Introduction
Diabetes mellitus (DM) can cause serious health
complications and lead to chronic damage and dysfunction of organs,
including the eyes, kidneys, cardiovascular system and nervous
system. Diabetic foot (DF) is one of the most serious complications
of DM. It is a major cause of morbidity and disability in patients
with DM (1). Disorders associated
with DF include ulceration, infection, vascular disease, Charcot
arthropathy and neuropathic fracture. Slight foot injuries in
patients with DM, particularly in elderly individuals with a long
duration of DM, can develop into ulcers because of the high blood
glucose levels at the wounds, and may ultimately lead to
amputation.
DFU is characterized by chronic poor blood flow in
the lower extremities (2). The study
of vascular disease of the lower extremity is important in DFU
research. In the course of DFU, vascular disease can lead to
alterations in the expression levels of regulatory factors such as
hypoxia-inducible factor-1α, vascular endothelial growth factor and
transforming growth factor (TGF)-β1 (3,4). TGF-β1
is important in the morphological changes, proliferation,
differentiation and healing of cells. Madhyastha et al
(5) reported that TGF-β1 induced the
expression of miRNA-21 under high-glucose conditions, and this
effect was dependent on NF-κB activation; therefore, it was
suggested that manipulation of the TGF-β1/NF-κB/miRNA-21 pathway
may serve as an innovative approach in the development of
therapeutics to treat diabetic ulcers. Previous studies have
investigated the regulation of the expression of genes associated
with the TGF-β1 pathway by miRNA-145 in gallbladder cancer
(6), cardiac myofibroblast
differentiation (7), lung fibrosis
(8) and cystic fibrosis (9). However, to the best of our knowledge,
there have been no previous reports on the correlation between the
expression levels of miRNA-145 and TGF-β1 in patients with
DFUs.
In the present study, the expression levels of
TGF-β1 and miRNA-145 were detected in the serum, dorsalis pedis
arteries and muscles of the amputated limbs of patients with DFUs,
in order to investigate whether miRNA-145 regulates the expression
of TGF-β1 in DFU patients.
Materials and methods
Patients
A total of 26 patients with DFUs (16 males and 10
females; mean age, 61.6±5.1 years; age range, 53–68 years) with
amputation, and 15 trauma patients (9 males and 6 females; mean
age, 52.3±7.8 years; age range, 46–61 years) undergoing amputation,
at Laiwu City People's Hospital (Laiwu, China) were enrolled in the
present study between January 2013 and August 2014. The present
study was approved by the Ethics Committee of Laiwu City People's
Hospital and prior written informed consent was obtained from all
patients.
Peripheral blood samples were collected in the early
morning from fasted patients prior to amputation. The serum was
separated and stored at −80°C. The dorsalis pedis arteries and
muscles with ulcers were collected from the amputated limbs of the
patients shortly following amputation and stored in liquid
nitrogen.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the samples of dorsalis
pedis arteries and muscles using TRIzol reagent (Yisheng
Biotechnology Co., Ltd., Shanghai, China) and the serum using an
miRNeasy Serum/Plasma kit (Jianlun Biological Technology Co., Ltd.,
Guangzhou, China). Total RNA (4 µl) was reverse transcribed to form
cDNA using a TIANscript RT kit (Tiangen Biotech Co., Ltd., Beijing,
China). RT-qPCR analysis of miRNA and mRNA levels was performed
using the iQ5 Real-Time PCR detection system (Bio-Rad Technologies,
Inc., Hercules, CA, USA) with miRcute miRNA qPCR detection and
SuperReal PreMix (SYBR Green) kits (both from Tiangen Biotech Co.,
Ltd.). The 2−ΔΔCq method was used to calculate the
expression levels of the TGF-β1 gene and miRNA-145 relative to the
endogenous control genes: β-actin and U6 small nuclear RNA (RNU6),
respectively. PCR reactions were conducted using the CFX96 Touch™
thermal cycler (Bio-Rad Technologies, Inc.), in which the following
primers were used: TGF-β1, 5′-GGACACCAACTATTGCTTCAG-3′ and
5′-TCCAGACTCCAAATGTAG-3′; β-actin, 5′-TTCCAGCCTTCCTTCCTGG-3′ and
5′-TTGCGCTCAGGAGGAGGAAT-3′; miRNA-145,
5′-ACACTCCAGCTGGGGTCCAGTTTTCCCAGGAA-3′ and
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG-3′; RNU6,
5′-ACACTCCAGCTGGGTTCGTGAAGCGTTCCA-3′ and
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG-3′ (Sangon Biotech Co.,
Ltd., Shanghai, China). PCR procedures for TGF-β1 and β-actin
comprised template denaturation at 95°C for 2 min, then 30 cycles
of 94°C for 45 sec, 55°C for 55 sec and 72.0°C for 1 min, and a
final extension at 72°C for 10 min. PCR procedures for miRNA-145
and RNU6 comprised template denaturation at 95°C for 30 sec, then
40 cycles of 95°C for 5 sec, 55°C for 34 sec and 72.0°C for 34 sec,
and a final extension at 72°C for 10 min.
Western blot analysis
Total proteins were harvested from the samples and
the concentrations of the proteins were determined using a
Bicinchoninic Acid Protein Assay kit (Real-Times Biotechnology Co.,
Ltd., Beijing, China). Then, 20 µg total proteins were separated by
10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(Beyotime Institute of Biotechnology, Haimen, China), and then
transferred to polyvinylidene difluoride membranes (Bio-Rad
Laboratories, Inc.) for western blotting. After blocking with 5%
non-fat milk for 1 h at room temperature, the membranes were
incubated at 4°C overnight with rabbit anti-human TGF-β1 (1:500;
cat. no. ab92486; Abcam, Cambridge, MA, USA) and rabbit anti-human
β-actin (1:5,000; cat. no. ab8227; Abcam) polyclonal antibodies.
After washing with phosphate-buffered saline three times, the
membranes were incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (1:3,000; cat. no. ab6721;
Abcam) for 1 h at room temperature. Bound antibodies were detected
using an enhanced chemiluminescence system (Abcam). The mean
normalized optical density (OD) of the TGF-β1 protein band relative
to the OD of the β-actin band from the same individual was
calculated using Image Lab software, version 3.0 (Bio-Rad
Laboratories, Inc.).
Statistical analysis
All data were processed using SPSS software, version
18.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as the mean
± standard deviation. A normality test was performed on all data.
One-way analysis of variance was conducted in order to determine
the statistical significance of differences. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
The lesions of peripheral arterial disease (PAD) in
the DFU patients include arterial intimal thickening (≥1 mm),
single or multiple artery plaques, artery stenosis and occlusion.
PAD with one of these four types of lesions was designated as mild,
with two as moderate and three or more as severe (10). In the present study, all 26 DFU
patients had previously been diagnosed with PAD, of which 1 patient
had mild PAD, 6 patients had moderate PAD and 19 patients had
severe PAD.
Expression of TGF-β1 mRNA in DFU
patients
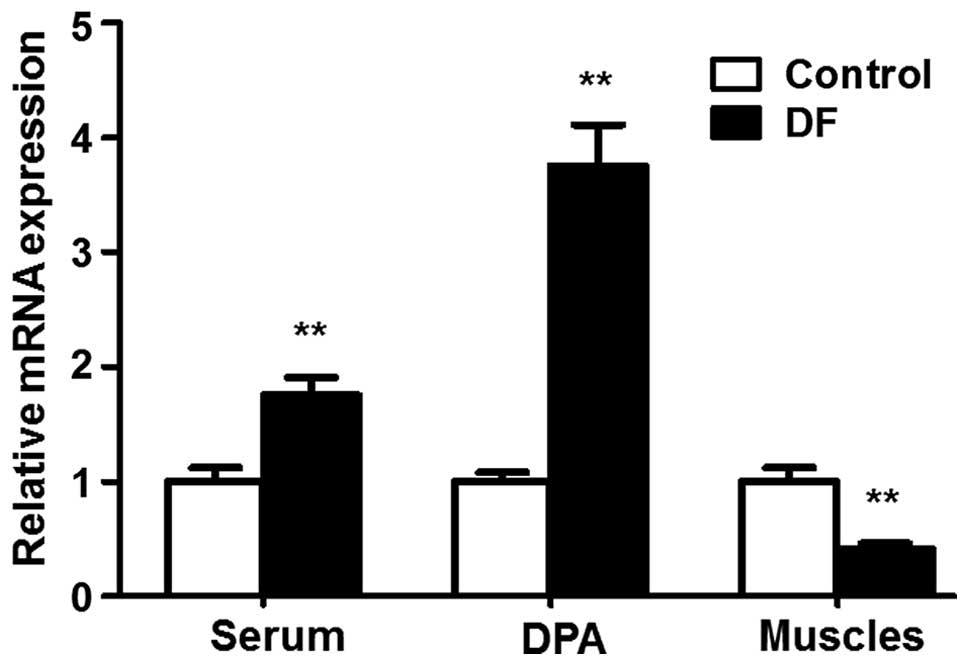
To investigate the expression of TGF-β1 in DFU
patients, the levels of TGF-β1 mRNA were detected using RT-qPCR
analysis. The expression level of TGF-β1 in the serum of DFU
patients was significantly higher than that in the serum of the
control trauma patients (1.00±0.12 vs. 1.75±0.16, respectively;
P<0.01; Fig. 1). TGF-β1 mRNA
expression was also detected in the dorsalis pedis arteries of the
amputated limbs. Consistent with the observations in the serum, the
expression level of TGF-β1 mRNA in the dorsalis pedis arteries of
DFU patients was significantly higher than that in the
corresponding arteries of the control (1.00±0.08 vs. 3.75±0.36,
respectively; P<0.01, Fig. 1).
Notably, the expression level of TGF-β1 mRNA was significantly
lower in the ulcerated muscles of the amputated limbs of DFU
patients compared with those in the amputated leg muscles of the
control patients (1.00±0.12 vs. 0.41±0.05, respectively; P<0.01;
Fig. 1). These results reveal a
distinct expression pattern of TGF-β1 mRNA in DFU patients.
Expression of TGF-β1 protein in DFU
patients
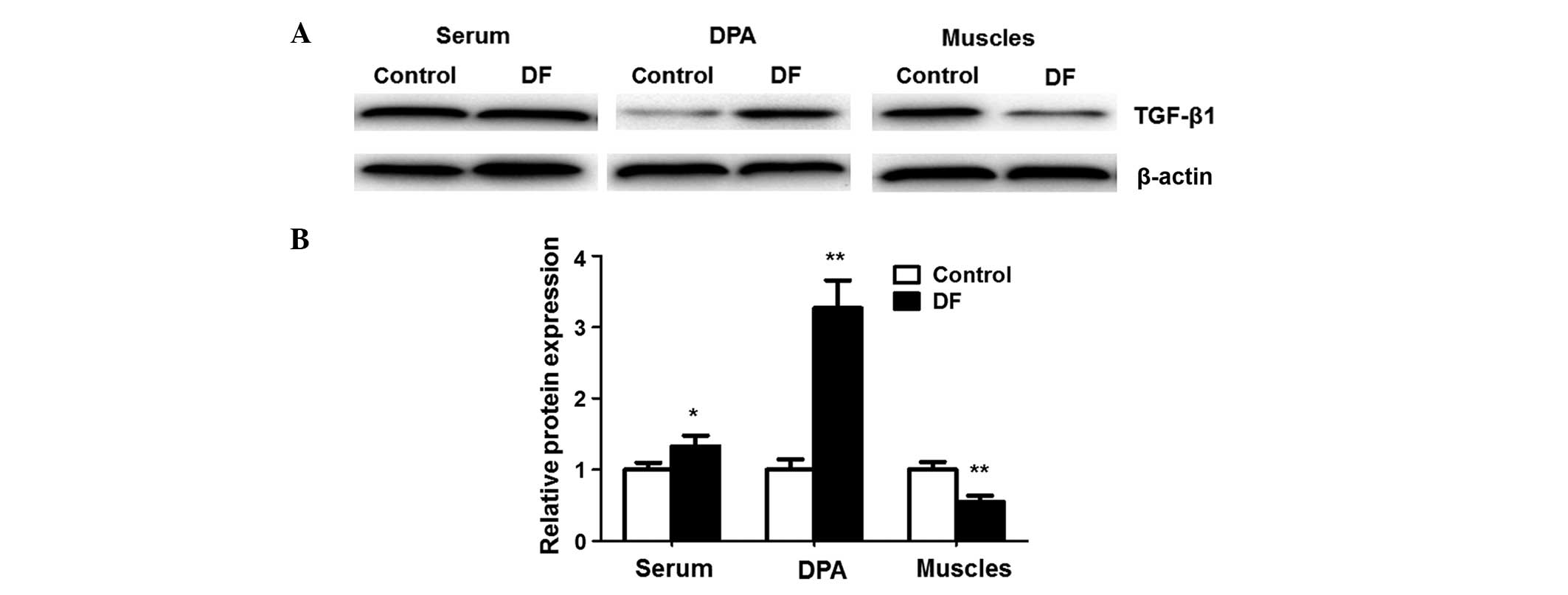
To confirm the results of the TGF-β1 mRNA expression
analysis described above, the levels of TGF-β1 protein were
detected using western blot analysis. As shown in Fig. 2, compared with the control, the
expression of TGF-β1 protein in DFU patients was significantly
higher in the serum (1.00±0.10 vs. 1.33±0.15, respectively;
P<0.05) and the dorsalis pedis arteries (1.00±0.15 vs.
3.27±0.39, respectively; P<0.01), but significantly lower in the
ulcerated muscles of the amputated limb (1.00±0.11 vs. 0.55±0.09,
respectively; P<0.01). These results indicate that the
expression level of TGF-β1 protein correlates well with the TGF-β1
mRNA expression level, and further confirms the expression pattern
of TGF-β1 in DFU patients.
Expression of miRNA-145 in DFU
patients
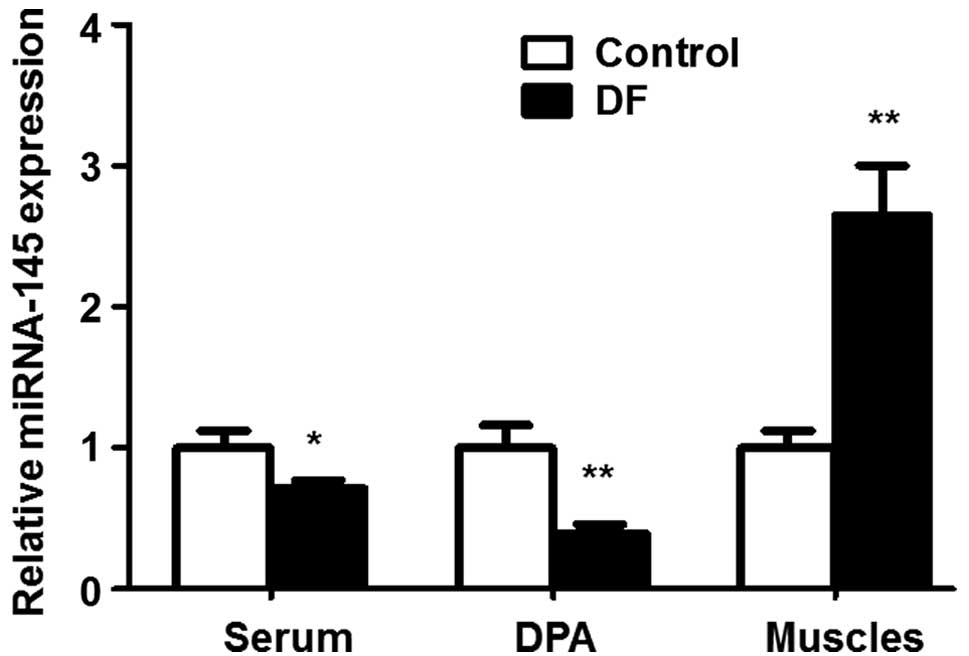
In order to investigate whether the expression of
TGF-β1 is negatively regulated by miRNA-145 in patients with DFUs,
miRNA-145 expression levels were evaluated using RT-qPCR analysis.
As shown in Fig. 3, compared with
the control, in the DFU patients the expression of miRNA-145 was
significantly lower in the serum (1.00±0.12 vs. 0.71±0.06,
respectively; P<0.05) and the dorsalis pedis arteries (1.00±0.16
vs. 0.39±0.07, respectively; P<0.01), but significantly higher
in the ulcerated muscles (1.00±0.12 vs. 2.65±0.35, respectively;
P<0.01). These results indicate that an inverse correlation
exists between miRNA-145 and TGF-β1 expression levels in patients
with DFUs.
Discussion
In the present study, the expression levels of
TGF-β1 and miRNA-145 were investigated in patients with DFUs and
control trauma patients undergoing amputation. The results unveiled
a distinct expression pattern of TGF-β1 in the DFU patients. An
inverse correlation of the expression of miR-145 with the
expression of TGF-β1 was also observed in the patients with
DFUs.
Peripheral artery disease (PAD) is an important
factor leading to DFU. Hao et al reported that DFUs are more
evident in patients with PAD, which is further reflected by a
significantly greater number of underlying cases of infection and
disabling comorbidity (11). PAD was
evident in all 26 patients with DFUs enrolled in the present study.
Janda et al observed a significant correlation of increased
TGF-β1 expression levels with atherosclerotic changes in the
carotid arteries of patients requiring peritoneal dialysis
(12). In the present study, it was
found that the levels of TGF-β1 expression in the serum and
dorsalis pedis arteries of the amputated limbs of DFU patients were
significantly higher than those in the control patients. The
results also suggest that a correlation exists between increased
TGF-β1 expression and vascular diseases in DFU patients.
It has been reported that TGF-β1 can stimulate the
secretion of extracellular matrix (ECM) and plays important roles
in the morphological changes, proliferation and differentiation of
mononuclear cells, brain cells and osteocytes (13–15).
In vivo studies have suggested that the upregulation of
TGF-β1 can promote wound healing and the formation of typical
granulation tissues (16,17). In the present study, TGF-β1
expression levels were observed to be significantly decreased in
the ulcerated muscles of the amputated limbs of DFU patients. This
may be one of the factors affecting the healing of DFUs.
TGF-β1 expression can be regulated by, for example,
nemo-like kinase and Smad (18,19). It
has been reported that TGF-β1 may also be post-transcriptionally
regulated by miRNA (20). The
present study provides direct evidence of the inverse correlation
of the expression of miRNA-145 and TGF-β1 in DFU patients, which is
consistent with the aforementioned studies. These results suggest
that miRNA-145 regulates the expression of TGF-β1 in DFU
patients.
DF is the most common cause of hospitalization of
diabetic patients. The incidence of DF is particularly high in the
elderly populations of developing countries. Every year, 4 million
diabetic patients develop DFUs. Worldwide, one person undergoes DF
amputation every 30 sec (21).
However, 85% of these amputations could be avoided (21), and it is important to identify
reliable and stable molecular markers of DFU in diabetic patients.
In the present study, it was found that the expression of miRNA-145
is negatively correlated with TGF-β1 expression. Since TGF-β1 plays
important roles in DFU development (5), miRNA-145 might be a potential marker or
therapeutic target for the diagnosis or treatment of DFU. The
effects of miRNA-145 on DFUs and the underlying mechanisms merit
further investigation.
Acknowledgements
The authors of the present study would like to thank
Dr Fenglian Liu, director of Laiwu City People's Hospital, for help
in the choice of the research topic, the design and guidance of the
experiments, analysis of the data and the writing and revision of
this manuscript.
References
|
1
|
Rdeini WM, Agbenorku P and Mitish VA:
Strategy of surgical management of peripheral neuropathy form of
diabetic foot syndrome in Ghana. Plast Surg Int.
2014:1850232014.PubMed/NCBI
|
|
2
|
Cakmak T, Metin S, Yaman H, Demirbas S,
Yildiz S, Turker T and Akin A: The effect of hyperbaric oxygen
therapy on ischemia-modified albumin levels in people with diabetes
with foot ulcers. Undersea Hyperb Med. 41:277–281. 2014.PubMed/NCBI
|
|
3
|
Zhang J, Guan M, Xie C, Luo X, Zhang Q and
Xue Y: Increased growth factors play a role in wound healing
promoted by noninvasive oxygen-ozone therapy in diabetic patients
with foot ulcers. Oxid Med Cell Longev. 2014:2734752014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thangarajah H, Vial IN, Grogan RH, Yao D,
Shi Y, Januszyk M, Galiano RD, Chang EI, Galvez MG, Glotzbach JP,
et al: HIF-1alpha dysfunction in diabetes. Cell Cycle. 9:75–79.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Madhyastha R, Madhyastha H, Pengjam Y,
Nakajima Y, Omura S and Maruyama M: NFkappaB activation is
essential for miR-21 induction by TGFβ1 in high glucose conditions.
Biochem Biophys Res Commun. 451:615–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Letelier P, García P, Leal P, Álvarez H,
Ili C, López J, Castillo J, Brebi P and Roa JC: miR-1 and miR-145
act as tumor suppressor microRNAs in gallbladder cancer. Int J Clin
Exp Pathol. 7:1849–1867. 2014.PubMed/NCBI
|
|
7
|
Wang YS, Li SH, Guo J, Mihic A, Wu J, Sun
L, Davis K, Weisel RD and Li RK: Role of miR-145 in cardiac
myofibroblast differentiation. J Mol Cell Cardiol. 66:94–105. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang S, Cui H, Xie N, Icyuz M, Banerjee S,
Antony VB, Abraham E, Thannickal VJ and Liu G: miR-145 regulates
myofibroblast differentiation and lung fibrosis. FASEB J.
27:2382–2391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Megiorni F, Cialfi S, Cimino G, De Biase
RV, Dominici C, Quattrucci S and Pizzuti A: Elevated levels of
miR-145 correlate with SMAD3 down-regulation in cystic fibrosis
patients. J Cyst Fibros. 12:797–802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu WF, Wu WQ, Chen LQ, Yang WP and Wen H:
Analysis of the incidence of diabetic lower extremities vascular
disease, pathological changes and the related factors. J Pract Med.
26:2323–2325. 2010.
|
|
11
|
Hao D, Hu C, Zhang T, Feng G, Chai J and
Li T: Contribution of infection and peripheral artery disease to
severity of diabetic foot ulcers in Chinese patients. Int J Clin
Pract. 68:1161–1164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Janda K, Krzanowski M, Dumnicka P,
Kuśnierz-Cabala B, Kraśniak A and Sułowicz W: Transforming growth
factor beta 1 as a risk factor for cardiovascular diseases in
end-stage renal disease patients treated with peritoneal dialysis.
Clin Lab. 60:1163–1168. 2014.PubMed/NCBI
|
|
13
|
Cicha I, Yilmaz A, Klein M, Raithel D,
Brigstock DR, Daniel WG, Goppelt-Struebe M and Garlichs CD:
Connective tissue growth factor is overexpressed in complicated
atherosclerotic plaques and induces mononuclear cell chemotaxis in
vitro. Arterioscler Thromb Vasc Biol. 25:1008–1013. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng L, He X, Wang Y, Tang Y, Zheng C, Cai
H, Liu J, Wang Y, Fu Y and Yang GY: MicroRNA-210 overexpression
induces angiogenesis and neurogenesis in the normal adult mouse
brain. Gene Ther. 21:37–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanaan RA, Aldwaik M and Al-Hanbali OA:
The role of connective tissue growth factor in skeletal growth and
development. Med Sci Monit. 12:RA277–RA281. 2006.PubMed/NCBI
|
|
16
|
Hameedaldeen A, Liu J, Batres A, Graves GS
and Graves DT: FOXO1, TGF-β regulation and wound healing. Int J Mol
Sci. 15:16257–16269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okizaki S, Ito Y, Hosono K, Oba K, Ohkubo
H, Amano H, Shichiri M and Majima MS: Suppressed recruitment of
alternatively activated macrophages reduces TGF-β1 and impairs
wound healing in streptozotocin-induced diabetic mice. Biomed
Pharmacother. 70:317–325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao Z, Zhang J, Peng X, Dong Y, Jia L, Li
H and Du J: The Notch γ-secretase inhibitor ameliorates kidney
fibrosis via inhibition of TGF-β/Smad2/3 signaling pathway
activation. Int J Biochem Cell Biol. 55:65–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi Y, Ye K, Wu H, Sun Y, Shi H and Huo K:
Human SMAD4 is phosphorylated at Thr9 and Ser138 by interacting
with NLK. Mol Cell Biochem. 333:293–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martin J, Jenkins RH, Bennagi R, Krupa A,
Phillips AO, Bowen T and Fraser DJ: Post-transcriptional regulation
of transforming growth factor beta-1 by microRNA-744. PLoS One.
6:e250442011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barshes NR, Gold B, Garcia A, Bechara CF,
Pisimisis G and Kougias P: Minor amputation and palliative wound
care as a strategy to avoid major amputation in patients with foot
infections and severe peripheral arterial disease. Int J Low Extrem
Wounds. 13:211–219. 2014. View Article : Google Scholar : PubMed/NCBI
|