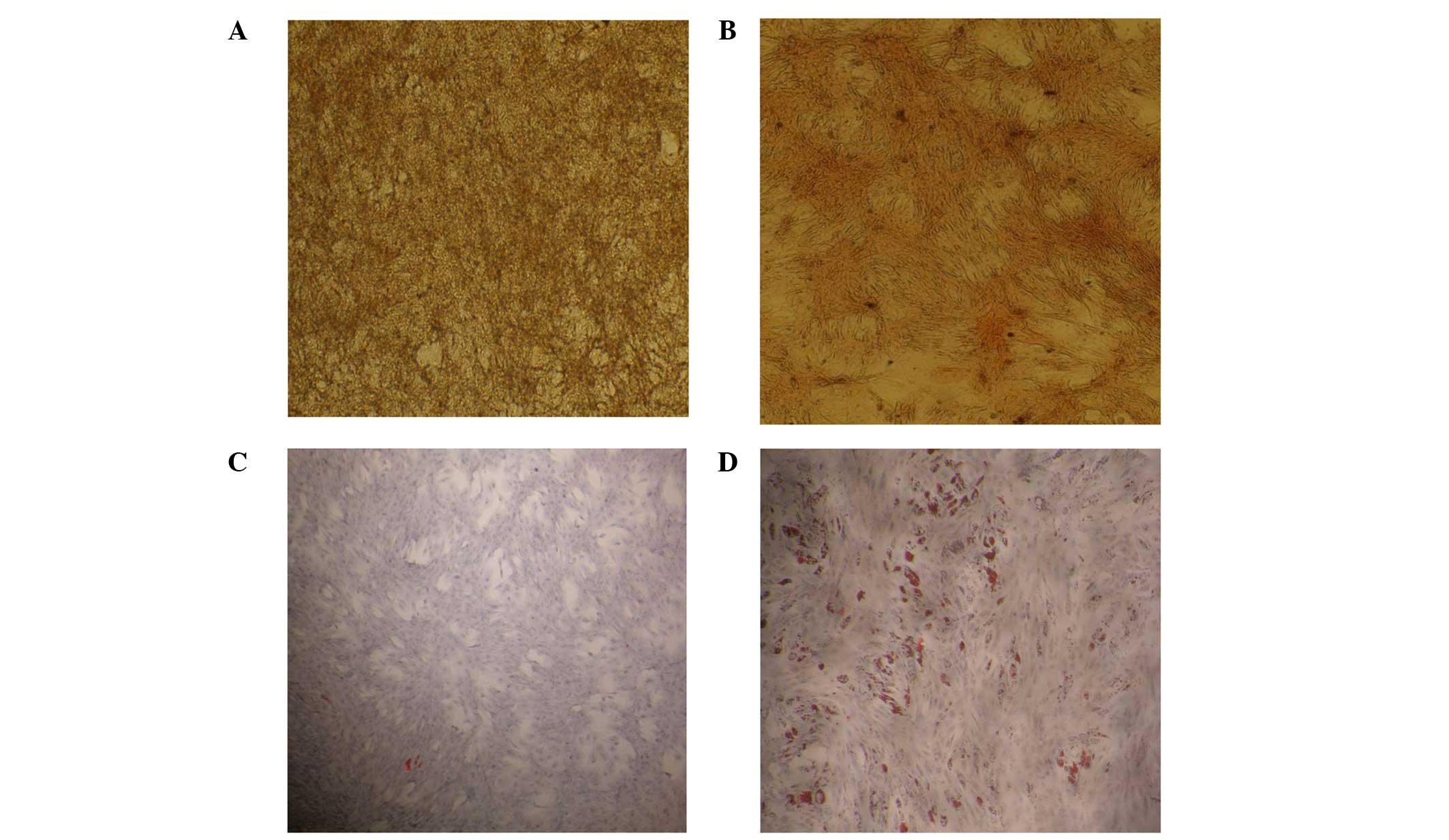
ADSC differentiation capability
ADSCs were observed to differentiate into osteocytes
and adipocytes in vitro (Fig.
1) following culture in osteogenic differentiation and
adipogenic differentiation media, respectively.
Effect of ADSCs on IRI-induced renal
dysfunction as evaluated by serum creatinine levels and creatinine
clearance
IRI resulted in a significant increase in serum
creatinine levels on days 3 and 7 and a significant reduction in
creatinine clearance on days 1, 3 and 7 compared with those in
sham-operated rats. These changes were attenuated by the use of
ADSCs (Table I).
 | Table I.Biochemical values in the three groups
at different time points. |
Table I.
Biochemical values in the three groups
at different time points.
| Variable | Sham control
(n=6) | Positive control
(n=6) | ADSC (n=8) | P-value |
|---|
| Serum creatinine,
mg/dl |
|
|
|
|
| Day
1 |
0.45±0.24 |
1.48±1.30 |
0.70±0.12 | 0.07 |
| Day
3 |
0.40±0.24 |
1.54±1.36a |
0.63±0.11a,b | 0.014 |
| Day
7 |
0.42±0.22 |
0.68±0.08a |
0.57±0.09 | 0.013 |
| Day
14 |
|
|
0.54±0.11c |
|
| Creatinine clearance,
ml/min |
|
|
|
|
| Day
1 |
2.58±1.99 |
0.19±0.18a |
0.52±0.33a,b | ≤0.001 |
| Day
3 |
3.22±3.18 |
0.26±0.07a |
0.52±0.25a,b | ≤0.001 |
| Day
7 |
3.78±2.80 |
0.57±0.45a,c,d |
0.93±0.81a | 0.002 |
| Day
14 |
|
|
1.22±0.26c,d |
|
| Serum MDA,
nmol/ml |
|
|
|
|
| Day
1 |
8.60±2.90 |
36.68±2.70a |
36.93±5.18a | ≤0.001 |
| Day
3 |
8.90±1.90 |
497.60±70.80a,c |
41.12±5.20a,b | ≤0.001 |
| Day
7 |
10.23±2.14 |
40.7±6.01a |
39.58±9.09a | ≤0.001 |
| Day
14 |
|
|
35.60±6.80 |
|
| Tissue MDA,
nmol/g |
|
|
|
|
| Day
1 |
13.10±5.34 |
155.90±22.87a |
91.47±36.75a,b | ≤0.001 |
| Day
3 |
13.40±2.17 |
199.80±80.50a |
154.00±49.30a,c | ≤0.001 |
| Day
7 |
10.78±3.50 |
140.00±87.90a |
118.45±29.27a,d | 0.002 |
| Day
14 |
|
|
103.20±30.70d |
|
Effect of ADSCs on IRI-induced
oxidative stress as evaluated by MDA levels
IRI resulted in a significant increase in plasma and
tissue MDA levels on days 1, 3 and 7 compared with those in
sham-operated rats, indicating the occurrence of significant lipid
peroxidation secondary to oxidative stress. This was partially
ameliorated in the serum by ADSCs at day 3 and in the tissues at
days 3 and 7, but remained significantly elevated in the serum and
the tissues in comparison with the sham group (Table I).
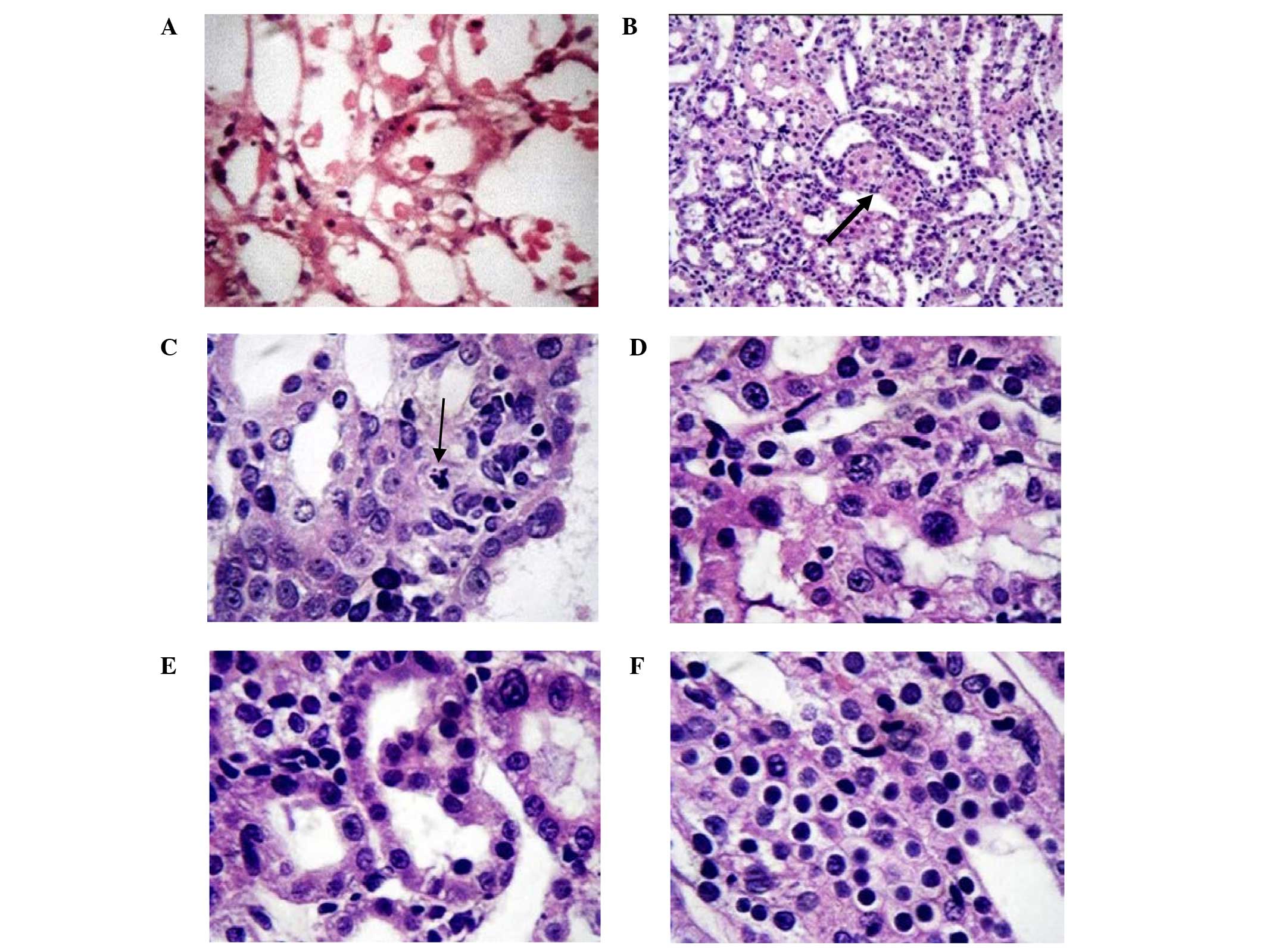
Effect of ADSCs on IRI-induced renal
histopathological changes
The sham group showed an intact brush border with no
evidence of necrotic tubules or regenerative changes. The pathology
scores were zero for all layers in the sham group.
Histopathological changes occurring following IRI
with and without ADSCs treatment are shown in Fig. 2. The histopathological changes in the
cortex, ISOM and OSOM generated the highest injury scores, which
were significantly evident in the positive control group. The use
of ADSCs was associated with significantly lowered injury scores at
days 1 and 3, but not on day 7. No significant histopathological
differences were observed in the inner medulla between different
groups (Tables II and III).
 | Table II.Pathological changes in scores for
different kidney zones at various time intervals in positive
control and ADSC-treated animals. |
Table II.
Pathological changes in scores for
different kidney zones at various time intervals in positive
control and ADSC-treated animals.
|
| Cortex | Outer stripe outer
medulla | Inner stripe outer
medulla | Inner medulla |
|---|
|
|
|
|
|
|
|---|
| Score | Positive control
(n=6) | ADSCs (n=8) | P-value | Positive control
(n=6) | ADSCs (n=8) | P-value | Positive control
(n=6) | ADSCs (n=6) | P-value | Positive control
(n=6) | ADSC (n=8) | P-value |
|---|
| Injury score |
|
|
|
|
|
|
|
|
|
|
|
|
| Day
1 | 2.5 (1–4) | 0 (0–3) | 0.028 | 4 (3–4) | 0 (0–4) | 0.016 | 3 (1–4) | 0 | 0.001 | 0 | 0 |
|
| Day
3 | 1.5 (1–2) | 0a | 0.001 | 2.5 (0–5) | 0 (0–4) | 0.045 | 1
(0–1)a | 0 | 0.008 | 0 (0–1) | 0 | 0.09 |
| Day
7 | 0.5
(0–1)b | 0 (0–3) | 0.43 | 0.5
(0–2)a | 0
(0–2)a | 0.81 | 0
(0–1)b | 0
(0–1) | 0.78 | 0 (0–2) | 0 | 0.2 |
| Day
14 |
| 0 (0–1) |
|
| 0 |
|
| 0 |
|
| 0
(0–1) |
|
| Regeneration
score |
|
|
|
|
|
|
|
|
|
|
|
|
| Day
1 | 0 | 0 |
| 1 (0–2) | 0 | 0.014 | 0 | 0 |
| 0 | 0
(0–2) | 0.4 |
| Day
3 | 0 | 0 |
| 1 (0–5) | 0 (0–2) | 0.11 | 0 (0–1) | 0
(0–1) | 0.84 | 0 | 0
(0–2) | 0.09 |
| Day
7 | 0 (0–1) | 0 (0–1) | 0.81 | 0 (0–2) | 1
(0–4)a | 0.11 | 0 (0–1) | 0
(0–3) | 0.32 | 0 (0–1) | 0
(0–5) | 0.2 |
| Day
14 |
| 0 |
|
| 0 |
|
| 0.5
(0–1)a |
|
| 1.5
(0–3)a |
|
| Chronic score |
|
|
|
|
|
|
|
|
|
|
|
|
| Day
1 | 0 | 0 |
| 0 | 0 |
| 0 | 0 |
| 0 | 0 |
|
| Day
3 | 0 | 0 |
| 0 | 0 |
| 0 | 0 |
| 0 | 0 |
|
| Day
7 | 0 | 0 |
| 0 | 0 |
| 0 | 0 |
| 0 | 0 |
|
| Day
14 |
| 0 |
|
| 0 |
|
| 0 |
|
| 0 |
|
 | Table III.Total pathological changes in all
kidney zones. |
Table III.
Total pathological changes in all
kidney zones.
| Score | Positive control
(n=6) | ADSC (n=8) | P-value |
|---|
| Injury score |
|
|
|
| Day
1 | 9 (7–11) | 0 (0–6) | 0.002 |
| Day
3 | 5 (1–9) | 0
(0–4)a | 0.007 |
| Day
7 | 1
(0–6)b | 0 (0–5) | 0.7 |
| Day
14 |
| 0 (0–2) |
|
| Regeneration
score |
|
|
|
| Day
1 | 1 (0–2) | 0 (0–2) | 0.12 |
| Day
3 | 1.5 (0–5) | 0 (0–5) | 0.08 |
| Day
7 | 0 (0–5) | 2
(0–10)a | 0.08 |
| Day
14 |
| 2.5 (0–3) |
|
| Chronic score |
|
|
|
| Day
1 | 0 | 0 |
|
| Day
3 | 0 | 0 |
|
| Day
7 | 0 | 0 |
|
| Day
14 |
| 0 |
|
| Total pathology
score |
|
|
|
| Day
1 | 9.5 (8–13) | 0 (0–6) | 0.002 |
| Day
3 | 6.5 (1–13) | 0 (0–9) | 0.013 |
| Day
7 | 1
(0–11)a | 2 (0–11) | 0.35 |
| Day
14 |
| 2.5
(0–5)a |
|
These data demonstrated that the injury induced by
IRI primarily affects the cortex and outer medulla and is
potentially reversible and attenuated by the use of ADSCs.
Discussion
The pathophysiological process of AKI following IRI
leads to functional and structural changes that are centered around
the proximal tubule cells and endothelium (15). Renal ischemia induces endothelial
stress, which leads to swelling of the renal vasculature and
consequent narrowing of the lumen with no-reflow phenomenon
following the restoration of the renal blood flow (6). Moreover, the constant communication
between the tubular epithelium and the vascular endothelium via
‘cellular cross-talk’ plays a role in the pathogenesis of AKI
associated with IRI (6). When
exposed to IRI the renal tubular epithelium generates mediators
such as tumor necrosis factor (TNF)-α, transforming growth factor-β
and interleukin (IL)-6 that may affect the endothelium directly or
by the potentiation of an inflammatory response (6). Although the clinical management of
patients with AKI has significantly improved in recent years,
specific therapies to enhance kidney repair are lacking. Recovery
following acute injury is critical for minimizing patient morbidity
and mortality in the hospital setting (16).
The emerging field of regenerative medicine is
progressing rapidly and is supported by a large number of studies
demonstrating that stem cells have the capacity to substitute for
damaged or lost differentiated cells in various organs and tissues
(17–20).
Semedo et al (21) demonstrated an anti-inflammatory
pattern in MSC-treated animals, indicating the potential of MSCs to
modulate IRI, leading to the earlier regeneration of damaged renal
tissue.
The aim of the present study was to address the role
of ADSCs in AKI secondary to renal IRI. In this study, one million
ADSCs were injected into the rats 4 h following the surgical IRI
modeling procedure, and rats were sacrificed on days 1, 3, 7 and 14
after this. The surgery entailed clamping of the renal pedicle for
45 min in anesthetized rats to cause severe IRI injury in the
kidney.
The damaging effects of renal IRI comprise a complex
interrelated sequence of events, eventually resulting in both
apoptosis and necrosis of the renal cells (5). Thus an ideal modality to manage IRI
injury should work by multiple mechanisms.
In the present study, the use of ADSCs was found to
be capable of ameliorating renal dysfunction, as demonstrated by
improvements of serum creatinine levels and creatinine clearance,
and improvement of the histological indices of injury in the renal
cortex and outer medulla. Moreover, the use of ADSCs partially
ameliorated oxidative stress and lipid peroxidation as reflected by
changes in the levels of MDA in the serum and renal tissue. The
results are concordant with the results of previous studies. Chen
et al (16) demonstrated that
ADSC therapy minimized kidney injury following IRI by suppressing
oxidative stress and the inflammatory response. Furuichi et
al (22) also showed that ADSCs
were able to ameliorate AKI induced secondary to IRI in a mouse
model via the suppression of cytokines (IL-1β and TNF-α) and
chemokines (microphage inflammatory protein-1α), which led to an
anti-inflammatory activity and alleviation of tubular necrosis.
Based on the ability of cyclosporine to reduce the generation of
reactive oxygen species Chen et al (7) administered cyclosporine in addition to
ADSCs to mitigate AKI in the setting of IRI in rats, and this
combined treatment showed an improved protective effect against
acute IRI compared with either treatment alone.
By contrast, in a study conducted in a mouse model
by Jiang et al (23), it was
concluded that transplantation using hematopoietic stem cells or an
MSC cell line did not improve the renal repair process. In
addition, the use of stem cells is currently limited by the
possible risks associated with the use of stem cell therapy, and
controversy exists regarding the exact mechanism underlying the
effects of the therapy; thorough scientific exploration is required
to assess mechanism, safety profile, reproducibility and methods
for monitoring the administered stem cells (8).
Notably, ADSCs exhibit both an early anti-acute
insult effect and late regenerative activities, as demonstrated by
the results of the present study. Table
I indicates a significantly intact renal clearance in the ADSC
group at day 1 following renal ischemia-reperfusion, as compared
with that of the untreated positive control group. This
observation, combined with a notably lower level of MDA in the
renal tissue at day 1 is indicative of an early protective
mechanism of ADSCs, which may act through antioxidative activity
with a consequent anti-inflammatory effect. The histopathological
evaluations confirm this finding, as indicated by the significantly
lower injury score for the outer stripe of the outer medulla at the
day-1 scoring.
The late regenerative stimulant activity of ADSCs is
indicated by the markedly significant improvement in the creatinine
clearance by the day-14 measurement without a proportional
reduction of the tissue MDA level, together with a significant
improvement in the regenerative score of the OSOM by day 7 in
comparison with the score on day 1.
In conclusion, the results of the present study
demonstrate the ability of ADSCs to ameliorate the renal injury and
dysfunction associated with IRI in rats by early protective
antioxidative activity and later regenerative stimulant activity.
However, the major limitations of this study are that molecular
studies of the renal tissues were not conducted, and the underlying
mechanisms involved in the therapeutic effect of ADSCs against
renal IRI remain descriptive. Further investigations, therefore,
are warranted to clarify the exact mechanisms underlying the
effects of the ADSCs. Since satisfactory treatment modalities for
renal IRI are currently lacking, stem cell therapy may be promising
provided that gaps in knowledge are filled to assure a safe
transition to use in humans.
Acknowledgements
The present study was supported by the Mansoura
University Research and Technology Funding Unit (grant no.
012-09).
References
|
1
|
Palevsky PM: Epidemiology of acute renal
failure: The tip of the iceberg. Clin J Am Soc Nephrol. 1:6–7.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Palevsky PM, Molitoris BA, Okusa MD, Levin
A, Waikar SS, Wald R, Chertow GM, Murray PT, Parikh CR, Shaw AD, et
al: Design of clinical trials in acute kidney injury: Report from
an NIDDK workshop on trial methodology. Clin J Am Soc Nephrol.
7:844–850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Giraud S, Favreau F, Chatauret N,
Thuillier R, Maiga S and Hauet T: Contribution of large pig for
renal ischemia-reperfusion and transplantation studies: The
preclinical model. J Biomed Biotechnol. 2011:5321272011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jang HR, Ko GJ, Wasowska BA and Rabb H:
The interaction between ischemia-reperfusion and immune responses
in the kidney. J Mol Med (Berl). 87:859–864. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sheridan AM and Bonventre JV:
Pathophysiology of ischemic acute renal failure. Contrib Nephrol.
7–21. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brodsky SV and Goligorsky MS: Endothelium
under stress: Local and systemic messages. Semin Nephrol.
32:192–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen YT, Yang CC, Zhen YY, Wallace CG,
Yang JL, Sun CK, Tsai TH, Sheu JJ, Chua S, Chang CL, et al:
Cyclosporine-assisted adipose-derived mesenchymal stem cell therapy
to mitigate acute kidney ischemia-reperfusion injury. Stem Cell Res
Ther. 4:622013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bagul A, Frost JH and Drage M: Stem cells
and their role in renal ischaemia reperfusion injury. Am J Nephrol.
37:16–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Si YL, Zhao YL, Hao HJ, Fu XB and Han WD:
MSCs: Biological characteristics, clinical applications and their
outstanding concerns. Ageing Res Rev. 10:93–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bassi G, Pacelli L, Carusone R, Zanoncello
J and Krampera M: Adipose-derived stromal cells (ASCs). Transfus
Apher Sci. 47:193–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zuk PA: Stem cell research has only just
begun. Science. 293:211–212. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lotfy A, Salama M, Zahran F, Jones E,
Badawy A and Sobh M: Characterization of mesenchymal stem cells
derived from rat bone marrow and adipose tissue: A comparative
study. Int J Stem Cells. 7:135–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bunnell BA, Flaat M, Gagliardi C, Patel B
and Ripoll C: Adipose-derived stem cells: Isolation, expansion and
differentiation. Methods. 45:115–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jablonski P, Howden BO, Race DA, Birrel
CS, Marshall VC and Tange J: An experimental model for assessment
of renal recovery from warm ischemia. Transplantation. 35:198–204.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Semedo P, Palasio CG, Oliveira CD, Feitoza
CQ, Gonçalves GM, Cenedeze MA, Wang PM, Teixeira VP, Reis MA,
Pacheco-Silva A and Câmara NO: Early modulation of inflammation by
mesenchymal stem cell after acute kidney injury. Int
Immunopharmacol. 9:677–682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen YT, Sun CK, Lin YC, Chang LT, Chen
YL, Tsai TH, Chung SY, Chua S, Kao YH, Yen CH, et al:
Adipose-derived mesenchymal stem cell protects kidneys against
ischemia-reperfusion injury through suppressing oxidative stress
and inflammatory reaction. J Transl Med. 9:512011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Donovan PJ and Gearhart J: The end of the
beginning for pluripotent stem cells. Nature. 414:92–97. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goodell MA: Stem-cell ‘plasticity’:
Befuddled by the muddle. Curr Opin Hematol. 10:208–213. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Herzog EL, Chai L and Krause DS:
Plasticity of marrow-derived stem cells. Blood. 102:3483–3493.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weissman IL: Stem cells: Units of
development, units of regeneration, and units of evolution. Cell.
100:157–168. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Semedo P, Wang PM, Andreucci TH, Cenedeze
MA, Teixeira VP, Reis MA, Pacheco-Silva A and Câmara NO:
Mesenchymal stem cells ameliorate tissue damages triggered by renal
ischemia and reperfusion injury. Transplant Proc. 39:421–423. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furuichi K, Shintani H, Sakai Y, Ochiya T,
Matsushima K, Kaneko S and Wada T: Effects of adipose-derived
mesenchymal cells on ischemia-reperfusion injury in kidney. Clin
Exp Nephrol. 16:679–689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang Y, Jahagirdar BM, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, et al: Pluripotency of mesenchymal stem cells
derived from adult marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|