Introduction
Bone marrow mesenchymal stem cells (BMSCs) are
fibrocyte-like stem cells that exist alongside hematopoietic stem
cells within the marrow cavity. BMSCs are highly self-renewable
with multipotential differentiation (1); they may develop into osteoblasts,
chondrocytes and adipose cells through directional differentiation,
and at present it is understood that all osteoblasts are derived
from BMSCs (2).
Peroxisome proliferator-activated receptor γ (PPARγ)
is a PPAR subtype that contributes towards the regulation of cell
differentiation, proliferation and apoptosis (3,4). To
date, it is understood that the PPARγ subtype is the primary
regulator of fat differentiation, which serves a key regulatory
role in the direction of BMSC differentiation (5). In the marrow cavity, osteoblasts and
adipocytes are derived from BMSCs, and it is understood that there
is an association between their expression levels (6). Previous studies demonstrate that
PPARγ-mediated adipogenic differentiation of BMSCs directly affects
the differentiation of osteoblasts (7,8).
Osteoclasts are derived from hematopoietic stem cells in bone
marrow, and are responsible for increased bone resorption and
osteoporosis; this is demonstrated through an increase of fat in
bone marrow cavities that occurs in every type of osteoporosis
(9,10).
Soy isoflavone is a class 1 secondary metabolite
that is formed during the growth of soybeans. In total, 12 types of
natural isoflavones exist in soybeans, including daidzin, daidzein,
genistin, genistein, glycitin and glycitein (11). Of these, genistein possesses the
highest level of activity (12).
Genistein possesses a number of bioactivities (13); it is an effective antioxidant, a
protein tyrosine activating enzyme inhibitor and a phytoestrogen
(14). In recent years, increasing
evidence indicates that genistein may aid in the prevention and
treatment of breast cancer, prostatic cancer, post-menopause
syndrome, osteoporosis and angiocardiopathy (15,16). In
the present study, the mechanisms underlying the effect of
genistein on the suppression of human BMSC adipogenic
differentiation and the enhancement of BMSC osteogenic potential
were investigated.
Materials and methods
Reagents
Dulbeccos modified Eagles medium (DMEM) and fetal
bovine serum (FBS) were provided by Gibco (Thermo Fisher
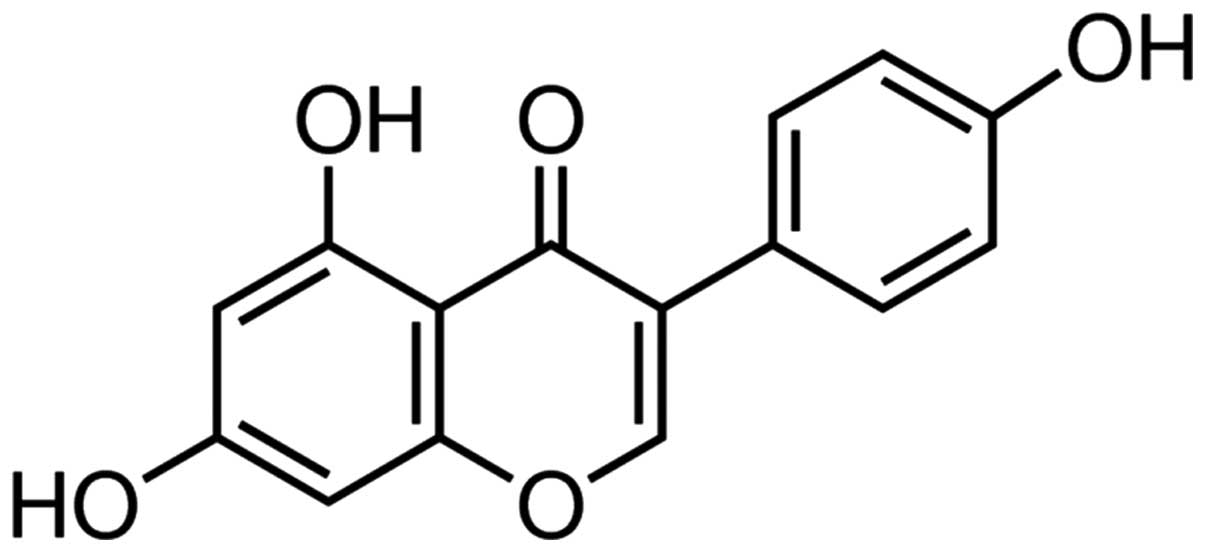
Scientific, Inc., Waltham, MA, USA), and genistein (Fig. 1; ≥98%, high-performance liquid
chromatography) and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were provided by Sigma-Aldrich (St. Louis, MO, USA).
BMSC culture and identification
The use of animals in the present study was approved
by the Animal Care and Use Committee of the Chinese People's
Liberation Army General Hospital (Beijing, China). A total of 20
male and female Sprague-Dawley rats (age, 2–4 weeks; 100±10 g; male
= 24, female = 24; purchased from Charles River Laboratories,
Wilmington, MA, USA) were used to isolate marrow-derived BMSCs and
were housed in an animal quarter (humidity, 60–70%; temperature,
23±1°C; 12-h light-dark cycle) with ad libitum access to
food and water. Rats were sacrificed by cervical dislocation and
BMSCs were isolated according to a previously described method
(17). Briefly, bone marrow was
flushed from the femur and tibia with saline, and placed into 25
cm2 flasks with DMEM supplemented with 10% FBS, 100 units
penicillin and 100 µg-ml streptomycin (both purchased from
Sigma-Aldrich), and was incubated at 37°C in a humidified
atmosphere containing 5% CO2 for 1 day. Following the
incubation period, nonadherent cells were removed and adherent
cells were washed with phosphate-buffered saline (PBS; Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China). Next, adherent cells
were incubated with DMEM for 2 h at 37°C and washed with PBS once
they reached 80–90% confluence. The cells were detached using 0.25%
trypsin (Nanjing Sunshine Biotechnology, Co., Ltd., Nanjing, China)
and the remaining cells were incubated in new 25 cm2 flasks.
BMSCs were treated with genistein (0, 5, 10 and 20
µm) for 1, 2 or 3 days or GW9662 (1 mM; Invitrogen; Thermo Fisher
Scientific, Inc.), a PPARγ inhibitor. BMSCs were then fixed using
5% pre-cooled paraformaldehyde (Sinopharm Chemical Reagent Co.,
Ltd.) for 10–15 min at 4°C, and cultured with hematoxylin and eosin
staining (Invitrogen; Thermo Fisher Scientific, Inc.) for 10 min.
Next, BMSCs were washed using tap and distilled water for 5–10 min.
Stained BMSCs were dehydrated with 95% ethanol for 1–2 min and
xylene (Shangbeijia Biological Technology Co., Ltd.) was applied
for 5–10 min until transparent. BMSCs were analyzed using a
microscope (TE2000; Nikon Corporation, Tokyo, Japan).
Grouping and cell proliferation
assay
BMSCs were seeded in 96-well plates and incubated
with different concentrations of genistein (0, 5, 10 and 20 µm) for
1, 2 and 3 days or GW9662 (1 mM). In the MTT cell proliferation
assay, BMSCs were incubated with 20 µl MTT for 4 h at 37°C in a
humidified atmosphere containing 5% CO2. Once the medium
was removed, 150 µl dimethyl sulfoxide was added for 10 min at room
temperature. The optical density was read at 570 nm (Epoch
Microplate Spectrophotometer; BioTek Instruments, Inc., Winooski,
VT, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) of runt-related transcription
factor 2 (Runx2), collagen type I (Col I) and osteocalcin (OC)
Following treatment with genistein, total RNA (1 µg)
was extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and BMSC cDNA (1 µg) was transcribed using RT-PCR
Quick Master Mix (Toyoba Co., Ltd., Dalian, China) according to the
manufacturers protocol. qPCR was performed using LightCycler480
SYBR Green I Master (Roche Diagnostics, Indianapolis, IN, USA) at
94°C for 45 sec, followed by 40 cycles of 95°C for 30 sec, 60°C for
45 sec and 72°C for 30 sec, and then 4°C for 10 min. The primer
sequences are listed in Table I.
Samples were quantified using the 2−ΔΔCq method
(18).
 | Table I.Design of primer sequences. |
Table I.
Design of primer sequences.
| Gene | Forward primer
sequence | Reverse primer
sequence |
|---|
| Runx2 |
5-CAGTTCCTAACGGGCACCAT-3 |
5-TTAGGGTCTCGGAGGGAAGG-3 |
| Col I |
5-TGACCTCAAGATGTGCCACT-3 |
5-GGGAGTTTCCATGAAGCCAC-3 |
| OC |
5-CATGAGAGCCCTCACA-3 |
5-AGAGCGACACCCTAGAC-3 |
| β-actin |
5-GCTCTCCAGAACATCACTCCTGCC-3 |
5-CGTTGTCATACCAGGAAATGAGCTT-3 |
Enzyme-linked immunosorbent assay of
alkaline phosphatase (ALP) and triglyceride (TG)
Following the treatment of BMSCs with genistein or
GW9662, the activity of ALP and TG in cells was detected using an
ALP and TG determination kit (Beyotime Institute of Biotechnology,
Haimen, China) according to the manufacturers protocol. The optical
density was read using a microplate reader (LabSystems Miltiskan MS
Plate Reader; Thermo Fisher Scientific, Inc.) at 405 nm.
Western blotting for PPARγ
Following the application of genistein or GW9662 to
BMSCs, equal quantities of protein were analyzed using a BCA
protein assay kit (Beyotime Institute of Biotechnology), according
to the manufacturers instructions. Protein was separated using 10%
sodium dodecyl sulfate polyacrylamide (Sinopharm Chemical Reagent
Co., Ltd.) gel electrophoresis (110 V; 45 min) and transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membrane was blocked in 5% nonfat milk-PBS-Tween 20
solution (Shanghai Macklin Biochemical Co., Ltd.) for 1 h at room
temperature, followed by separate incubation with polyclonal
antibodies specific for PPAR (dilution, 1:1,000; goat anti-mouse;
sc-1985; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
β-actin (dilution, 1:2,000; goat anti-mouse; sc-1616; Santa Cruz)
at 4°C overnight. The membranes were incubated for 1 h at room
temperature with horseradish peroxidase-conjugated secondary
antibody (goat anti-mouse IgG; dilution, 1:5,000; sc-45101; Santa
Cruz) in 5% nonfat milk-PBS-Tween 20. The membranes were visualized
using enhanced chemiluminescence (Thermo Fisher Scientific, Inc.),
and analyzed using a Gel-Doc 2000 imaging scanner (Bio-Rad
Laboratories, Inc.).
Statistical analysis
All data are expressed as the mean ± standard error.
Statistical analysis of data was performed using one-way analysis
of variance and the statistical software package SPSS version 17.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Authenticating of BMSCs
The chemical structure of genistein is presented in
Fig. 1. Fig. 2 demonstrates that the morphology of
cultured BMSCs are spindle-shaped with serial subcultivation,
homogeneity and multiplicity. The cell nucleus of stained BMSCs
appeared dark blue (Fig. 2).
Effect of genistein on cell
proliferation in BMSCs
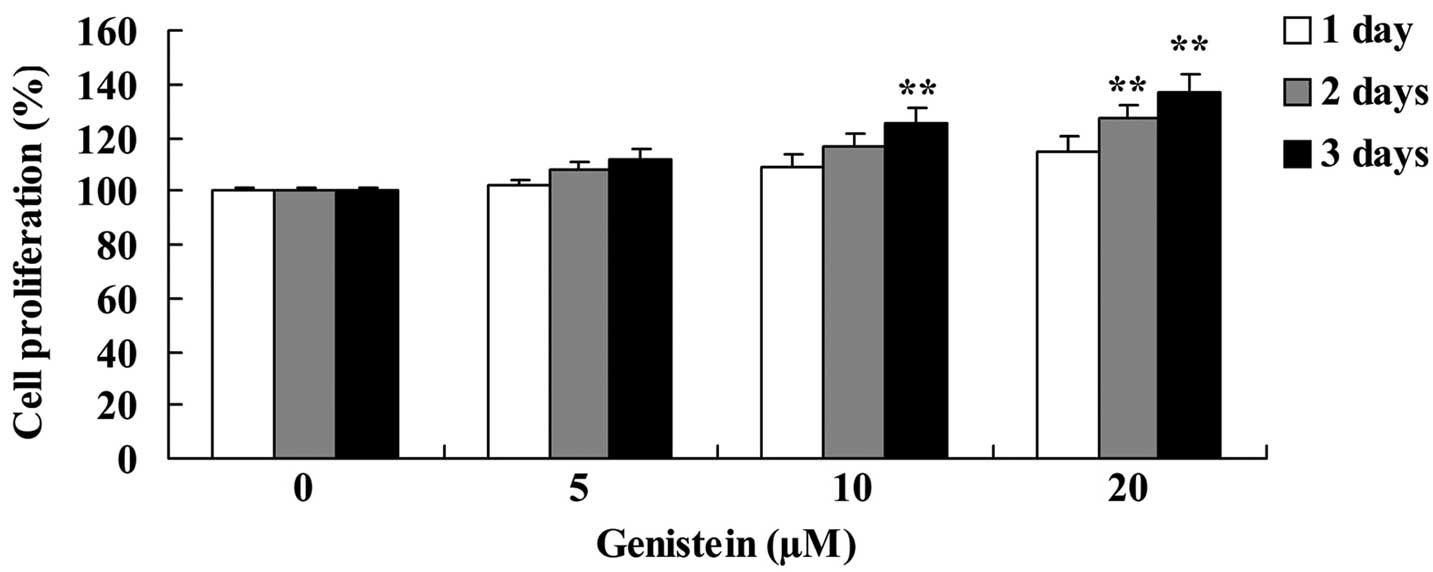
Analysis indicated that genistein increased BMSC
cell proliferation in a time- and dose-dependent manner (Fig. 3). When cells were treated with 20 µm
genistein for 2 and 3 days, and 10 µm genistein for 3 days, BMSC
cell proliferation was significantly increased compared with
untreated BMSC cells (P<0.01; Fig.
3).
Effect of genistein on Runx2, Col I
and OC mRNA expression in BMSCs
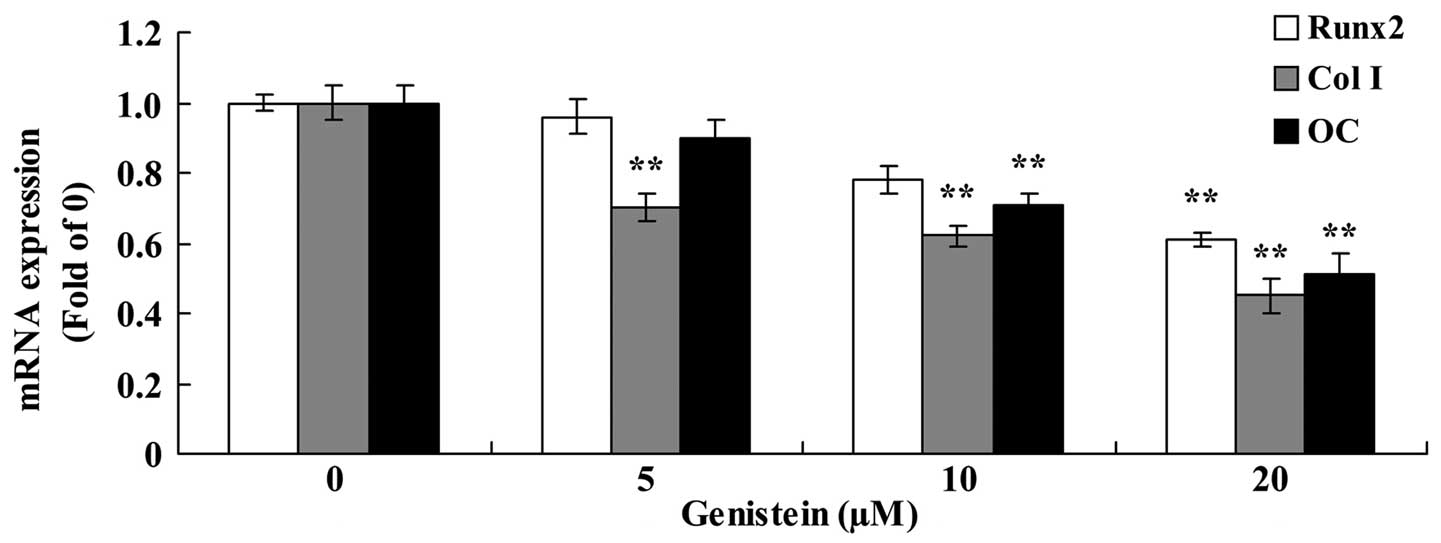
As presented in Fig.
4, the expression of Runx2, Col I and OC mRNA were
significantly reduced following treatment with genistein. In BMSCs,
the expression of Runx2 mRNA was significantly inhibited following
treatment with 20 µm genistein for 2 days, the expression of Col I
mRNA was significantly reduced following treatment with 5, 10 and
20 µm genistein for 2 days, and the expression of OC mRNA was
significantly reduced following treatment with 10 and 20 µm
genistein for 2 days (P<0.01; Fig.
4).
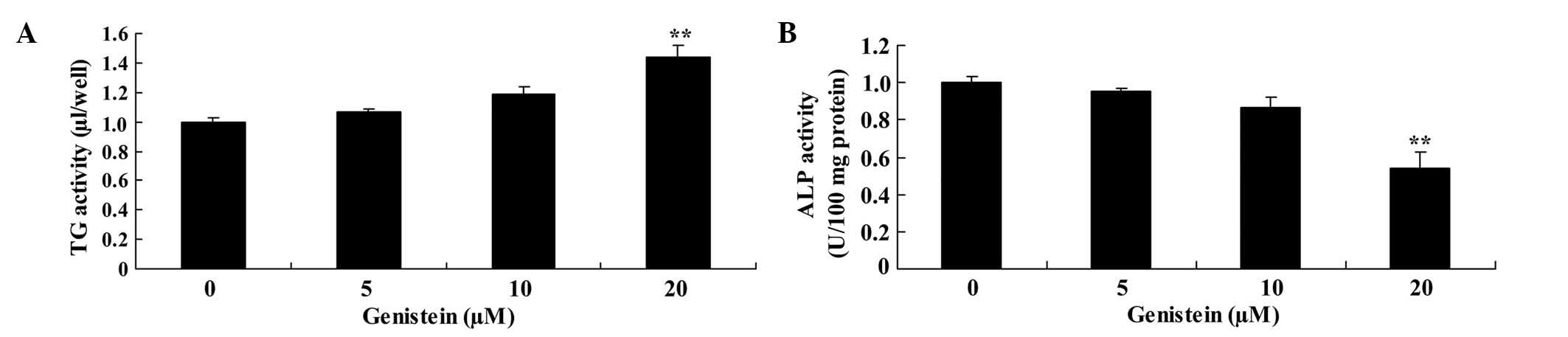
Effect of genistein on ALP and TG
activity in BMSCs
Compared with BMSCs in the absence of genistein, the
activity of ALP was significantly inhibited and the activity of TG
was significantly enhanced following treatment with 20 µm genistein
(P<0.01; Fig. 5).
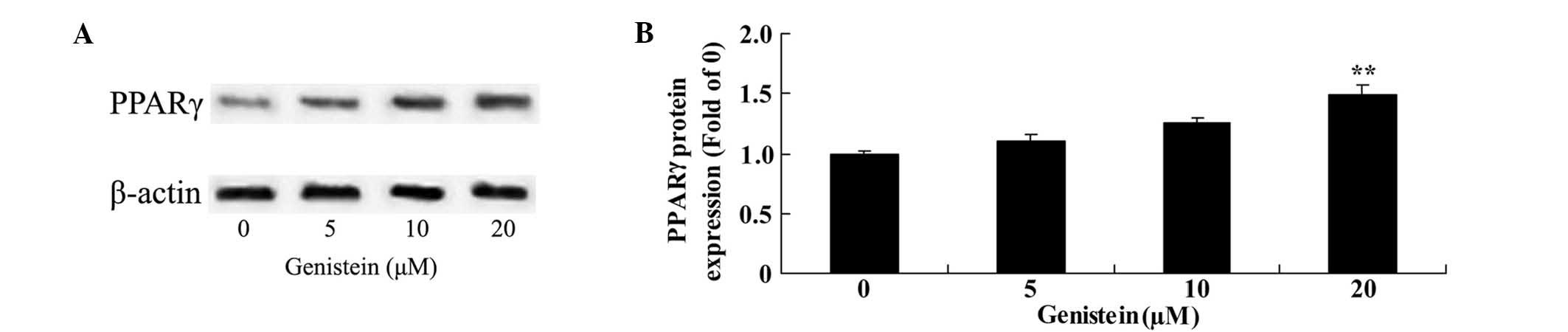
Effect of genistein on PPARγ protein
expression in BMSCs
To observe the mechanism of genistein on BMSCs, the
protein expression of PPARγ was analyzed using western blotting.
The results demonstrated that the protein expression of PPARγ was
significantly increased following BMSC pretreatment with 20 µm
genistein for 2 days (P<0.01; Fig.
6).
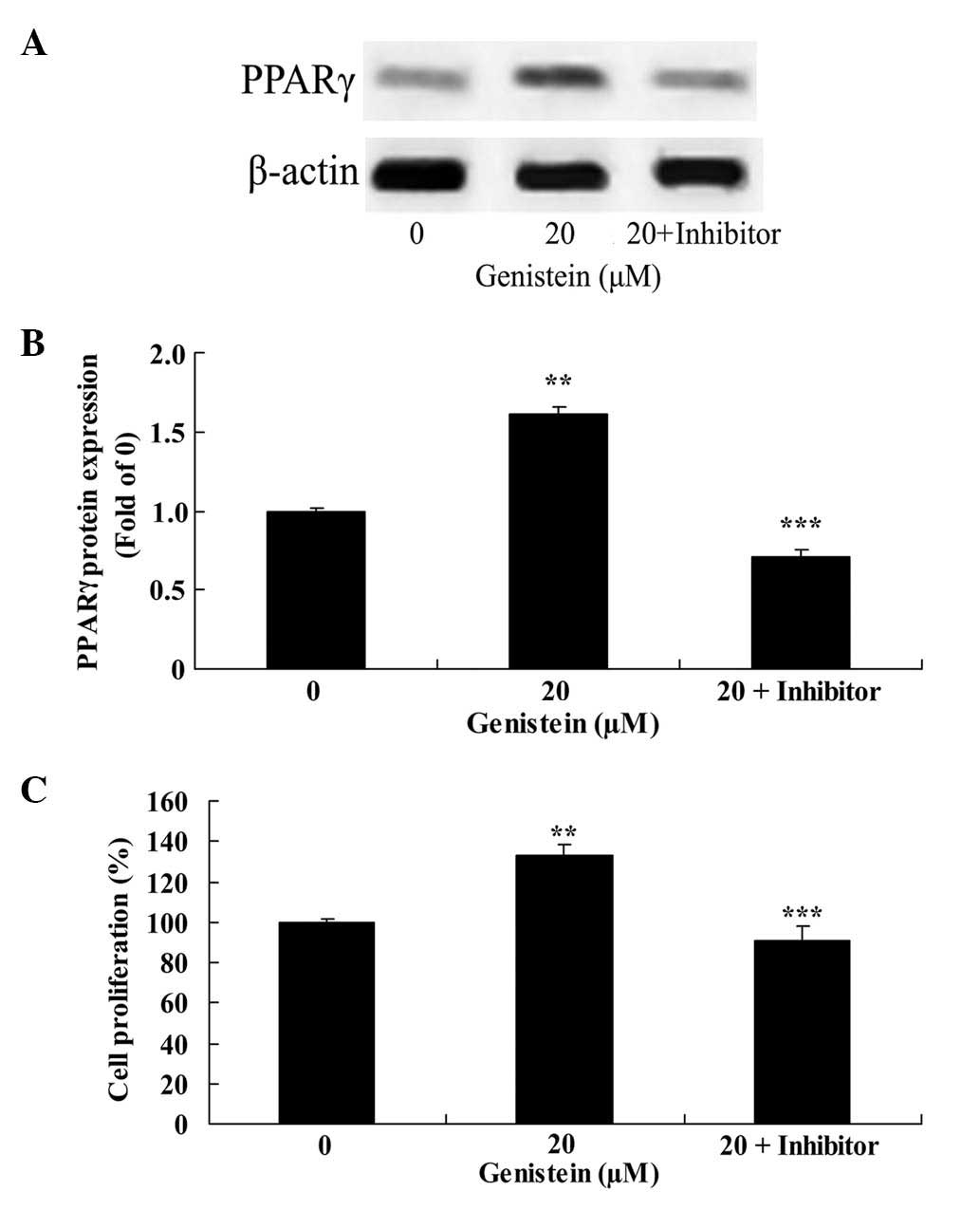
Effect of PPARγ downregulation on
genistein-induced BMSC cell proliferation
GW9662 significantly inhibited PPARγ protein
expression in BMSCs treated with 20 µm genistein for 2 days,
compared with BMSCs treated only with 20 µm genistein (P<0.01;
Fig. 7A and B). In addition, GW9662
significantly reduced the effect of 20 µm genistein on BMSC cell
proliferation compared with cells treated only with 20 µm genistein
(P<0.01; Fig. 7C).
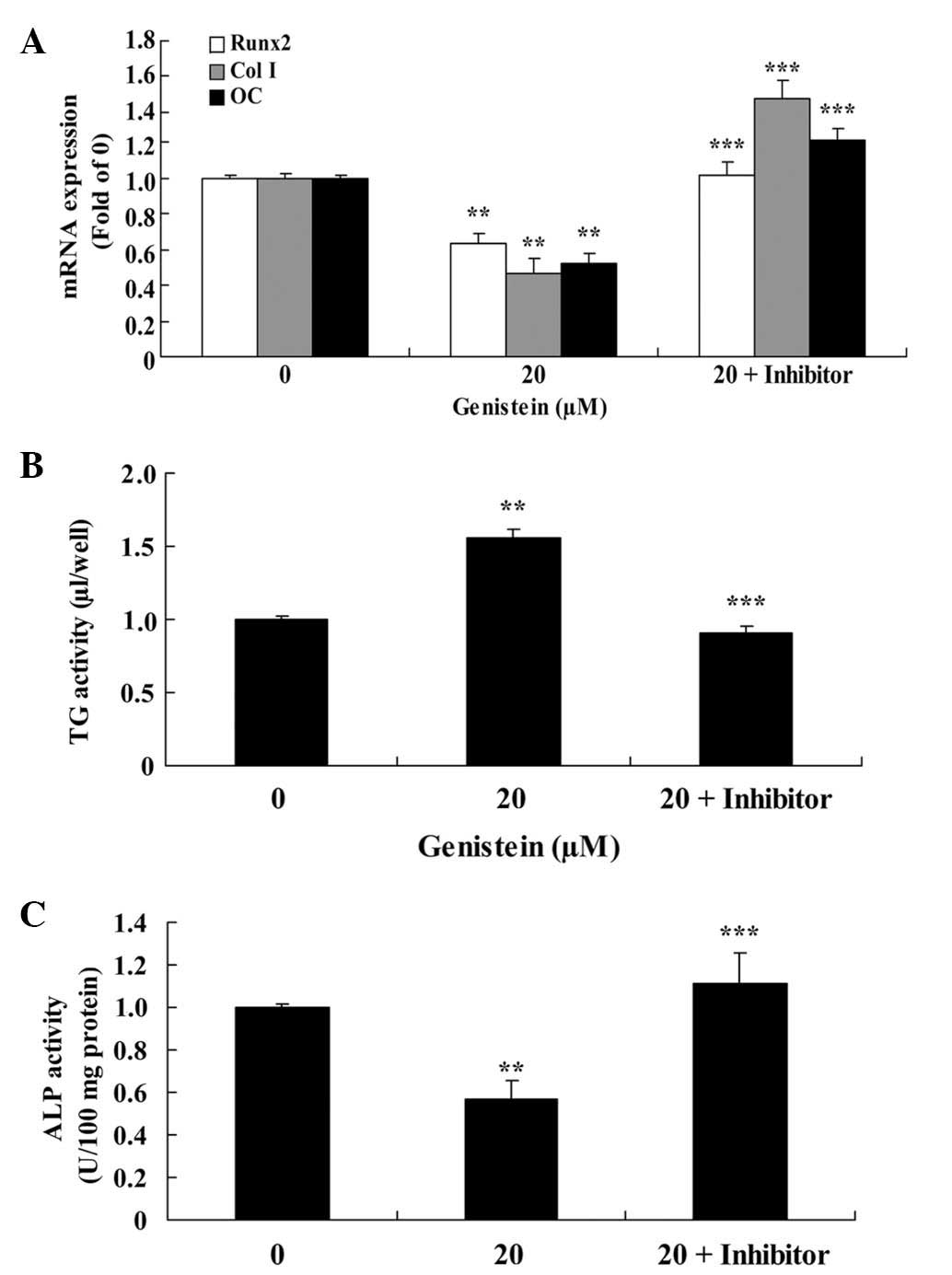
Effect of PPARγ downregulation on
Runx2, Col I and OC mRNA expression and the activity of ALP and TG
in BMSCs
As presented in Fig.
8A, the effect of 20 µm genistein on the expression of Runx2,
Col I and OC mRNAs was significantly reduced following pretreatment
with a PPARγ inhibitor for 2 days (P<0.01), compared with cells
treated only with 20 µm genistein for 2 days. In addition, the
genistein-induced increase in TG activity and reduction in ALP
activity were significantly inhibited by a PPARγ inhibitor
(P<0.01; Fig. 8B and C).
Discussion
A number of local factors and hormones are required
to transmit signals to transcription factors when BMSCs
differentiate in osteogenesis, and these control the expression of
specific genes during each stage of BMSC differentiation, thus
controlling the osteoblast at each stage (1,2). Once
mesenchymal stem cells become osteoblasts, the osteogenic cells
proliferate as a result of mitotic growth factors, which ensure
that a sufficient quantity of osteoblasts are generated for
osteogenesis (19). Meanwhile,
osteogenic cells undergo differentiation into mature osteoblasts
during osteogenesis (20).
OC, ALP, Runx2 and Col I are derived during the
synthesis of osteoblasts. At different stages of osteogenesis, only
part of the aforementioned proteins and factors may be generated
(21). With the generation of OC,
Runx2 and Col I, osteoblasts will enter into different stages of
differentiation (22). Thus, the
synthesis and secretion of such proteins are significant markers of
osteoblast differentiation, and affect the biological function and
performance of osteoblasts (23).
In the present study, genistein significantly
accelerated BMSC cell proliferation, reduced Runx2, Col I and OC
mRNA expression, and inhibited the activity of ALP and increased
the activity of TG, which suggests that genistein has the potential
to be used as a BMSC inductive agent. In particular, Relic et
al (24) reported that genistein
induces adipogenesis through activating PPARγ pathway.
BMSCs possess a multipotent differentiation
potential that allows them to differentiate into a variety of cell
osteoblasts, including chondrocytes, adipocytes and myoblasts, by a
number of induction pathways, and this directional differentiation
can alter when the differentiation induction system changes
(25). When osteogenesis- and
chondrogenesis-inducing factors are present in BMSC culture
systems, the expression of PPARγ is significantly increased and an
increased number of adipocytes are generated (3). Osteoblasts are able to generate a
variety of cytokines that regulate the differentiation and
apoptosis of osteoclasts in various stages of differentiation
proliferation, maturation and mineralization (26). Therefore, the effect of the PPARγ
gene and its ligand on osteoblasts may alter the levels of
cytokines in the bone marrow microenvironment, resulting in a
direct or indirect influence on the differentiation and function of
osteoclasts (3,8).
Results from the present study demonstrated that
BMSC pretreatment with 20 µm genistein significantly activates
PPARγ protein expression. Similarly, a previous report observed
that genistein inhibits human osteosarcoma MG-63 cells by
activating the PPARγ signaling pathway (27). In addition, Chatterjee et al
(28) demonstrated that genistein
prevents Alzheimers disease-associated inflammation through
increasing PPARγ expression in cultured astrocytes (29).
PPAR was identified as a substance that may be
activated by a peroxysome proliferation stimulator of a fatty
acid-like compound (30). It was
observed in vitro and in vivo that PPAR served an
important role in the regulation of BMSC differentiation (31). A number of studies report that the
PPARγ genes possesses an influence on the pathogenesis of
osteoporosis induced by microgravity; under simulated microgravity
conditions, the expression of PPARγ is increased, thus enhancing
the activity of PPARγ, as well as its target gene adipsin and
recombinant human leptin (32,33). The
present study observed that downregulation of PPARγ reduced the
effect of genistein-induced BMSC cell growth, inhibited
genistein-induced adipogenic differentiation and suppressed the
osteogenic potential of BMSCs.
In conclusion, the results from the current study
demonstrate that genistein promotes cell growth, induces adipogenic
differentiation and suppresses the osteogenic potential of BMSCs by
upregulating PPARγ expression. To conclude, genistein may be a
potential therapeutic agent for the treatment of orthopedic
diseases.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China subsidization project (no.
81301564), the Army Medical Science Youth Training Project (no.
13QNP184), the ‘Twelfth Five-Year Plan’ Science and Technology
Research Project of Jilin Province Department of Education (no.
141) and the Jilin Province Science and Technology Department
Project (no. 20130624003JC).
References
|
1
|
Katsuda T, Tsuchiya R, Kosaka N, Yoshioka
Y, Takagaki K, Oki K, Takeshita F, Sakai Y, Kuroda M and Ochiya T:
Human adipose tissue-derived mesenchymal stem cells secrete
functional neprilysin-bound exosomes. Sci Rep. 3:11972013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dumont N, Boyer L, Émond H, Celebi-Saltik
B, Pasha R, Bazin R, Mantovani D, Roy DC and Pineault N: Medium
conditioned with mesenchymal stromal cell-derived osteoblasts
improves the expansion and engraftment properties of cord blood
progenitors. Exp Hematol. 42:741–752.e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawai M, Green CB, Lecka-Czernik B, Douris
N, Gilbert MR, Kojima S, Ackert-Bicknell C, Garg N, Horowitz MC,
Adamo ML, et al: A circadian-regulated gene, Nocturnin, promotes
adipogenesis by stimulating PPAR-gamma nuclear translocation. Proc
Natl Acad Sci USA. 107:10508–10513. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lv FH, Gao JZ, Teng QL and Zhang JY:
Effect of folic acid and vitamin B12 on the expression of PPARγ,
caspase-3 and caspase-8 mRNA in the abdominal aortas of rats with
hyperlipidemia. Exp Ther Med. 6:184–188. 2013.PubMed/NCBI
|
|
5
|
Zhou Y, Zhu ZL, Guan XX, Hou WW and Yu HY:
Reciprocal roles between caffeine and estrogen on bone via
differently regulating cAMP-PKA pathway: The possible mechanism for
caffeine-induced osteoporosis in women and estrogens antagonistic
effects. Med Hypotheses. 73:83–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu T, Huang Y, Wang H, Ma Y and Guan W:
Multi-lineage potential research of bone marrow-derived stromal
cells (BMSCs) from cattle. Appl Biochem Biotechnol. 172:21–35.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan J, Li J and Fan Q: Naringin promotes
differentiation of bone marrow stem cells into osteoblasts by
upregulating the expression levels of microRNA-20a and
downregulating the expression levels of PPARgamma. Mol Med Rep.
12:4759–4765. 2015.PubMed/NCBI
|
|
8
|
Weivoda MM and Hohl RJ: Geranylgeranyl
pyrophosphate stimulates PPARγ expression and adipogenesis through
the inhibition of osteoblast differentiation. Bone. 50:467–476.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao J, Ou G, Yang N, Ding K, Kream BE,
Hamrick MW, Isales CM and Shi XM: Impact of targeted PPARγ
disruption on bone remodeling. Mol Cell Endocrinol. 410:27–34.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Chen L, Yin Q, Gao H, Dong P,
Zhang X and Kang J: Reciprocal interferences of TNF-α and
Wnt1-β-catenin signaling axes shift bone marrow-derived stem cells
towards osteoblast lineage after ethanol exposure. Cell Physiol
Biochem. 32:755–765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirayama K, Matsuzuka Y, Kamiya T,
Ikeguchi M, Takagaki K and Itoh K: Metabolism of isoflavones found
in the Pueraria thomsonii flower by human intestinal
microbiota. Biosci Microflora. 30:135–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SH, Kim CW, Jeon SY, Go RE, Hwang KA
and Choi KC: Chemopreventive and chemotherapeutic effects of
genistein, a soy isoflavone, upon cancer development and
progression in preclinical animal models. Lab Anim Res. 30:143–150.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagaraju GP, Zafar SF and El-Rayes BF:
Pleiotropic effects of genistein in metabolic, inflammatory, and
malignant diseases. Nutr Rev. 71:562–572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Menze ET, Esmat A, Tadros MG, Abdel-Naim
AB and Khalifa AE: Genistein improves 3-NPA-induced memory
impairment in ovariectomized rats: Impact of its antioxidant,
anti-inflammatory and acetylcholinesterase modulatory properties.
PLoS One. 10:e01172232015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Duan Y, Zhang X, Ye Y, Ge B and
Chen J: Genistein induces apoptosis by the inactivation of the
IGF-1R-p-Akt signaling pathway in MCF-7 human breast cancer cells.
Food Funct. 6:995–1000. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liao MH, Tai YT, Cherng YG, Liu SH, Chang
YA, Lin PI and Chen RM: Genistein induces oestrogen receptor-α gene
expression in osteoblasts through the activation of
mitogen-activated protein kinases-NF-κB-activator protein-1 and
promotes cell mineralisation. Br J Nutr. 111:55–63. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng X, Yu SP, Taylor T, Ogle M and Wei L:
Protective effect of apelin on cultured rat bone marrow mesenchymal
stem cells against apoptosis. Stem Cell Res (Amst). 8:357–367.
2012. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pauksch L, Hartmann S, Szalay G, Alt V and
Lips KS: In vitro assessment of nanosilver-functionalized PMMA bone
cement on primary human mesenchymal stem cells and osteoblasts.
PLoS One. 9:e1147402014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee S, Cho HY, Bui HT and Kang D: The
osteogenic or adipogenic lineage commitment of human mesenchymal
stem cells is determined by protein kinase C delta. BMC Cell Biol.
15:422014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Antoniou J, Wang HT, Alaseem AM, Haglund
L, Roughley PJ and Mwale F: The effect of Link N on differentiation
of human bone marrow-derived mesenchymal stem cells. Arthritis Res
Ther. 14:R2672012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nichols RA Jr, Niagro FD, Borke JL and
Cuenin MF: Mechanical stretching of mouse calvarial osteoblasts
in vitro models changes in MMP-2 and MMP-9 expression at the
bone-implant interface. J Oral Implantol. May 11–2015.(Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu HM, Yang L, Wang Z, Liu YW, Fan JZ, Fan
J, Liu J and Luo ZJ: Overexpression of integrin α2 promotes
osteogenic differentiation of hBMSCs from senile osteoporosis
through the ERK pathway. Int J Clin Exp Pathol. 6:841–852.
2013.PubMed/NCBI
|
|
24
|
Relic B, Zeddou M, Desoroux A, Beguin Y,
de Seny D and Malaise MG: Genistein induces adipogenesis but
inhibits leptin induction in human synovial fibroblasts. Lab
Invest. 89:811–822. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arrigoni C, De Luca P, Gilardi M, Previdi
S, Broggini M and Moretti M: Direct but not indirect co-culture
with osteogenically differentiated human bone marrow stromal cells
increases RANKL-OPG ratio in human breast cancer cells generating
bone metastases. Mol Cancer. 13:2382014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choudhary S, Goetjen A, Estus T,
Jacome-Galarza CE, Aguila HL, Lorenzo J and Pilbeam C: Serum
amyloid A3 secreted by preosteoclasts inhibits parathyroid
hormone-stimulated cAMP signaling in murine osteoblasts. J Biol
Chem. 23–Dec;2015.(Epub ahead of print). pii jbc. M115.686576.
PubMed/NCBI
|
|
27
|
Song M, Tian X, Lu M, Zhang X, Ma K, Lv Z,
Wang Z, Hu Y, Xun C, Zhang Z and Wang S: Genistein exerts growth
inhibition on human osteosarcoma MG-63 cells via PPARγ pathway. Int
J Oncol. 46:1131–1140. 2015.PubMed/NCBI
|
|
28
|
Chatterjee G, Roy D, Khemka VK,
Chattopadhyay M and Chakrabarti S: Genistein, the isoflavone in
soybean, causes amyloid beta peptide accumulation in human
neuroblastoma cell line: Implications in Alzheimers disease. Aging
Dis. 6:456–465. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Valles SL, Dolz-Gaiton P, Gambini J,
Borras C, Lloret A, Pallardo FV and Viña J: Estradiol or genistein
prevent Alzheimers disease-associated inflammation correlating with
an increase PPAR gamma expression in cultured astrocytes. Brain
Res. 1312:138–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhuang H, Zhang X, Zhu C, Tang X, Yu F,
Shang GW and Cai X: Molecular mechanisms of PPARγ governing MSC
osteogenic and adipogenic differentiation. Curr Stem Cell Res Ther.
31–May;2015.(Epub ahead of print).
|
|
31
|
Zhou H, Yang X, Wang N, Zhang Y and Cai G:
Tigogenin inhibits adipocytic differentiation and induces
osteoblastic differentiation in mouse bone marrow stromal cells.
Mol Cell Endocrinol. 270:17–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang L, Li L, Gao H and Li Y: Effect of
pioglitazone on transdifferentiation of preosteoblasts from rat
bone mesenchymal stem cells into adipocytes. J Huazhong Univ Sci
Technolog Med Sci. 32:530–533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee NJ, Doyle KL, Sainsbury A, Enriquez
RF, Hort YJ, Riepler SJ, Baldock PA and Herzog H: Critical role for
Y1 receptors in mesenchymal progenitor cell differentiation and
osteoblast activity. J Bone Miner Res. 25:1736–1747. 2010.
View Article : Google Scholar : PubMed/NCBI
|