Introduction
Hypoxemia following arthroplasty has been reported
to be a common postoperative complication (1–3). In 32%
of hypoxic patients, hypoxemia was the initial symptom to develop
and is a predicting factor for major complications, and is defined
as life-threatening if left untreated (1–3). It has
been suggested that a hypoxemic episode may contribute to
myocardial ischemia, infarction, wound infection (2,4,5), mental confusion (6,7),
heterotopic ossification and idiopathic arthrofibrosis (8) following the surgical procedure.
Myocardial ischemia is more likely to occur if an episode of
hypoxemia is prolonged (>5 min) and severe [perioperative
peripheral oxygen saturation (SpO2) <85%] (5). The morbidity associated with hypoxia is
exacerbated by delirium, which commonly follows total hip
arthroplasty (THA) in patients with femoral neck fractures
(7).
Anemia is commonly encountered during the
perioperative period (3,5,7). Blood
loss may occur from the osteotomy surfaces of the distal femoral
and proximal tibial bones, from the soft tissue-injury and from the
dredged marrow cavity (9). However,
long-term anticoagulation therapy and early rehabilitation of joint
function have also resulted in postoperative anemia (10).
Questions persist as to whether hypoxia indicates
that a patient is anemic and whether anemia causes a decline in
arterial oxygen pressure accompanied by hypoxemia. The purpose of
the present study was to determine the association between
hypoxemia and anemia following arthroplasty.
Materials and methods
Ethical approval
The present study was approved by the Institutional
Review Board on Human Studies of the Ethical Committee of the
China-Japan Friendship Hospital (Beijing. China), and was conducted
in accordance with the Helsinki Declaration of 1975 (11). All patients were required to sign an
informed consent form prior to participation in the study. The
written-informed consent forms were provided by the patients and
stored in the hospital database for clinical research.
Patient characteristics
A total of 135 patients (male, 53; female, 82; mean
age, 59.7 years; age range, 24–83 years) who underwent arthroplasty
at the China-Japan Friendship Hospital from January to May 2013
were retrospectively studied. The inclusion criteria were as
follows: Primary arthroplasty, no previous history of severe lung
disease, normal preoperative pulmonary function, and availability
of complete medical records. Patients were divided into 5 groups
according to the type of arthroplasty they underwent (Table I): Unilateral total knee arthroplasty
(TKA), bilateral TKA, unilateral THA, bilateral THA or unilateral
unicompartmental knee arthroplasty (UKA). Patient charts were
reviewed in order to obtain the required data, including body mass
index, preoperative diagnosis, type of anesthesia, medical
complications, and anticoagulant options (either low molecular
weight heparin or rivaroxaban). Height and weight were recorded
preoperatively. SpO2 and hemoglobin (Hb) levels were measured by
routine blood tests perioperatively. Room-air SpO2 was measured
prior to the surgical procedure using a pulse oximeter (Portable
PC-60C Non-invasive Pulse Oximeter; Shenzhen Creative Industry Co.,
Ltd., Guangdong, China). Oxygen saturation was measured
preoperatively, intraoperatively, and on postoperative days (POD)
1–6 using a pulse oximeter. Nursing surveillance included recording
of pulse oximetry with routine vital signs every 6 h following
arthroplasty. Hypoxemia was defined as SpO2<90%. A complete
blood count was performed for all patients pre- and postoperatively
via routine blood tests. A detailed data sheet recording the exact
circumstances surrounding a hypoxic event, the general information
and the final outcome was completed for each patient. No patient
developed a pulmonary embolism.
 | Table I.Characteristics of the patients who
underwent arthroplasty. |
Table I.
Characteristics of the patients who
underwent arthroplasty.
| Characteristic | U-THA | B-THA | U-TKA | B-TKA | U-UKA |
|---|
| Number of
patients | 51 | 15 | 42 | 22 | 5 |
| Gender
(male/female) | 33/18 | 8/7 | 7/35 | 5/17 | 0/5 |
| Age (years) | 54.3±13.5 | 47.3±13.3 | 67.1±8.4 | 66.0±7.4 | 59.8±9.5 |
| Height (cm) | 165.9±6.9 | 165.3±5.7 | 161.5±6.5 | 163.2±7.2 | 158.8±4.0 |
| Weight (kg) | 67.5±11.5 | 71.4±12.4 | 70.4±9.7 | 74.3±12.3 | 66.3±9.5 |
| BMI
(kg/m2) | 24.5±3.5 | 26.1±3.8 | 26.9±2.9 | 27.9±4.2 | 26.3±4.1 |
| Diagnosis
(OA/RA/ONFH) | 12/4/35 | 1/0/14 | 42/0/0 | 22/0/0 | 5/0/0 |
| Anesthesia
(ESA/GA) | 12/39 | 1/14 | 9/33 | 0/22 | 1/4 |
| Anticoagulants
(LMWH/R) | 45/6 | 15/0 | 36/6 | 20/2 | 4/1 |
Surgical procedures
All surgical procedures were performed by or under
the supervision of a fellowship-trained arthroplasty surgeon. Hip
arthroplasty at the China-Japan Friendship Hospital is performed
with the patient in the lateral decubitus position using a modified
posterolateral approach and uncemented components. TKA and UKA are
performed on an exsanguinated limb under tourniquet pressure, using
a medial parapatellar arthrotomy and cement fixation (12). The postoperative protocol for
arthroplasty patients included deep venous thrombosis prophylaxis
with subcutaneous administration of 4,000–6,000 U low
molecular-weight heparin (Glaxo Wellcome Production, Evreux,
France) or oral administration of 10 mg rivaroxaban (Bayer AG,
Leverkusen, Germany). All arthroplasty patients were placed on
oxygen during the immediate postoperative period and weaned over
the following 24 h. Patients were mobilized early in the
postoperative period and were encouraged to ambulate with assistive
devices on POD 2–3.
Statistical analysis
Data are presented as the mean ± standard deviation.
All data analyses were performed using SPSS 16.0 (SPSS, Inc.,
Chicago, IL, USA). In all patients, SpO2 levels and Hb were
measured and drawn into a tendency chart preoperatively,
intraoperatively and on POD 1–6. Variations in these parameters
were identified, and associations between the changes in SpO2
(ΔSpO2) and Hb (ΔHb) levels on POD 1 and 3 were analyzed using
Pearson's correlation test for each category of arthroplasty.
P<0.05 was considered to indicate a statistically significant
result.
Results
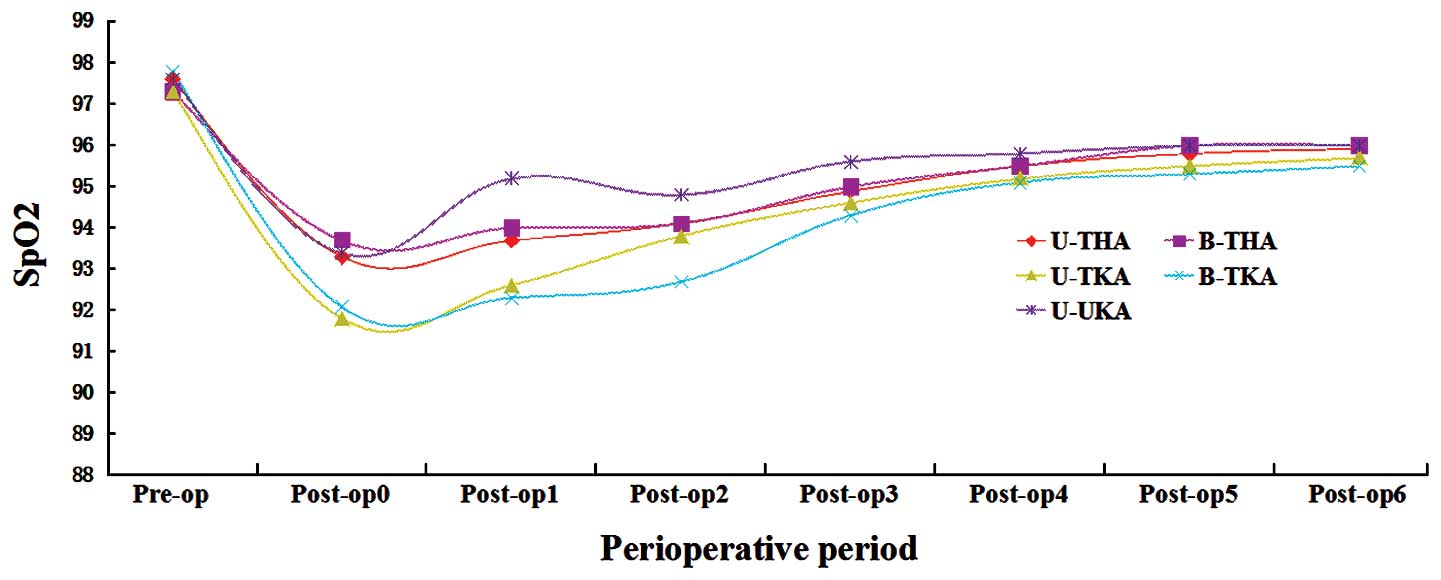
The perioperative SpO2 curve for each
surgical category demonstrated that SpO2 was typically
lowest on the day of surgery (POD 0). Marked hypoxemia was observed
on POD 0–2, although the recovery curve demonstrated a stable
increase in SpO2 on POD 3–5 (Fig. 1). The analysis of ΔHb revealed trends
among the 5 groups, with the lowest Hb value observed most
frequently on POD 2–3 following arthroplasty. Hb levels increased
consistently over POD 4–5 (Fig.
2).
ΔSpO2 was not associated with ΔHb
(P>0.05). The results of the Pearson's correlation tests between
ΔSpO2 and ΔHb on POD 1 and 3 are shown in Table II. For unilateral THA, the
ΔSpO2 on POD 1 and 3 were 3.9±1.7 and 2.7±1.7%,
respectively, and the ΔHb on POD 1 and 3 were 12.6±9.1 and 27.3±9.2
g/l, respectively. There was a negative correlation between
ΔSpO2 and ΔHb (on POD 1, r=0.236 and P=0.096; on
POD 3, r=−0.113 and P=0.430). Similar results were observed
for the bilateral THA (on POD 1, r=−0.192 and P=0.494; on
POD 3 and r=−0.168 and P=0.548). For unilateral TKA, the
ΔSpO2 on POD 1 and 3 were 4.7±1.8 and 2.7±1.7%,
respectively, and the ΔHb on POD 1 and 3 were 20.0±10.8 and
32.3±11.4 g/l, respectively. Correlation analyses showed that the
ΔSpO2 and ΔHb were not closely associated (on POD 1,
r=0.244 and P=0.119; on POD 3, r=−0.072 and P=0.652).
Similar results were observed for bilateral TKA (on POD 1,
r=0.238 and P=0.286; on POD 3, r=0.374 and P=0.087).
For U-UKA, the results of the correlation analyses demonstrated
that the ΔHb levels on POD 1 were not associated with the
ΔSpO2 levels (r=−0.164; P=0.792). However, the
opposite was observed for POD 3 (r=0.942; P=0.017), which
may have been due to the relatively small sample size of the U-UKA
group.
 | Table II.Results of Pearson's correlation
tests between ΔSpO2 and ΔHb on the postoperative first
and third days. |
Table II.
Results of Pearson's correlation
tests between ΔSpO2 and ΔHb on the postoperative first
and third days.
| Variable | U-THA | B-THA | U-TKA | B-TKA | U-UKA |
|---|
| Patient nos. | 51 | 15 | 42 | 22 | 5 |
|
Post-op1 |
|
|
|
|
|
|
ΔSpO2 | 3.9±1.7 | 3.3±1.5 | 4.7±1.8 | 5.5±2.3 | 2.4±1.5 |
|
ΔHb | 12.6±9.1 | 14.0±14.7 | 20.0±10.8 | 18.8±9.3 | 12.6±9.1 |
|
r-value | 0.236 | −0.192 | 0.244 | 0.238 | −0.164 |
|
P-value | 0.096 | 0.494 | 0.119 | 0.286 | 0.792 |
|
Post-op3 |
|
|
|
|
|
|
ΔSpO2 | 2.7±1.7 | 2.3±2.3 | 2.7±1.7 | 3.5±2.2 | 2.0±1.6 |
|
ΔHb | 27.3±9.2 | 20.5±11.4 | 32.3±11.4 | 38.5±8.4 | 27.3±9.2 |
|
r-value | −0.113 | −0.168 | −0.072 | 0.374 | 0.942 |
|
P-value | 0.430 | 0.548 | 0.652 | 0.087 | 0.017 |
Discussion
Due to the lack of consistency in the literature
with regard to the definition and monitoring of hypoxemia, marked
discrepancies in reported incidences of hypoxemia following
arthroplasty have been found. The incidence of hypoxia in clinical
settings is generally unknown. A previous study reported a 42%
incidence of hypoxemia on POD 1 following THA (7). Austin et al (3) demonstrated that hypoxemia occurred more
frequently during POD 2, and that its incidence was relatively low
(4%). Hong et al (13)
reported a case of hypoxemia in a healthy elderly male patient that
developed 2 h following cementation of the prosthesis during THA
under spinal anesthesia. However, increases in
ventilation-perfusion mismatch were demonstrated by Ereth et
al (14) to occur within 30 min
following insertion of the femoral prosthesis. The lowest
SpO2 value was shown to often occur on the day of
surgery, with significant hypoxemia on POD 0–2 followed by a stable
increase in the first 3–5 days (14). However, Mimura et al (15) reported that even on POD 5, oxygen
tension was decreased by >10% in 5 of the 10 post-TKA patients.
It was also noted that none of these patients developed clinical
dyspnea, and only one patient was diagnosed with pulmonary embolism
using a ventilation/perfusion scintigraphy (15).
Postoperative hypoxemia results most frequently from
one of two possible physiological mechanisms: Hypoventilation or
abnormal gas exchange (16). These
mechanisms include pulmonary embolism, pulmonary edema, and
pneumonia. Hypoxemia also varies according to the physical status
and cardiovascular reserve of the patient (7,16).
Postoperative hypoxemia can be secondary to numerous conditions
(1,3,7),
including the effects of narcotics, postoperative atelectasis,
fluid overload, and hypoventilation. Blood loss following revision
surgery requires abundant blood transfusion and high fluid
resuscitation (9). Intravenous fluid
overload in a weak, malfunctioning heart predisposes a patient to
pulmonary edema, which may result in respiratory failure and
hypoxemia (3). In the present study,
a significant difference was identified in ΔSpO2 between
hip and knee arthroplasties. The results of previous studies
suggested that a thrombogenic process is created from venous stasis
and intimal wall damage caused by tourniquet use (17,18).
Hypoxemia may result from venous embolization that occurs in
conjunction with intramedullary hypertension in the femur during
insertion of the prosthesis in patients undergoing arthroplasty
(14). A second explanation is based
on the results of transesophageal echocardiographic studies that
demonstrated that fat embolic loads are greater following cemented
TKA than following uncemented THA (19). Within knee arthroplasty, the
incidence of filling defects was greater when both the femoral and
tibial canals were cannulated than when only one or no bone was
cannulated (17,20). These results are concordant with
those of a previous study which examined lower cardiac embolic load
with computer-assisted surgery (20). Perioperative monitoring for embolism
may be helpful in assessing patients in whom cardiorespiratory
function deteriorates. Due to the lack of a validated screening
tool (17,21), the poor sensitivity of the clinical
signs and symptoms of thromboembolic complications, and the
possibility of a fatal outcome, clinical decision-making on the
course of action for the hypoxemic patient is difficult (3). The value of pulse oximetry and blood
gases in evaluating pulmonary embolism remains unclear. Episodic
hypoxemic events have been described in sleeping postoperative
patients who had received analgesic therapy such as opioids
(22). In addition, patients with
obstructive sleep apnea had a higher and more transient hypoxia;
however, the incidence of complications experienced by these
patients was not increased following appropriate oxygen
supplementation (4,23).
The results of the present study indicated that
ΔSpO2 was inconsistent with a decrease in Hb levels.
There appeared to be little correlation between the two random
variables. Arthroplasty is usually associated with significant
blood loss (9,10). Documented complications of
perioperative anemia include tachycardia, hypotension, and
increased risk of perioperative myocardial infarction (24). The oxygen-carrying capacity of blood
is likely best determined by the mass of circulating red blood
cells (3,16,25).
Anemia is a condition characterized by a decrease in the
oxygen-carrying capacity of blood. Under most circumstances, Hb
concentration is a good indicator of red cell mass, but changes in
plasma volume may lead to discrepancies in the results. In patients
undergoing surgical procedures and critically ill patients,
fluctuations in the plasma volume often occur due to the use fluid
resuscitation to treat patients with fluid resuscitation for
hypovolemia and increased capillary leakage (25). Some investigators have hypothesized
that anemia-induced tissue hypoxia elicits the same responses as
exposure to low fractions of inspired oxygen or systemic hypoxia
(26,27). There are important differences
between anemia and hypoxia in terms of blood chemistry (28). During anemia, a reduction in blood
oxygen content occurs as a result of reduced Hb levels while
arterial oxygenation and oxyhemoglobin saturation remain high.
During hypoxia, arterial oxygenation is reduced, which results in
oxyhemoglobin desaturation at normal Hb levels (26). At reduced Hb levels, optimal oxygen
loading of Hb in the lung is observed with anemia (29). The mechanisms underlying these
effects are thought to include a nitric oxide-mediated increase in
minute ventilation and ventilation/perfusion matching, which
optimizes Hb saturation to ~100% (29). SpO2 serves as a
cardiopulmonary oxygen transfer measurement, not a measure of
tissue perfusion. A severely anemic person may have an
SpO2of 100% but a very low oxygen-carrying capacity.
This is the cause of anemia-associated problems with wound healing,
organ failure and other complications (9,24).
Severe anemia is associated with marked stability of pulmonary gas
exchange (30). Anemia results in
improved gas exchange in the normal lung due to an improvement in
overall ventilation/perfusion matching. In turn, this may be a
result of favorable changes in pulmonary blood flow distribution,
as assessed by the fractal dimension and spatial correlation of
blood flow and as a result of increased nitric oxide availability
(26,27). The increase in cardiac output results
from a composite increase in both heart rate and stroke volume,
which acts to increase cardiac output and thereby maintain overall
systemic oxygen delivery and consumption with low Hb levels
(31,32).
There were limitations to the present study. The
majority of the patients in the study underwent general anesthesia,
which may account for the perioperative decrease in SpO2
levels. Medical comorbidities were not considered in the analysis.
The sample sizes were relatively small, since there were numerous
type of surgical procedures and deep venous thrombosis prophylaxis
and anesthesia that were used. Further studies with larger cohorts
are required in order to draw conclusions regarding blood loss.
In conclusion, SpO2 levels should not
serve as a clinical indicator of the incidence and severity of
anemia in patients who have undergone primary arthroplasty. To a
point, postoperative anemia does not affect SpO2 levels.
Hypoxemia alone was not found to be specific for a particular
etiology, and further diagnostic studies are required.
Acknowledgements
The authors of the present study would like to thank
the Department of Epidemiology and Bio-statistics at the School of
Public Health, Peking University (Beijing, China) for assistance
with statistical analyses, and to Miss Fan Meng at the China-Japan
Friendship Hospital for help with data collection. The present
study was supported by grants from the National Natural Science
Foundation of China (grant no. 81372013) and the Research Fund of
the China-Japan Friendship Hospital (grant nos. 2013-MS-27 and
2014-4-QN-29).
References
|
1
|
Galway UA and Gugliotti D: Sudden hypoxia
during knee surgery. Cleve Clin J Med. 79:401–409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clarke MT, Longstaff L, Edwards D and
Rushton N: Tourniquet-induced wound hypoxia after total knee
replacement. J Bone Joint Surg Br. 83:40–44. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Austin L, Pulido L, Ropiak R, Porat M,
Parvizi J and Rothman RH: Hypoxemia after total joint arthroplasty:
A problem on the rise. J Arthroplasty. 23:1016–1021. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lewer BM, Larsen PD, Torrance JM and
Galletly DC: Artefactual episodic hypoxaemia during postoperative
respiratory monitoring. Can J Anaesth. 45:182–185. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gill NP, Wright B and Reilly CS:
Relationship between hypoxaemic and cardiac ischaemic events in the
perioperative period. Br J Anaesth. 68:471–473. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin GM, Chen YJ, Li YH, Jaiteh LE and Han
CL: The effect of hypoxia-hypercapnia on neuropsychological
function in adult respiratory distress syndrome. Am J Respir Crit
Care Med. 186:1307–1308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clayer M and Bruckner J: Occult hypoxia
after femoral neck fracture and elective hip surgery. Clin Orthop
Relat Res. 265–271. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Freeman TA, Parvizi J, Dela Valle CJ and
Steinbeck MJ: Mast cells and hypoxia drive tissue metaplasia and
heterotopic ossification in idiopathic arthrofibrosis after total
knee arthroplasty. Fibrogenesis Tissue Repair. 3:172010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sehat KR, Evans RL and Newman JH: Hidden
blood loss following hip and knee arthroplasty. Correct management
of blood loss should take hidden loss into account. J Bone Joint
Surg Br. 86:561–565. 2004.PubMed/NCBI
|
|
10
|
Parvizi J, Pour AE, Peak EL, Sharkey PF,
Hozack WJ and Rothman RH: One-stage bilateral total hip
arthroplasty compared with unilateral total hip arthroplasty: A
prospective study. J Arthroplasty. 21(6 Suppl 2): S26–S31. 2006.
View Article : Google Scholar
|
|
11
|
Shephard DA: The 1975 Declaration of
Helsinki and consent. Can Med Assoc J. 115:1191–1192.
1976.PubMed/NCBI
|
|
12
|
Gao F, Guo W, Sun W, Li Z, Wang W, Wang B
and Cheng L: Mechanisms and influencing factors of hypoxemia after
joint arthroplasty. Zhonghua Yi Xue Za Zhi. 94:655–659. 2014.(In
Chinese). PubMed/NCBI
|
|
13
|
Hong CL, Liu HP, Wu CY, Ho AC, Shyr MH,
Wong CH and Chun HS: Delayed hypoxemia after bone cement insertion
during total hip replacement under spinal anesthesia-a case report.
Acta Anaesthesiol Sin. 41:47–51. 2003.PubMed/NCBI
|
|
14
|
Ereth MH, Weber JG, Abel MD, Lennon RL,
Lewallen DG, Ilstrup DM and Rehder K: Cemented versus noncemented
total hip arthroplasty-embolism, hemodynamics, and intrapulmonary
shunting. Mayo Clin Proc. 67:1066–1074. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mimura M, Yamazaki Y, Yamamoto H and
Namiki A: Postoperative hypoxia and hyperfibrinolysis in patients
after total knee replacement. Masui. 47:190–194. 1998.(In
Japanese). PubMed/NCBI
|
|
16
|
Jones JG, Sapsford DJ and Wheatley RG:
Postoperative hypoxaemia: Mechanisms and time course. Anaesthesia.
45:566–573. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gandhi R, Salonen D, Geerts WH, Khanna M,
McSweeney S and Mahomed NN: A pilot study of computed
tomography-detected asymptomatic pulmonary filling defects after
hip and knee arthroplasties. J Arthroplasty. 27:730–735. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kato N, Nakanishi K, Yoshino S and Ogawa
R: Abnormal echogenic findings detected by transesophageal
echocardiography and cardiorespiratory impairment during total knee
arthroplasty with tourniquet. Anesthesiology. 97:1123–1128. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hagio K, Sugano N, Takashina M, Nishii T,
Yoshikawa H and Ochi T: Embolic events during total hip
arthroplasty: An echocardiographic study. J Arthroplasty.
18:186–192. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Church JS, Scadden JE, Gupta RR, Cokis C,
Williams KA and Janes GC: Embolic phenomena during
computer-assisted and conventional total knee replacement. J Bone
Joint Surg Br. 89:481–485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lawton RL, Morrey BF and Narr BJ: Validity
of index of suspicion for pulmonary embolism after hip
arthroplasty. Clin Orthop Relat Res. 180–192. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Catley DM, Thornton C, Jordan C, Lehane
JR, Royston D and Jones JG: Pronounced, episodic oxygen
desaturation in the postoperative period: Its association with
ventilatory pattern and analgesic regimen. Anesthesiology.
63:20–28. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Berend KR, Ajluni AF, Núñez-García LA,
Lombardi AV and Adams JB: Prevalence and management of obstructive
sleep apnea in patients undergoing total joint arthroplasty. J
Arthroplasty. 25(Suppl 6): S54–S57. 2010. View Article : Google Scholar
|
|
24
|
Liu X, Zhang X, Chen Y, Wang Q, Jiang Y
and Zeng B: Hidden blood loss after total hip arthroplasty. J
Arthroplasty. 26:1100–1105.e1. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McLellan SA, McClelland DB and Walsh TS:
Anaemia and red blood cell transfusion in the critically ill
patient. Blood Rev. 17:195–208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hare GM, Tsui AK, Ozawa S and Shander A:
Anaemia: Can we define haemoglobin thresholds for impaired oxygen
homeostasis and suggest new strategies for treatment? Best Pract
Res Clin Anaesthesiol. 27:85–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hare GM, Freedman J and Mazer David C:
Review article: Risks of anemia and related management strategies:
Can perioperative blood management improve patient safety? Can J
Anaesth. 60:168–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsui AK, Marsden PA, Mazer CD, Adamson SL,
Henkelman RM, Ho JJ, Wilson DF, Heximer SP, Connelly KA, Bolz SS,
et al: Priming of hypoxia-inducible factor by neuronal nitric oxide
synthase is essential for adaptive responses to severe anemia. Proc
Natl Acad Sci USA. 108:17544–17549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deem S, Hedges RG, McKinney S, Polissar
NL, Alberts MK and Swenson ER: Mechanisms of improvement in
pulmonary gas exchange during isovolemic hemodilution. J Appl
Physiol (1985). 87:132–141. 1999.PubMed/NCBI
|
|
30
|
Deem S, Alberts MK, Bishop MJ, Bidani A
and Swenson ER: CO2 transport in normovolemic anemia: Complete
compensation and stability of blood CO2 tensions. J Appl Physiol
(1985). 83:240–246. 1997.PubMed/NCBI
|
|
31
|
Weiskopf RB, Viele MK, Feiner J, Kelley S,
Lieberman J, Noorani M, Leung JM, Fisher DM, Murray WR, Toy P and
Moore MA: Human cardiovascular and metabolic response to acute,
severe isovolemic anemia. JAMA. 279:217–221. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ragoonanan TE, Beattie WS, Mazer CD, Tsui
AK, Leong-Poi H, Wilson DF, Tait G, Yu J, Liu E, Noronha M, et al:
Metoprolol reduces cerebral tissue oxygen tension after acute
hemodilution in rats. Anesthesiology. 111:988–1000. 2009.
View Article : Google Scholar : PubMed/NCBI
|