Introduction
Legg-Calvé-Perthes disease (LCPD) is a
noninflammatory, aseptic, self-limiting, idiopathic, avascular
necrosis of the femoral head, occurring in childhood, typically
between the ages of 4 and 10 years and causing permanent femoral
head deformity and premature osteoarthritis (1–3). The
disease was first described by three independent authors in the
year 1910: Perthes in Germany (4),
Legg in the United States (5) and
Calvé in France (6). The incidence
of LCPD varies between 5.1 and 16.9 per 100,000 in various regions
of the world (2,7). The etiology of LCPD remains unclear,
which makes it impossible to create prevention strategies or to
identify individuals at risk. A number of possible causes have been
proposed, including trauma, an inflammatory process, vascular
occlusion, hemodynamic alterations, endothelial injury,
thrombophilia and insulin-like growth factor-1 pathway abnormality
(2,8,9).
Nitric oxide (NO) is a multifunctional biomolecule
synthesized by three isoforms of the nitric oxide synthase (NOS)
enzyme, namely endothelial NOS (eNOS, also known as NOS III),
inducible NOS (iNOS or NOS II) and neuronal NOS (nNOS or NOS I). NO
participates in numerous physiological processes, including
angiogenesis, thrombosis, coagulation and fibrinolysis. The eNOS
gene is located on the long arm of chromosome 7 (7q35–36), has 26
exons and 25 introns, and encodes a protein of 1,203 amino acids
(10,11). The most examined and functionally
associated polymorphisms are G894T in exon 7 and 27-bp variable
number tandem repeat (VNTR) polymorphism in intron 4, respectively
(12,13). A number of studies have observed
associations between genetic polymorphisms in the eNOS gene and
cardiovascular diseases, including coronary artery disease, chronic
heart failure, hypertension, atherosclerosis, stroke, renal
diseases and avascular necrosis of the femoral head, mainly in
adult patients (14–20). Numerous studies have indicated that
disruption of the blood supply to the femoral head is a key
pathogenic event resulting in bone necrosis (2,21,22).
Thrombophilia and/or decreased fibrinolysis are possible mechanisms
that have been investigated as potential causes of LCPD (23–25). It
may be speculated that sequence variations in the eNOS gene could
influence nitric oxide production, and thereby affect the
progression of LCPD. To the best of our knowledge, no study has yet
investigated the association between eNOS polymorphism and the
pathogenesis of LCPD.
Therefore, in the present study, the aim was to
examine a relevance of the 27-bp VNTR polymorphism in intron 4 and
G894T polymorphism in exon 7 of the eNOS gene by conducting an
analysis of 80 LCPD Chinese patients and 100 healthy controls.
Materials and methods
Study subjects
A total of 80 patients with LCPD (70 boys and 10
girls, aged between 4 and 10 years) were consecutively enrolled in
this study at the Department of Orthopedics of the First Affiliated
Hospital of Guangxi Medical University (Nanning, China) from
October 2012 to September 2014. LCPD was diagnosed on the basis of
radiography and clinical presentation. Magnetic resonance imaging
(MRI) was used as a supplementary technique to identify the changes
of the femoral head in the early stage of LCPD. None of the
patients with LCPD had historical or clinical evidence of vascular
diseases, such as atherosclerosis, arterial thrombosis, coronary
artery disease, hypertension, renal diseases, ischemic heart
diseases, cerebrovascular diseases, or coexisting disorders that
may be associated with eNOS polymorphism. In addition, 100 healthy
age- and gender-matched children were included as controls, none of
whom had LCPD or clinical symptoms suggesting LCPD. This study was
approved by the Clinical Research Ethics Committee of the First
Affiliated Hospital of Guangxi Medical University. Written informed
consent was obtained from all participants or their parents.
DNA extraction and genotyping
For all subjects, peripheral blood samples were
collected into 2 ml EDTA tubes. Genomic DNA was extracted from
whole blood using a commercial kit (DP318-03; Tiangen Biotech Co.,
Ltd., Beijing, China), in accordance with the manufacturer's
protocol. The amplification of the eNOS 27-bp VNTR polymorphism of
intron 4 was performed by polymerase chain reaction (PCR) using the
following primers: Forward 5′-AGGCCCTATGGTAGTGCCTTT-3′ and reverse
5′-TCTCTTAGTGCTGTGGTCAC-3′. The PCR reactions were performed in a
total volume of 25 µl, which contained containing 500 µM each dNTP,
20 mM Tris-HCl (pH 8.3), 100 mM KCl, 3 mM MgCl2, 0.1
units Taq polymerase (Tiangen Biotech Co., Ltd.), 10 µM each primer
and 100 ng genomic DNA. The reaction mixture was heated to 94°C (3
min), followed by 30 cycles of 94°C (30 sec), 58°C (30 sec), and
72°C (1 min), and then a final extension at 72°C (5 min). The PCR
products were analyzed by electrophoresis in 2% agarose gels at 80
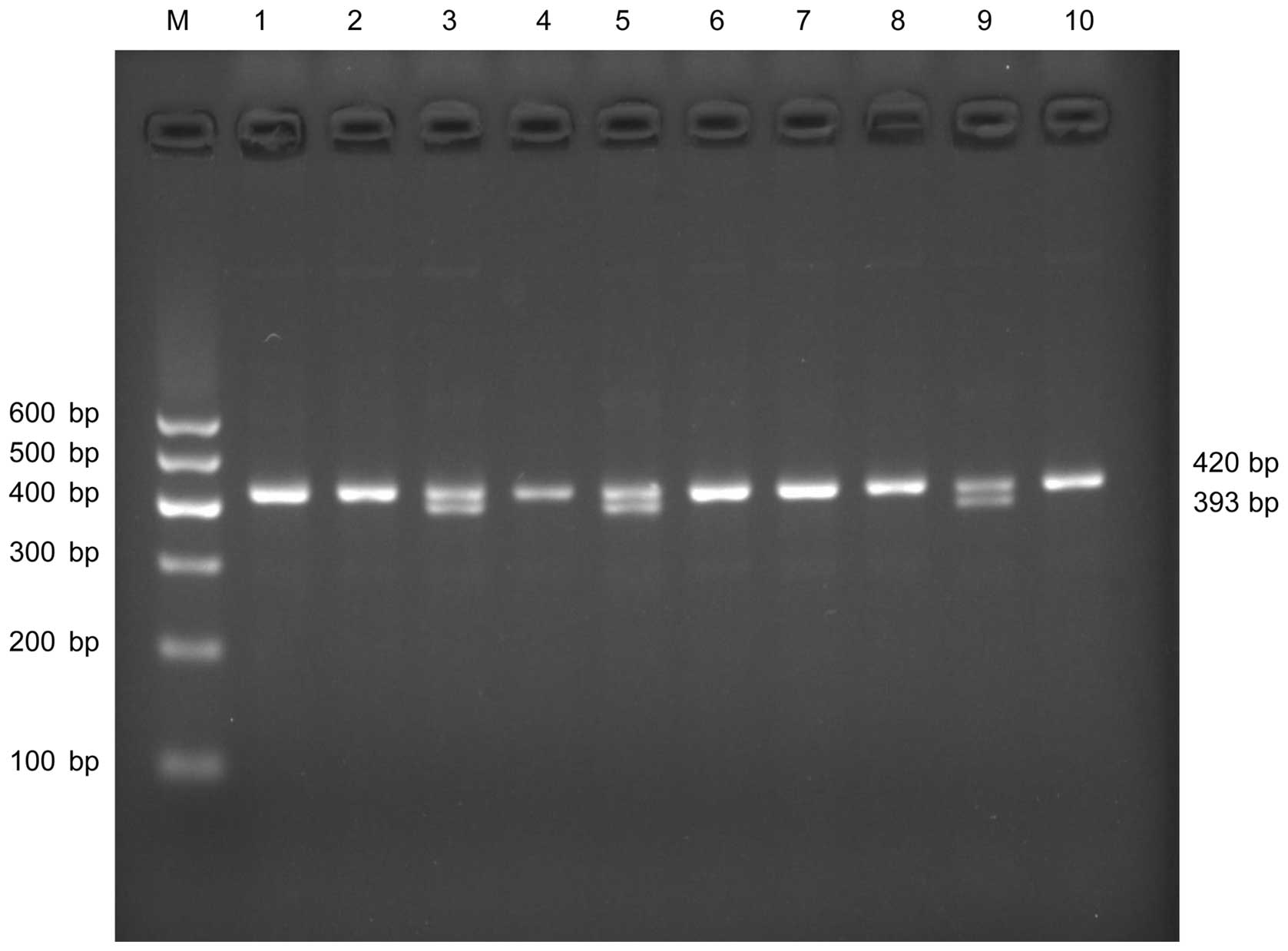
V for 40 min. A band size of 393 bp indicated a polymorphic
genotype (aa, 4 repeats). A 420-bp fragment represented the
wild-type genotype (bb, 5 repeats). Two fragments at 393 bp and 420
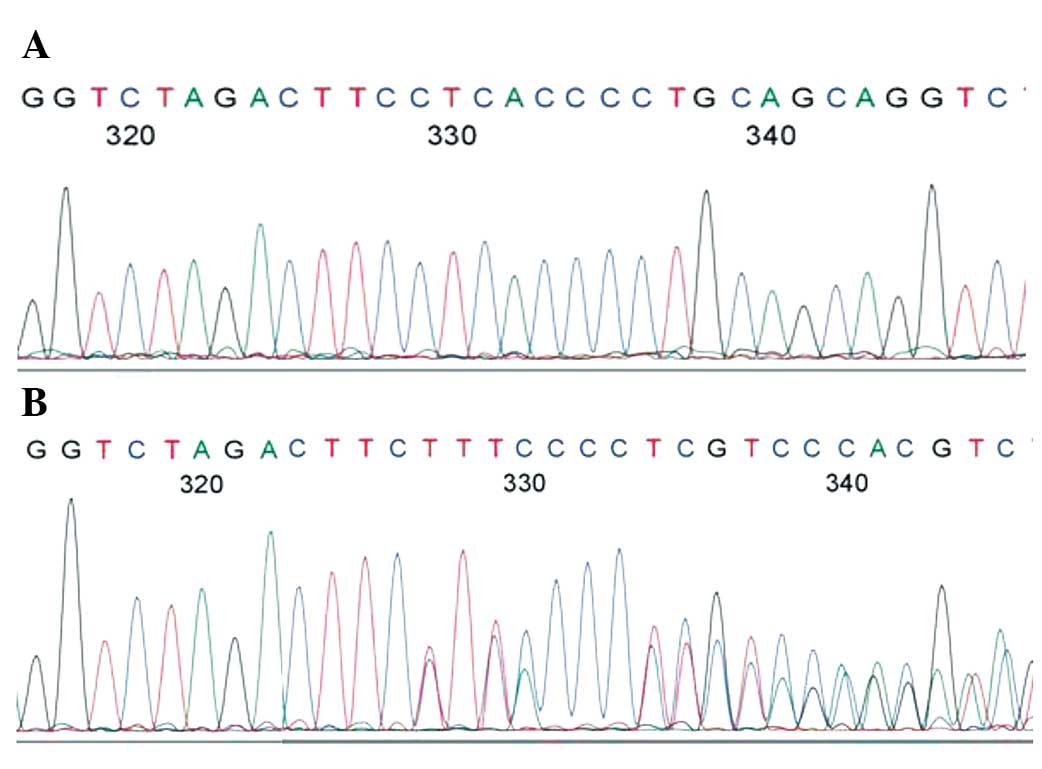
bp indicated a heterozygous genotype ab (Fig. 1). The PCR products were sequenced
using an ABI3730XL genetic analyzer (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) (Fig. 2).
The eNOS G894T polymorphism in exon 7 was determined by
PCR-restriction fragment length polymorphism (PCR-RFLP) analysis.
The forward primer was 5′-AAGGCAGGAGACAGTGGATGG-A-3′ and the
reverse primer was 5′-CCCAGTCAATCCCTTTGGTGCTCA-3′. PCR was
conducted to amplify 0.1 µg genomic DNA. The 25 µl reaction mixture
contained 500 µM each dNTP, 20 mM Tris-HCl (pH 8.3), 100 mM KCl, 3
mM MgCl2, 0.1 units Taq polymerase, and 10 µM sense and
antisense primers. PCR was performed by denaturation at 94°C for 3
min, followed by 30 cycles (30 sec at 94°C, 30 sec at 65°C and 60
sec at 72°C) and a final extension at 72°C for 5 min. This PCR
amplification yielded a 248-bp fragment. Following amplification,
PCR products were digested for 6 h using 2 units of BanII
restriction enzyme (Takara Biotechnology Co., Ltd., Dalian, China)
at 37°C. The final products of eNOS G894T polymorphism including
GG, GT and TT genotypes had band sizes of 163 bp/85 bp, 248 bp/163
bp/85 bp and 248 bp, respectively. The digestion products were
separated by electrophoresis on GoldView I-stained (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China) 2.5%
agarose gel (Fig. 3) with DNA marker
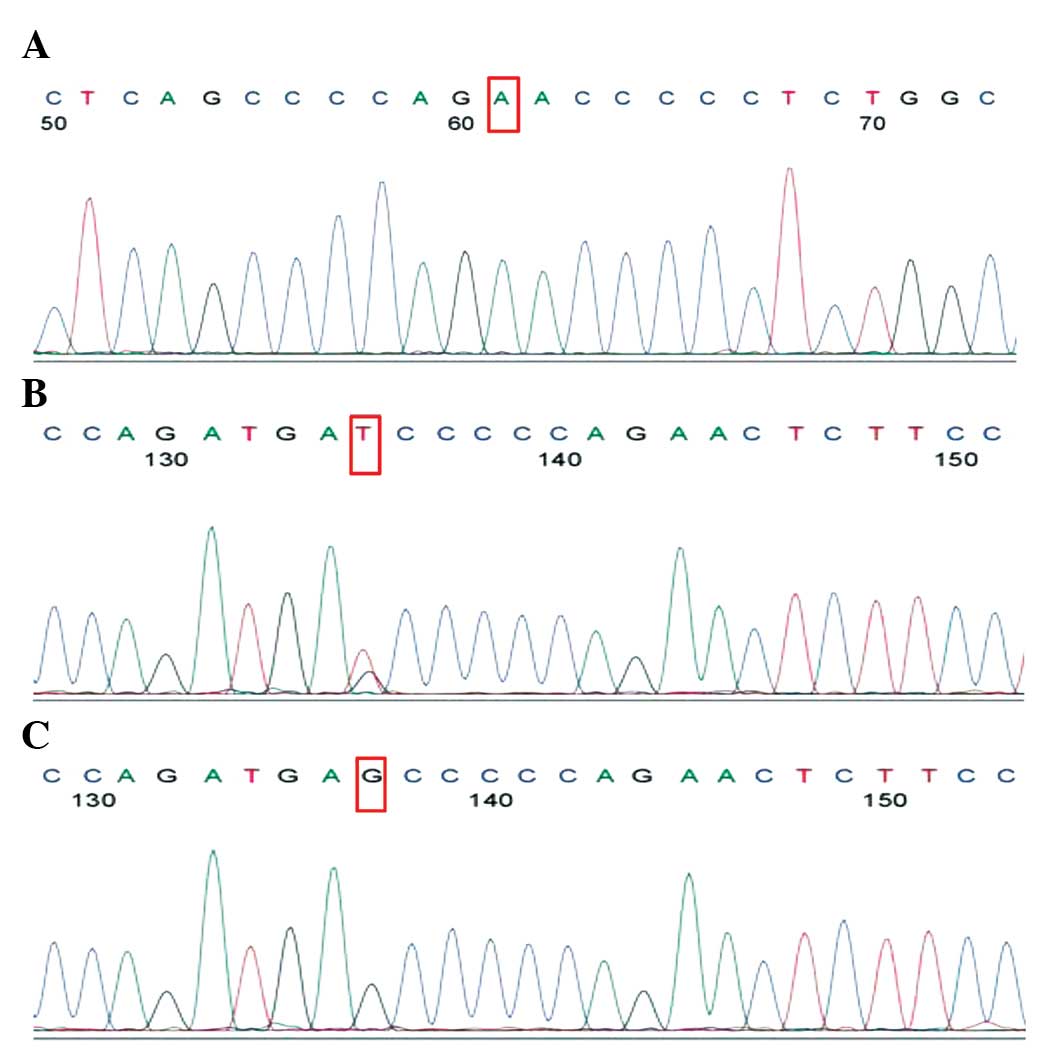
I (Tiangen Biotech Co., Ltd.). The eNOS G894T polymorphisms were
also sequenced using the ABI3730XL genetic analyzer (Fig. 4).
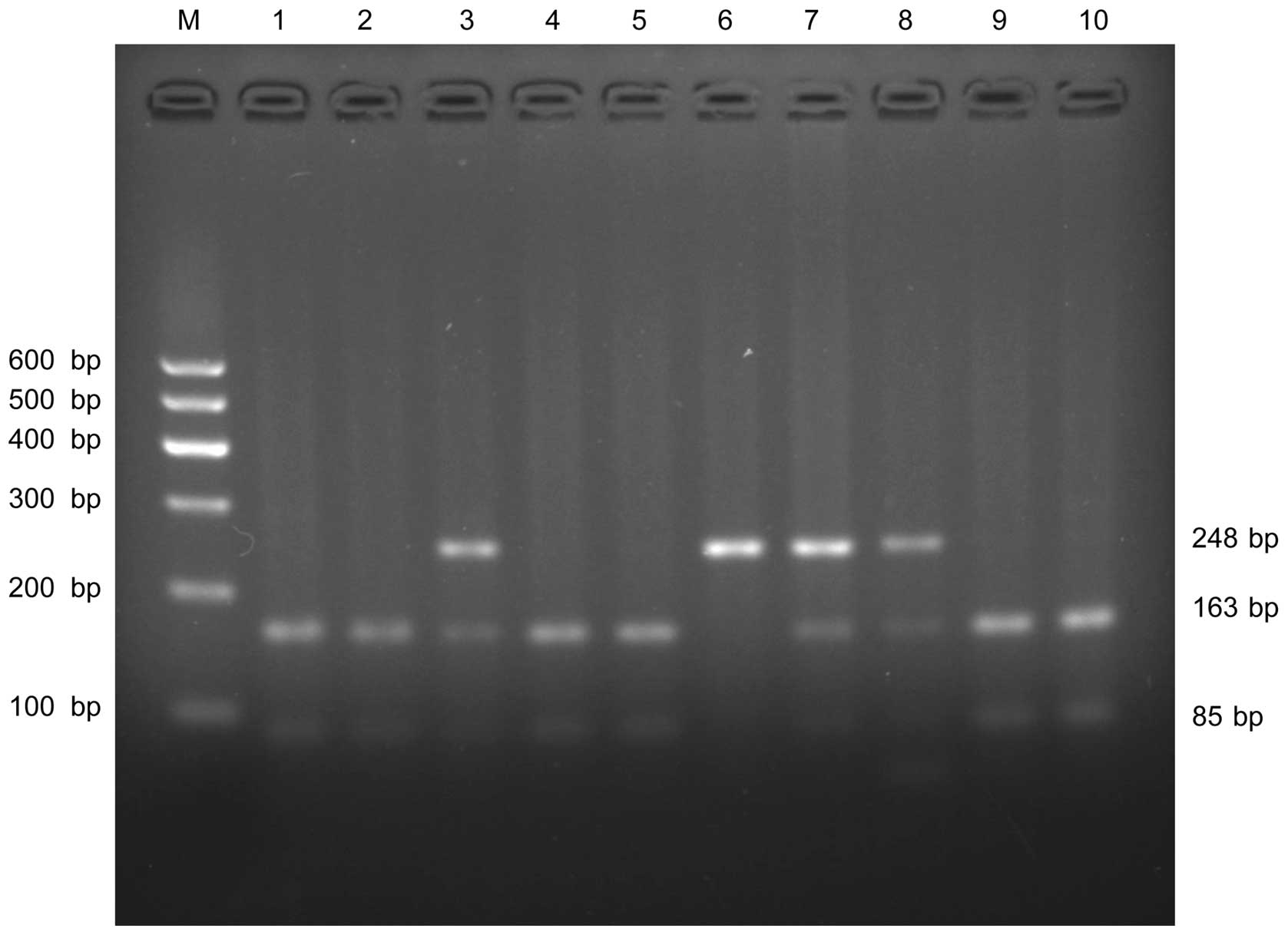 | Figure 3.Agarose gel electrophoresis result of
eNOS G894T polymorphism provided by PCR-RFLP. The PCR product was
digested with BanII restriction enzyme and visualized on a
2.5% agarose gel. Lanes 1, 2, 4, 5, 9, 10 show wild type GG
genotype (163 and 85 bp); lanes 3,7,8 show heterozygous GT (248,
163 and 85 bp); and lane 6: homozygous TT (248 bp). Lane M: DNA
marker. eNOS, endothelial nitric oxide synthase; PCR-RFLP,
polymerase chain reaction-restriction fragment length
polymorphism. |
Statistical analysis
Continuous data are presented as the mean ± standard
deviation. Categorical data are expressed in percentages. The
allelic frequencies were calculated by the chromosome counting
method. The genotype frequencies for eNOS polymorphism among
patients and healthy controls were assessed for Hardy-Weinberg
equilibrium (HWE) using the Pearson's Chi-square method. Odds
ratios (ORs) and 95% confidence intervals (CIs) were calculated
using relevant 2×2 contingency tables. The data was analyzed using
the SPSS statistical software package (version 16.0; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant result.
Results
General characteristics of the study
subjects
In this study, 80 LCPD patients and 100 controls
were included in the final analysis. LCPD was more common in boys
than in girls. The general characteristics of the study population
are summarized in Table I. There
were more boys than girls in both the patient group (87.5% boys)
and the gender-matched pediatric control group (85% boys). There
were no significant differences between the groups regarding age
and gender.
 | Table I.General characteristics of the study
subjects. |
Table I.
General characteristics of the study
subjects.
| Variable | LCPD (n=80) | Healthy control
(n=100) | P-value |
|---|
| Gender, n (%) |
|
|
|
| Male | 70 (87.5) | 85 (85.0) | >0.05 |
|
Female | 10 (12.5) | 15 (15.0) | >0.05 |
| Age,
yearsa | 7.3±2.0 | 6.8±2.1 | >0.05 |
| Site of LCPD, n
(%) |
|
|
|
|
Right | 38 (47.5) |
|
|
| Left | 30 (37.5) |
|
|
|
Bilateral | 12 (15.0) |
|
|
| Surgery |
|
|
|
| Yes | 49 |
|
|
| No | 31 |
|
|
eNOS gene polymorphisms in the case
and control groups
The genotypic and allelic frequencies of the two
eNOS gene polymorphisms in the case and control groups are shown in
Table II. The genotype frequencies
of the analyzed polymorphism were in HWE. The genotypic and allelic
frequencies of the two eNOS gene polymorphisms differed between the
control and patient groups. Representative electrophoresis results
and sequence diagrams for the eNOS 27-bp VNTR polymorphism are
shown in Figs. 1 and 2. The LCPD case group showed a higher
frequency of the ab genotype than the healthy control group (27.5
vs. 14%; OR, 2.33; 95% CI, 1.10–4.92; P=0.024). The frequency of
the a allele was significantly higher in the LCPD case group than
in the healthy controls (13.8 vs. 7%; OR, 2.12; 95% CI, 1.05–4.29;
P=0.034). Representative electrophoresis results and sequence
diagrams for the eNOS G894T polymorphism are shown in Figs. 3 and 4
The heterozygous genotype GT was observed in 35% of the patients
(28/80) and 17% (17/100) of the controls (OR, 2.67; 95% CI,
1.33–5.36; P=0.005). The frequency of allele T in the LCPD case
group (20%) was greater than that of the healthy control group
(10.5%), and the difference in frequency distribution between
alleles was significant (OR, 2.13; 95% CI, 1.18–3.87; P=0.011).
 | Table II.Comparison of genotypic distributions
and allele frequencies of the two eNOS gene polymorphisms between
the LCPD and healthy control groups. |
Table II.
Comparison of genotypic distributions
and allele frequencies of the two eNOS gene polymorphisms between
the LCPD and healthy control groups.
|
|
|
|
| LCPD vs. healthy
control |
|---|
|
|
|
|
|
|
|---|
| Polymorphism |
Genotype/Allele | LCPD (n=80) | Healthy control
(n=100) | OR | 95% CI | P-value |
|---|
| eNOS 27 bp VNTR, n
(%) | bb | 58 (72.5) | 86 (86.0) | 1 |
|
|
|
| ab | 22 (27.5) | 14 (14.0) | 2.33 | 1.10–4.92 | 0.024 |
|
| aa | 0 (0) | 0 (0) |
|
|
|
|
| b | 138 (86.2) | 186 (93.0) | 1 |
|
|
|
| a | 22 (13.8) | 14 (7.0) | 2.12 | 1.05–4.29 | 0.034 |
| eNOS G894T, n
(%) | GG | 50 (62.5) | 81 (81.0) | 1 |
|
|
|
| GT | 28 (35.0) | 17 (17.0) | 2.67 | 1.33–5.36 | 0.005 |
|
| TT | 2 (2.5) | 2 (2.0) | 1.62 | 0.22–11.87 | 0.632 |
|
| G | 128 (80.0) | 179 (89.5) | 1 |
|
|
|
| T | 32 (20.0) | 21 (10.5) | 2.13 | 1.18–3.87 | 0.011 |
Discussion
The precise pathogenetic basis of LCPD is currently
unknown. Repeated interruptions of the vascular blood supply to the
femoral head, which results in ischemic necrosis, appears to be a
key pathogenic event causing pathological and subsequent structural
changes to the growing femoral head (2,21,22).
However, at present, the exact pathogenetic basis of the reductions
in blood flow to the femoral head vasculature in patients with LCPD
is unclear. LCPD is considered to be a multifactorial disease, in
which genetic and environmental factors play a role (2). Since genetic background is a
determinant of LCPD, extensive genomics research is required to
identify polymorphic loci and elucidate the pathogenesis. eNOS gene
polymorphisms have been investigated as potential risk factors in
numerous vascular diseases, as aforementioned (14–20). To
the best of our knowledge, no studies have investigated the role of
eNOS gene polymorphism in the risk of LCPD. In the present study,
the association between eNOS gene polymorphism and the risk of LCPD
was analyzed in children from the Guangxi province of China, and it
was found that the frequency of an a allele and ab genotype in
intron 4 and allele 894T, GT genotype in exon 7 were significantly
higher in patients with LCPD than in healthy controls.
NO, synthesized by eNOS, has various roles in the
vasculature, which include the regulation of vascular tone and
blood pressure via vasodilation (26). NO also been suggested to be involved
in the modulation of angiogenesis (27). In a previous study, it was observed
that the development of angiogenesis in response to limb ischemia
was severely reduced in mice lacking the eNOS gene (28). Another study demonstrated that the
presence of a 27-bp VNTR polymorphism in intron 4 and G894T
polymorphism in exon 7 can cause changes in eNOS expression and
enzymatic activity; the NO levels in the plasma of subjects with
the a allele of eNOS in intron 4 were significantly lower than
those without the a allele (29).
The G894T polymorphism causes a structural change in the eNOS
protein that is associated with impaired eNOS activity (30). Therefore, allele a and G894T gene
polymorphisms can lead to low expression levels and activity of
eNOS. The present study also reveals that the 27-bp VNTR
polymorphism in intron 4 and G894T polymorphism in exon 7 may be
risk factors for LCPD, and indicates that NO produced by
constitutively expressed eNOS may play a protective role in the
pathogenesis of LCPD. The eNOS enzyme is constitutively expressed
in vascular endothelial cells. Endothelial cells are important in
the pathogenesis of diseases that involve thrombosis and/or
inflammation (31). Changes in
hemodynamics (22), vascular
thrombotic tendency due to hypercoagulability (32), and hypofibrinolysis (9,33,34) have
all been suggested to be significant factors in the pathogenesis of
LCPD. Intron 4 and G894T polymorphisms of eNOS have been shown to
decrease NO levels in human plasma (35,36). As
a result, polymorphisms of the eNOS gene and resultant reductions
in the quantity of synthesized NO are likely to lead to different
types of vascular diseases, as previously mentioned; these diseases
may share some common risks through inheritance or exposure.
Polymorphisms of the eNOS gene have been indicated to be risk
factors for avascular necrosis of the femoral head (20), an adult disease that has a similar
pathogenesis to LCPD. The finding in the present study that eNOS
polymorphism is associated with the pathogenesis of LCPD is,
therefore, not remarkable.
The present study has certain limitations: As all of
the samples are from the same hospital, potential selection bias
cannot be fully excluded. Also, in consideration of the low
incidence of LCPD, the sample size was small, and the rare aa
genotype was not found in the subjects investigated.
In summary, to the best of our knowledge, this is
the first report investigating an association between eNOS gene
polymorphism and the risk of LCPD. The results support the role of
eNOS in the pathogenesis of LCPD. However, as the sample size of
this study was relatively small, additional studies with larger
cohorts are required in order to confirm the observed
association.
Glossary
Abbreviations
Abbreviations:
|
CI
|
confidence interval
|
|
eNOS
|
endothelial nitric oxide synthase
|
|
HWE
|
Hardy-Weinberg equilibrium
|
|
LCPD
|
Legg-Calvé-Perthes disease
|
|
NO
|
nitric oxide
|
|
OR
|
odds ratio
|
|
PCR-RFLP
|
polymerase chain reaction-restriction
fragment length polymorphism
|
|
VNTR
|
variable number tandem repeat
|
References
|
1
|
Shah H: Perthes disease: Evaluation and
management. Orthop Clin North Am. 45:87–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim HK: Pathophysiology and new strategies
for the treatment of Legg-Calvé-Perthes disease. J Bone Joint Surg
Am. 94:659–669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng TL, Murphy CM, Cantrill LC, Mikulec
K, Carpenter C, Schindeler A and Little DG: Local delivery of
recombinant human bone morphogenetic proteins and bisphosphonate
via sucrose acetate isobutyrate can prevent femoral head collapse
in Legg-Calve-Perthes disease: A pilot study in pigs. Int Orthop.
38:1527–1533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perthes G: Concerning arthritis deformans
juvenilis. 1910. Clin Orthop Relat Res. 451:17–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Legg AT: An obscure affection of the hip
joint. 1910. Clin Orthop Relat Res. 451:11–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calvé J: On a particular form of
pseudo-coxalgia associated with a characteristic deformity of the
upper end of the femur. Clin Orthop Relat Res. 451:14–16. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wiig O, Terjesen T, Svenningsen S and Lie
SA: The epidemiology and aetiology of Perthes' disease in Norway. A
nationwide study of 425 patients. J Bone Joint Surg Br.
88:1217–1223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alpaslan AM, Aksoy MC and Yazici M:
Interruption of the blood supply of femoral head: An experimental
study on the pathogenesis of Legg-Calve-Perthes disease. Arch
Orthop Trauma Surg. 127:485–491. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Glueck CJ, Freiberg RA and Wang P: Role of
thrombosis in osteonecrosis. Curr Hematol Rep. 2:417–422.
2003.PubMed/NCBI
|
|
10
|
Marsden PA, Schappert KT, Chen HS, Flowers
M, Sundell CL, Wilcox JN, Lamas S and Michel T: Molecular cloning
and characterization of human endothelial nitric oxide synthase.
FEBS Lett. 307:287–293. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marsden PA, Heng HH, Scherer SW, Stewart
RJ, Hall AV, Shi XM, Tsui LC and Schappert KT: Structure and
chromosomal localization of the human constitutive endothelial
nitric oxide synthase gene. J Biol Chem. 268:17478–17488.
1993.PubMed/NCBI
|
|
12
|
Fairchild TA, Fulton D, Fontana JT,
Gratton JP, McCabe TJ and Sessa WC: Acidic hydrolysis as a
mechanism for the cleavage of the Glu (298)->Asp variant of
human endothelial nitric-oxide synthase. J Biol Chem.
276:26674–26679. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lembo G, De Luca N, Battagli C, Iovino G,
Aretini A, Musicco M, Frati G, Pompeo F, Vecchione C and Trimarco
B: A common variant of endothelial nitric oxide synthase
(Glu298Asp) is an independent risk factor for carotid
atherosclerosis. Stroke. 32:735–740. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abdel-Aziz TA and Mohamed RH: Association
of endothelial nitric oxide synthase gene polymorphisms with
classical risk factors in development of premature coronary artery
disease. Mol Biol Rep. 40:3065–3071. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Balligand JL, Feron O and Dessy C: eNOS
activation by physical forces: From short-term regulation of
contraction to chronic remodeling of cardiovascular tissues.
Physiol Rev. 89:481–534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Doshi AA, Ziolo MT, Wang H, Burke E,
Lesinski A and Binkley P: A promoter polymorphism of the
endothelial nitric oxide synthase gene is associated with reduced
mRNA and protein expression in failing human myocardium. J Card
Fail. 16:314–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Levinsson A, Olin AC, Björck L, Rosengren
A and Nyberg F: Nitric oxide synthase (NOS) single nucleotide
polymorphisms are associated with coronary heart disease and
hypertension in the INTERGENE study. Nitric Oxide. 39:1–7. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bellini MH, Figueira MN, Piccoli MF,
Marumo JT, Cendoroglo MS, Neto MC, Dalboni MA, Batista MC, Goes MA
and Schor N: Association of endothelial nitric oxide synthase gene
intron 4 polymorphism with end-stage renal disease. Nephrology
(Carlton). 12:289–293. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spoto B, Benedetto FA, Testa A, Tripepi G,
Mallamaci F, Maas R, Boeger RH and Zoccali C: An additive effect of
endothelial nitric oxide synthase gene polymorphisms contributes to
the severity of atherosclerosis in patients on dialysis. Am J
Hypertens. 20:758–763. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng L, Wang W, Ni J, Li Z and Xiao T:
The association of eNOS gene polymorphism with avascular necrosis
of femoral head. PloS One. 9:e875832014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferguson AB Jr: Segmental vascular changes
in the femoral head in children and adults. Clin Orthop Relat Res.
291–298. 1985.PubMed/NCBI
|
|
22
|
Launder WJ, Hungerford DS and Jones LH:
Hemodynamics of the femoral head. J Bone Joint Surg Am. 63:442–448.
1981.PubMed/NCBI
|
|
23
|
Woratanarat P, Thaveeratitharm C,
Woratanarat T, Angsanuntsukh C, Attia J and Thakkinstian A:
Meta-analysis of hypercoagulability genetic polymorphisms in
Perthes disease. J Orthop Res. 32:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aksoy MC, Aksoy DY, Haznedaroglu IC,
Sayinalp N, Kirazli S and Alpaslan M: Enhanced tissue factor
pathway inhibitor response as a defense mechanism against ongoing
local microvascular events of Legg-Calve-Perthes disease. Pediatr
Hematol Oncol. 22:391–399. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aksoy MC, Aksoy DY, Haznedaroglu IC,
Sayinalp N, Kirazli S and Alpaslan M: Thrombomodulin and GFC levels
in Legg-Calve-Perthes disease. Hematology. 13:324–328. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gamboa A, Okamoto LE, Diedrich A, Choi L,
Robertson D, Farley G, Paranjape S and Biaggioni I: Sympathetic
activation and nitric oxide function in early hypertension. Am J
Physiol Heart Circ Physiol. 302:H1438–H1443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cooke JP: NO and angiogenesis. Atheroscler
Suppl. 4:53–60. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murohara T, Asahara T, Silver M, Bauters
C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, et
al: Nitric oxide synthase modulates angiogenesis in response to
tissue ischemia. J Clin Invest. 101:2567–2578. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsukada T, Yokoyama K, Arai T, Takemoto F,
Hara S, Yamada A, Kawaguchi Y, Hosoya T and Igari J: Evidence of
association of the ecNOS gene polymorphism with plasma NO
metabolite levels in humans. Biochem Biophys Res Commun.
245:190–193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoshimura M, Yasue H, Nakayama M,
Shimasaki Y, Sumida H, Sugiyama S, Kugiyama K, Ogawa H, Ogawa Y,
Saito Y, et al: A missense Glu298Asp variant in the endothelial
nitric oxide synthase gene is associated with coronary spasm in the
Japanese. Hum Genet. 103:65–69. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Horstman LL, Jy W, Jimenez JJ and Ahn YS:
Endothelial microparticles as markers of endothelial dysfunction.
Front Biosci. 9:1118–1135. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eldridge J, Dilley A, Austin H, EL-Jamil
M, Wolstein L, Doris J, Hooper WC, Meehan PL and Evatt B: The role
of protein C, protein S and resistance to activated protein C in
Legg-Perthes disease. Pediatrics. 107:1329–1334. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hunt DM, Holmes Z, Pickering W and Cohen
H: Correspondence. J Bone Joint Surg Am. 80:604–606.
1998.PubMed/NCBI
|
|
34
|
Van Veldhuizen PJ, Neff J, Murphey MD,
Bodensteiner D and Skikne BS: Decreased fibrinolytic potential in
patients with idiopathic avascular necrosis and transient
osteoporosis of the hip. Am J Hematol. 44:243–248. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Marco KC, Antunes LM, Tanus-Santos JE
and Barbosa F Jr: Intron 4 polymorphism of the endothelial nitric
oxide synthase (eNOS) gene is associated with decreased NO
production in a mercury-exposed population. Sci Total Environ.
414:708–712. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saini V, Bhatnagar MK and Bhattacharjee J:
Endothelial nitric oxide synthase Glu298Asp (G894T) gene
polymorphism in coronary artery disease patients with type 2
diabetes mellitus. Diabetes Metab Syndr. 6:106–109. 2012.
View Article : Google Scholar : PubMed/NCBI
|