Introduction
Tooth loss is a common and serious health concern,
particularly in the elderly. Although the incidence of tooth loss
has declined in industrialized countries, the goal established by
the World Health Organization of retaining 20 teeth at the age of
80 years has not been widely achieved (1,2).
Mechanical stress has a crucial role in bone remodeling (3,4) and bone
loss may occur as a result of reduced mechanical force, which is
defined as disuse osteoporosis (5).
Occlusal hypofunction due to tooth loss has a negative impact on
jaw bone homeostasis (6). Tooth loss
may lead to a reduced bite force in the antagonistic tooth and
underlying alveolar bone during chewing. A lack of functional
occlusion may induce active alveolar bone loss, including
decreasing bone mass and volume (7,8). In
addition, in certain pathological conditions, such as estrogen
deficiency, functional occlusion has been revealed to slow down the
rate of bone loss (9), whereas
occlusal hypofunction was reported to accelerate bone loss
(10,11). However, the cellular and molecular
mechanisms underlying bone loss have yet to be fully
elucidated.
The Wnt/β-catenin signaling pathway has an essential
role in bone remodeling by regulating osteoblast differentiation
and function (12). Osteocytes,
which are the most abundant cells in the bone, have a dominant role
in sensing and transducing mechanical stress (13). Osteocytes express sclerostin, which
is a SOST gene-encoded soluble protein that is thought to diffuse
through the thick network of osteocyte canaliculi to reach the bone
surface (14,15). Previous studies have demonstrated
that sclerostin, which typically antagonizes the Wnt/β-catenin
signaling pathway by binding to the LRP5/6 receptor to inhibit bone
formation, is secreted less during mechanical stress, thus causing
an increase in bone production in this context (16–18). In
addition, sclerostin is able to promote osteoclastogenesis and
osteoclast resorptive activity by a receptor activator of nuclear
factor-κB ligand (RANKL)-dependent pathway (19). In a model of tail suspension
unloading-induced osteoporosis, decreased Wnt/β-catenin signaling
associated with upregulation of sclerostin was detected in the
femur (20).
Alveolar bone is highly adaptable to the development
of teeth and occlusal force, and is of unique character, as
compared with the long bone of limbs (6,7). Upon
occlusion, the components of the tooth-periodontal
ligament-alveolar bone complex behave in a synergistic manner
(21). The reaction of alveolar bone
to a mechanical stimulus may be affected by multiple components of
the periodontium (8). Occlusal
hypofunction has been reported to be a useful unloading model for
jaw bone research, since it has been associated with tooth loss in
patients requiring prosthodontic treatment (10,11).
However, to the best of our knowledge, the expression levels of
sclerostin in the occlusal hypofunction environment have not been
investigated to date. Therefore, the present study aimed to
investigate the roles of sclerostin and Wnt/β-catenin signaling in
the regulation of alveolar bone loss induced by occlusal
hypofunction.
Materials and methods
Establishment of a rat model of
occlusal hypofunction
A total of 14 male Sprague-Dawley rats (age, 10
weeks) were used, sourced from Chengdu Da Shuo Biological
Technology Co. Ltd. (Chengdu, China). All rats were fed a powder
diet (Chengdu Da Shuo Biological Technology Co. Ltd.), had free
access to drinking water and were maintained under a 12–12 h
light-dark cycle at a constant temperature of 23°C. The present
study was approved by the Ethics Committee of the West China
Hospital of Stomatology, Sichuan University (Chengdu, China). The
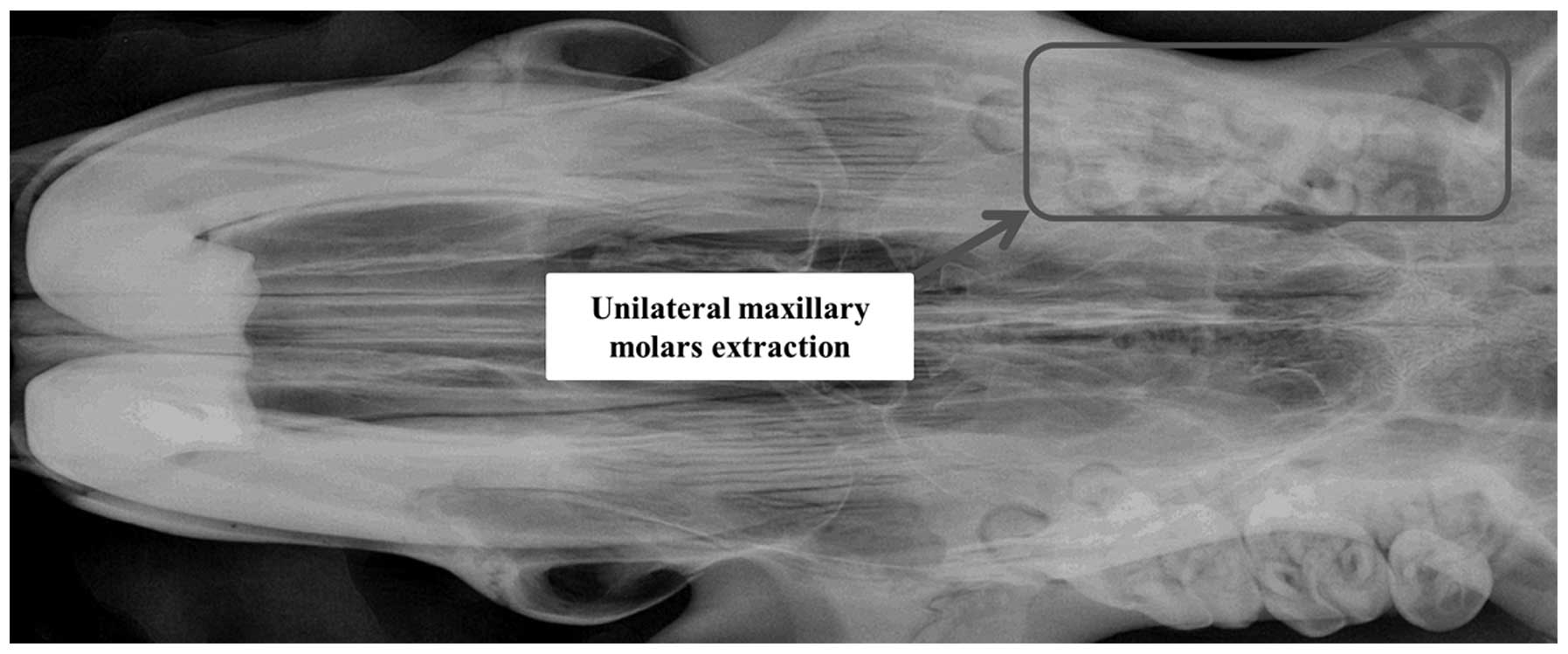
maxillary molars of the rats were extracted on the left side,
whilst the homolateral upper incisors were abraded, in order to
induce the occlusal hypofunction environment (Fig. 1). For each rat, the non-extraction
side was treated as the control group for comparisons with the
extraction side. During the surgical procedure, anesthesia was
administered intraperitoneally with 0.5% chloral hydrate (West
China Second University Hospital, Chengdu, China). The rats were
fed ad libitum throughout the experimental period. At week 8
after tooth extraction, all rats were sacrificed with an excess of
chloral hydrate and the mandibles were collected and stored in a
solution of 0.5% buffered formalin (Baoke Biotechnology, Inc.,
Chengdu, China).
Radiography and micro-computed
tomography (CT) analyses
The whole mandibular alveolar bone was examined. The
bone mineral density (BMD) of the alveolar bone was assessed by
high resolution X-ray radiography using a Faxitron MX-20 Digital
Radiography system (Faxitron Bioptics, LLC, Tucson, AZ, USA). The
morphological characteristics of the alveolar bone were evaluated
by micro-CT analysis. The specimens were placed in 10% buffered
formalin and scanned using a desktop micro-CT system (µCT 35;
Scanco Medical AG, Brüttisellen, Switzerland). The region of
interest in the alveolar bone was selected and the bone volume
(BV), total volume (TV) and the ratio of bone volume to total
volume (BV/TV) were measured.
Histological evaluation and osteoclast
activity
The alveolar bone samples were fixed overnight in
10% buffered formalin, decalcified in 0.5 mol/l ethylene
diaminetetraacetic acid (pH 7.2) for 2 weeks at room temperature,
and embedded in paraffin wax. Subsequently, 6-µm sections were
prepared using an RM2235 microtome (Leica Microsystems GmbH,
Wetzlar, Germany) and stained with hematoxylin and eosin (H&E;
Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China).
Tartrate-resistant acid phosphatase (TRAP) staining
was used to calculate the number of osteoclasts. Briefly, the
sections were stained using a TRAP Staining kit (cat. no. 387A;
Sigma-Aldrich, St. Louis, MO, USA) and the number of osteoclasts
was calculated by two independent investigators using an Eclipse
E100 microscope (Nikon Corporation, Toyko, Japan).
Immunohistochemistry
Demineralized, paraffin embedded sections of
alveolar bone were prepared in order to detect the protein
expression levels of sclerostin, β-catenin, osteoprotegrin (OPG)
and RANKL by immunohistochemical staining. Briefly, the specimens
were deparaffinized and treated with 3% hydrogen peroxide to
inhibit endogenous peroxidase activity. Subsequently, the fixed
sections were incubated with primary antibodies as follows:
Polyclonal rabbit anti-sclerostin (dilution, 1:50; cat. no.
ab63097; Abcam, Cambridge, UK), polyclonal rabbit anti-OPG
(dilution, 1:200; cat. no. ab73400; Abcam) and monoclonal rabbit
anti-β-catenin (dilution, 1:200; ab32572; Abcam) at 37°C for 2 h,
followed by incubation with horseradish peroxidase (HRP)-conjugated
goat anti-rabbit immunoglobulin G (dilution, 1:200; sc-2004; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) at 37°C for 30 min.
Alternatively, these were incubated with polyclonal goat anti-RANKL
(dilution, 1:200; cat. no. sc-7628; Santa Cruz Biotechnology,
Inc.), followed by HRP-conjugated rabbit anti-goat IgG within the
Polink-2 Plus Polymer HRP Detection system (cat. no. PV9003;
Zhongshan Bio-Tech, Co., Ltd, Beijing, China) for the corresponding
duration and temperatures. Finally, the sections were stained using
a 3,3′-diaminobenzidine tetrahydrochloride kit (OriGene
Technologies, Inc.). Immunostained sections were scanned using a
Nikon Eclipse 800 microscope (Nikon Corporation), and positive
areas representing the degree of antigen expression were calculated
using Image-Pro Plus version 6.0 image analysis software (Media
Cybernetics, Inc., Rockville, MD, USA). Expression levels are
presented as the mean optical density (MOD), which was calculated
using the following equation: MOD = Integrated optical density/size
of the study area.
Statistical analysis
All data are expressed as the mean ± standard
deviation. For quantitative outcomes, a paired t-test was used to
compare the differences between each group. All statistical
analyses were conducted using the SPSS software, version 17.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Alveolar bone histomorphometric
evaluation
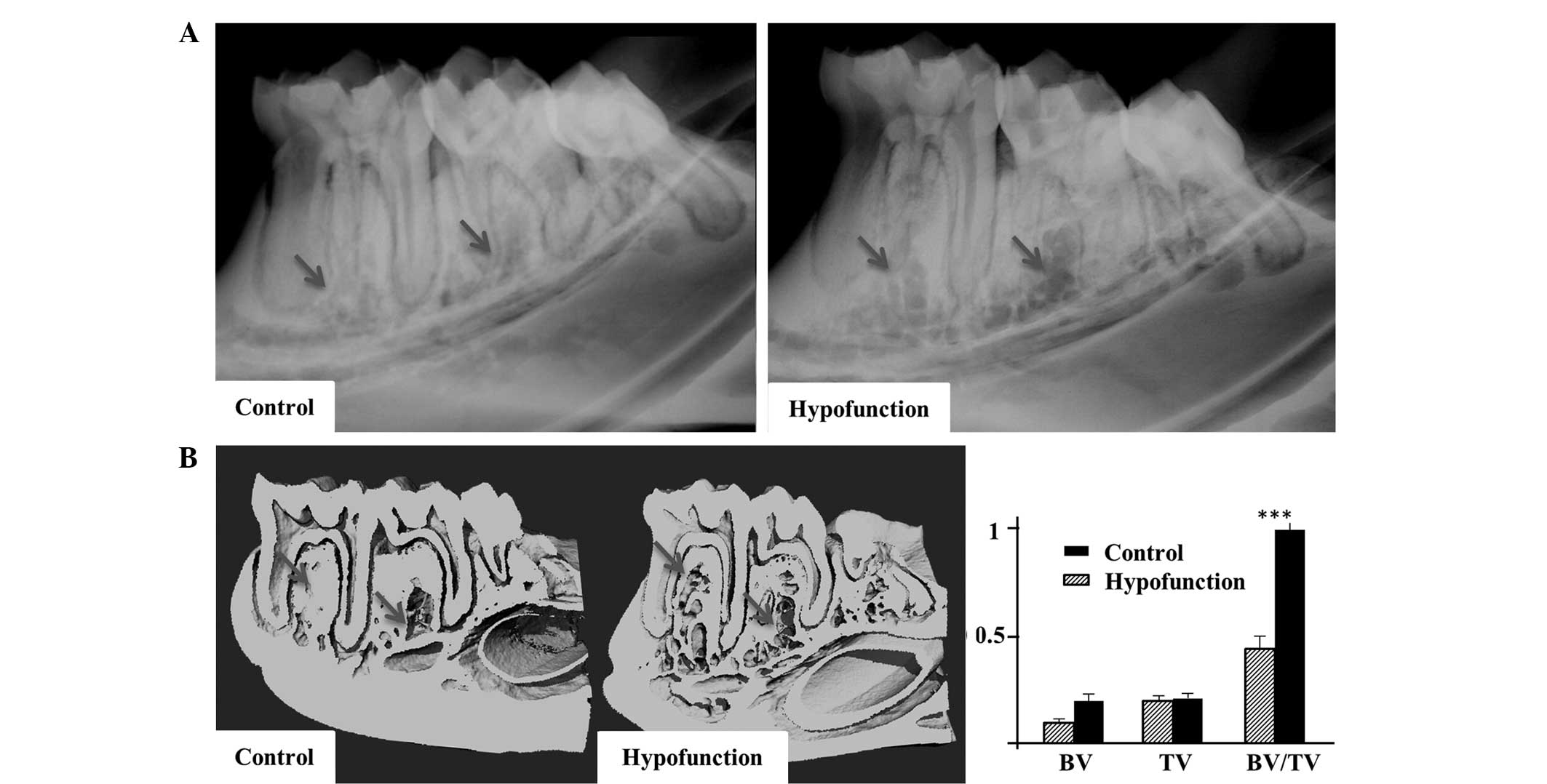
A decreased BMD and bone destruction were observed
in the alveolar apical area at the occlusal hypofunction side by
X-ray analysis (Fig. 2A). In
addition, high-resolution micro-CT images of the mandibles revealed
decreased trabecular and cortical bone masses at the occlusal
hypofunction side. Furthermore, development of a cavity and bone
architecture deterioration were detected at the occlusal
hypofunction side (Fig. 2B).
According to quantitative analysis, the BV and BV/TV values at the
occlusal hypofunction side were significantly lower, as compared
with those at the control side (P<0.001; Fig. 2B). These results were indicative of
severe bone loss at the occlusal hypofunction side.
Alveolar bone histological evaluation
and osteoclast activity
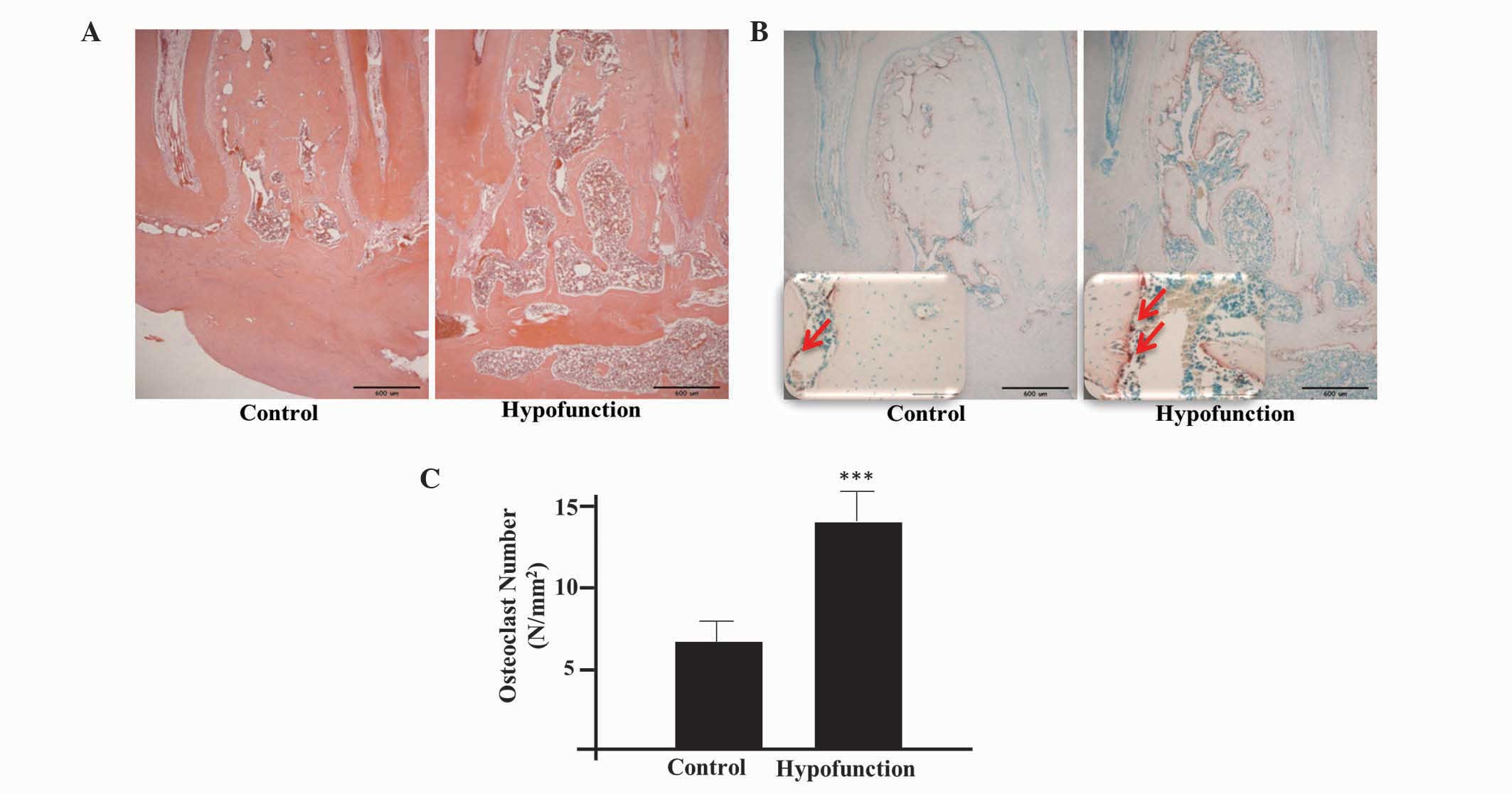
Bone deterioration and resorption were observed at
the occlusal hypofunction side by H&E staining. In addition, a
number of cavities were detected and the trabecular architecture
appeared to be deteriorated (Fig.
3A). As demonstrated by TRAP staining, the number of
osteoclasts was significantly higher at the occlusal hypofunction
side, as compared with the control side (P<0.001; Fig. 3B and C), thus indicating that active
bone resorption was occurring.
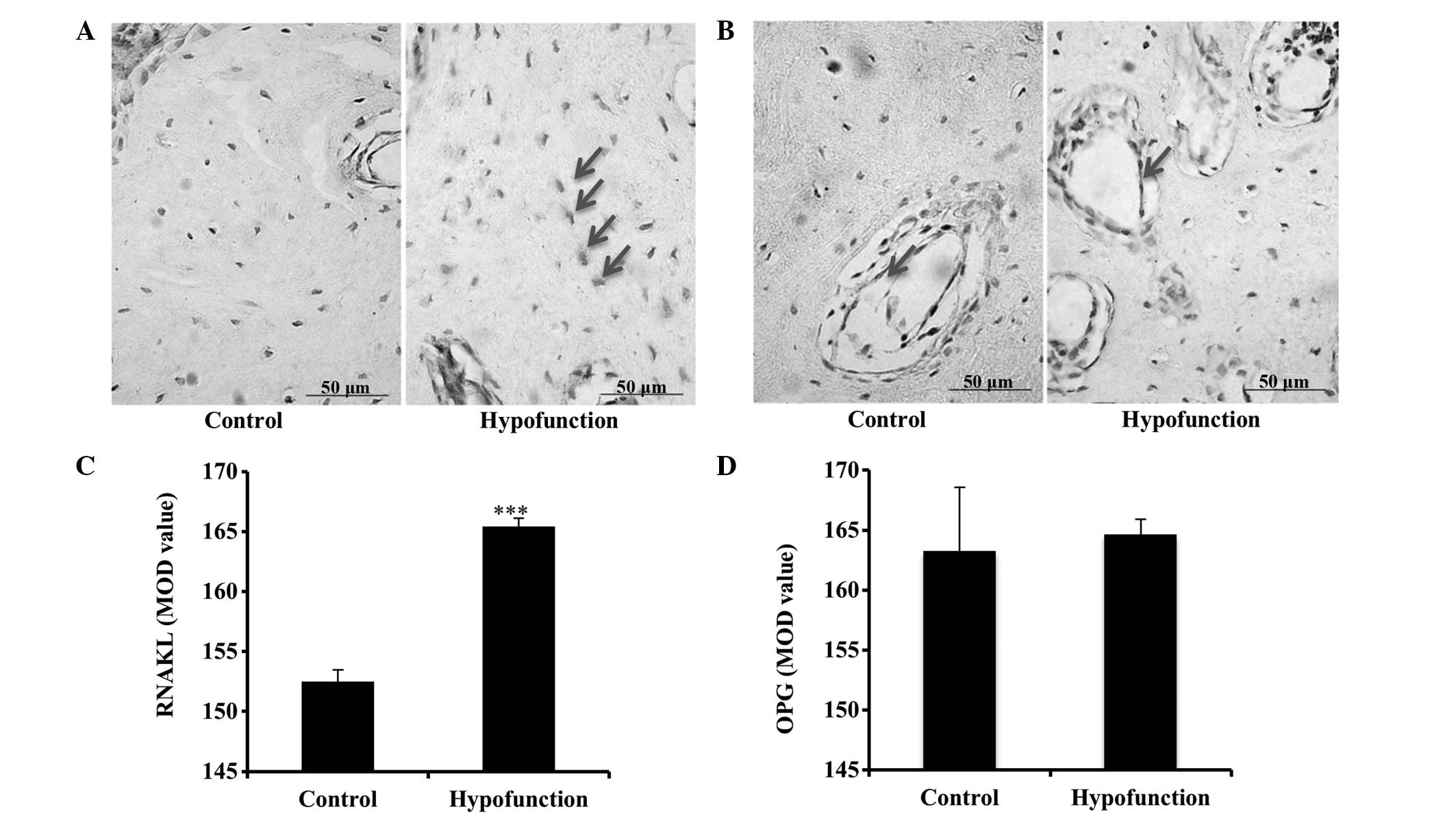
Immunohistochemical results
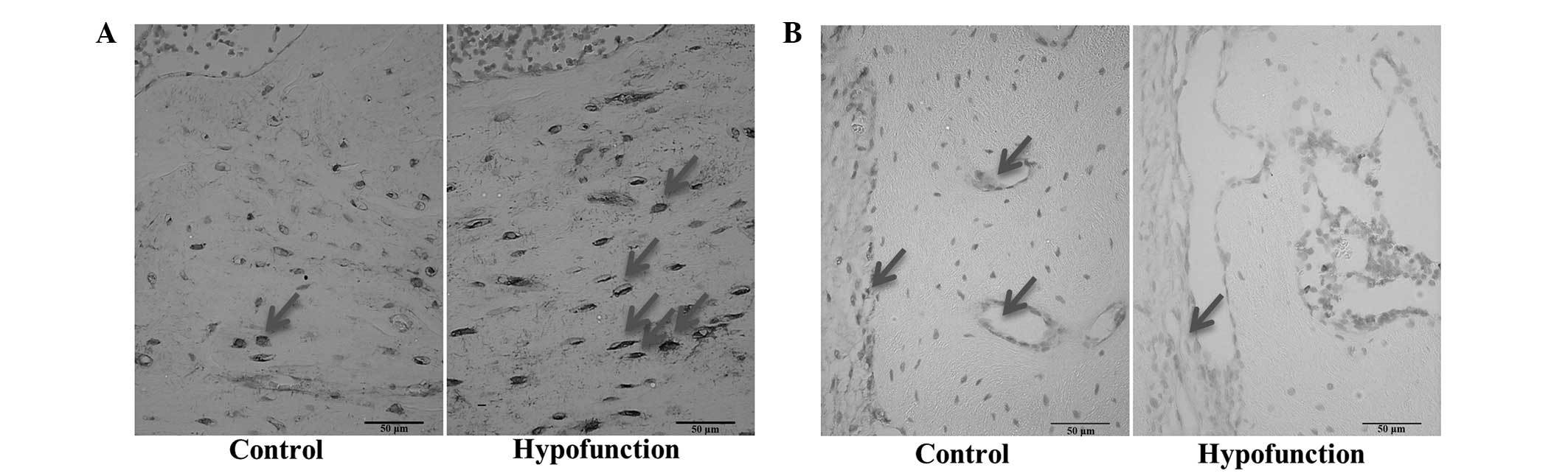
As compared with the control side, the protein
expression levels of β-catenin in the osteoblast cytoplasm were
decreased, and those of sclerostin in the alveolar bone were
increased, at the occlusal hypofunction side, as demonstrated by
immunohistochemical staining (Fig. 4A
and B). These results suggested that occlusal hypofunction
suppresses the Wnt signaling pathway. Furthermore, positive RANKL
immunoreactivity was detected in the osteoblasts and osteocytes,
while positive OPG immunoreactivity was observed in the
osteoblasts. At the hypofunction side, the protein expression
levels of RANKL in osteocytes were significantly increased
(P<0.001; Fig. 5), as compared
with the control side. By contrast, there was no significant
difference in the protein expression levels of OPG between the
sides (P>0.05; Fig. 5), although
stronger immunoreactivity was detected at the hypofunction
side.
Discussion
Tooth loss has been demonstrated to cause severe
damage and may impair the oral health-associated quality of life of
patients (22). Tooth removal may
result in alveolar bone resorption and promote structural and
compositional changes in the overlying soft tissue (23). In addition, tooth loss has been
reported to diminish functional occlusion in the antagonistic
tooth, which may lead to tooth extrusion and disuse osteoporosis of
the alveolar bone (7,8). Therefore, considering the potential
complications during prosthetic rehabilitation, an evaluation of
the magnitude of hard and soft tissue changes is essential prior to
comprehensive prosthodontic treatment (24). In the case that the prosthetics
rehabilitation is not applied immediately, these changes may
deteriorate and have an unfavorable impact on the oral health. On
the contrary, bone resorption can be reduced to some degree if the
prosthesis is applied to balance the occlusal loading on the
alveolar bone (25). In addition,
the functional and cosmetic results of prosthodontic treatment
depend on the quantity and quality of alveolar bone (26). Therefore, it is important to receive
timely prosthetic restoration when tooth loss occurs.
The bone remodeling process consists of bone
formation and bone resorption. Osteocytes serve a central role in
the bone remodeling process by sensing and transmitting external
mechanical loading information to effector cells, and function as
an orchestrator in the regulation of osteoblast and osteoclast
activities (27,28). Numerous cell signalling molecules
have been revealed to be modulated in osteocytes in response to
mechanical stress, including adenosine triphosphate, nitric oxide,
prostaglandin E2 and calcium channels (29). However, few of them have been
demonstrated to be a prerequisite for loading adaptation (29). Previous studies demonstrated that
loading reduces the secretion of sclerostin by osteocytes (30), which decreases the amount of
sclerostin able to bind Lrp5/Lrp6 to antagonize the Wnt signaling
pathway (31). Furthermore,
downregulation of sclerostin in osteocytes has been shown to be an
obligatory step in the osteogenic response to mechanical loading
(32). In unloading models, such as
in tail suspension, sclerostin expression levels increase, thereby
inhibiting the Wnt signaling pathway, inducing apoptosis,
suppressing osteoblast activity and decreasing the bone mass
(20). Therefore, the current
strategy of anti-sclerostin treatment for bone loss-associated
diseases, as a potential therapeutic approach, has been
investigated by numerous studies (33–36).
In the present study, evident bone loss and
architecture deterioration were detected at the occlusal
hypofunction side, as demonstrated using radiography, micro-CT and
H&E staining. A previous study reported that tail suspension
unloading resulted in the upregulation of sclerostin (20), which was similarly observed in the
present study in the occlusal hypofunction environment, since
occlusal hypofunction is also an unloading environment. Upon
extraction of the maxillary molars from the rats, the reduction in
the bite force exposed the mandible to unloading. RANKL and OPG are
recognized as positive and negative controllers of
osteoclastogenesis, respectively (37). In the present study, the protein
expression levels of RANKL were significantly increased in
osteocytes at the hypofunction side. This is consistent with the
study by Nakashima et al (38), which suggested that osteocytes are a
crucial in vivo source of RANKL required for
osteoclastogenesis. Conversely, the protein expression levels of
OPG were not significantly different at the hypofunction side, as
compared with the control side. It is hypothesized that the finding
of stronger staining for OPG at the hypofunction side in the
present study may have been the result of a negative feedback
mechanism aimed to control the RANKL-promoted osteoclastogenesis
process. These findings may suggest that bone resorption was
facilitated. In addition, decreased β-catenin and increased
sclerostin expression levels were observed in the occlusal
hypofunction environment. Since sclerostin is an antagonist of the
Wnt/β-catenin signaling pathway (39,40), the
upregulation of sclerostin at the occlusal hypofunction side may
have inhibited Wnt/β-catenin signaling activity, thereby reducing
bone formation, enhancing bone resorption and ultimately leading to
bone loss.
The association of occlusal hypofunction with bone
loss has been well-documented. The present study demonstrated that
sclerostin-mediated Wnt/β-catenin signaling inhibition may have a
crucial role in this process, and supplied one potential molecular
explanation for this phenomenon. The role of sclerostin-mediated
Wnt/β-catenin signaling in the regulation of unloading (tail
suspension)-induced bone responses has previously been described in
the long bone (20). However,
occlusal unloading differs from tail suspension unloading.
Typically, the mechanical stimulus is distributed among the teeth,
periodontal ligament, and throughout the alveolar bone. Therefore,
the loss of normal occlusal function leads not only to bone loss,
but also to atrophic alterations in the periodontal ligament
(41), which may also have an
important role in alveolar bone loss and remodeling due to
periodontal ligament fibroblasts responding to mechanical forces
(42). Thus, the occlusal
hypofunction model is required for jaw bone unloading
experiments.
Future studies are required to investigate the roles
of Wnt/β-catenin, as well as numerous other signaling pathways, in
the occlusal hypofunction status and their underlying mechanisms.
Sclerostin is emerging as a promising therapeutic target in bone
disease therapy (43). Various
animal studies demonstrated that the sclerostin antibody was
effective in preventing unloading-induced osteoporosis (44,45).
Thus, it can be hypothesized that anti-sclerostin treatment may be
able to protect against occlusal hypofunction-induced alveolar bone
loss, and its application in dental prosthetic rehabilitation may
be promising.
In conclusion, the present study demonstrated that
occlusal hypofunction-induced bone loss was associated with
sclerostin and Wnt/β-catenin signaling, in which upregulated
sclerostin may antagonize the activity of the Wnt/β-catenin
signaling pathway, thereby inhibiting bone formation and
accelerating bone resorption.
Acknowledgements
The present study was supported by the National
Nature Science Foundation of China (grant nos. 81371171, 11172190,
81541112 and 81371172) and the Science and Technology Department,
Sichuan, China (grant no. 2015FZ0074).
References
|
1
|
Recent advances in oral health. Report of
a WHO Expert Committee. World Health Organ Tech Rep Ser. 826:1–37.
1992.PubMed/NCBI
|
|
2
|
Müller F, Naharro M and Carlsson GE: What
are the prevalence and incidence of tooth loss in the adult and
elderly population in Europe? Clin Oral Implants Res. 18(Suppl 3):
S2–S14. 2007. View Article : Google Scholar
|
|
3
|
Harada S and Rodan G: Control of
osteoblast function and regulation of bone mass. Nature.
423:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jacobs CR, Temiyasathit S and Castillo AB:
Osteocyte mechanobiology and pericellular mechanics. Annu Rev
Biomed Eng. 12:369–400. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bikle DD and Halloran BP: The response of
bone to unloading. J Bone Miner Metab. 17:233–244. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimomoto Y, Chung CJ, Iwasaki HY,
Muramoto T and Soma K: Effects of occlusal stimuli on alveolar/jaw
bone formation. J Dent Res. 86:47–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
von Wowern N, Hjørting-Hansen E and
Stoltze K: Changes in bone mass in rat mandibles after tooth
extraction. Int J Oral Surg. 8:229–233. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ejiri S, Toyooka E, Tanaka M, Anwar RB and
Kohno S: Histological and histomorphometrical changes in rat
alveolar bone following antagonistic tooth extraction and/or
ovariectomy. Arch Oral Biol. 51:941–950. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuroda S, Mukohyama H, Kondo H, Aoki K,
Ohya K, Ohyama T and Kasugai S: Bone mineral density of the
mandible in ovariectomized rats: Analyses using dual energy X-ray
absorptiometry and peripheral quantitative computed tomography.
Oral Dis. 9:24–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elovic RP, Hipp JA and Hayes WC: Maxillary
molar extraction decreases stiffness of the mandible in
ovariectomized rats. J Dent Res. 73:1735–1741. 1994.PubMed/NCBI
|
|
11
|
Elovic RP, Hipp JA and Hayes WC: Maxillary
molar extraction causes increased bone loss in the mandible of
ovariectomized rats. J Bone Miner Res. 10:1087–1093. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Glass DA II and Karsenty G: Molecular
bases of the regulation of bone remodeling by the canonical Wnt
signaling pathway. Curr Top Dev Biol. 73:43–84. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tatsumi S, Ishii K, Amizuka N, Li M,
Kobayashi T, Kohno K, Ito M, Takeshita S and Ikeda K: Targeted
ablation of osteocytes induces osteoporosis with defective
mechanotransduction. Cell Metab. 5:464–475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weidauer SE, Schmieder P, Beerbaum M,
Schmitz W, Oschkinat H and Mueller TD: NMR structure of the Wnt
modulator protein sclerostin. Biochem Biophys Res Commun.
380:160–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Poole KE, van Bezooijen RL, Loveridge N,
Hamersma H, Papapoulos SE, Löwik CW and Reeve J: Sclerostin is a
delayed secreted product of osteocytes that inhibits bone
formation. FASEB J. 19:1842–1844. 2005.PubMed/NCBI
|
|
16
|
Bonewald LF and Johnson M: Osteocytes,
mechanosensing and Wnt signaling. Bone. 42:606–615. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Galli C, Passeri G and Macaluso GM:
Osteocytes and WNT: The mechanical control of bone formation. J
Dent Res. 89:331–343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paszty C, Turner CH and Robinson MK:
Sclerostin: A gem from the genome leads to bone-building
antibodies. J Bone Miner Res. 25:1897–1904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wijenayaka AR, Kogawa M, Lim HP, Bonewald
LF, Findlay DM and Atkins GJ: Sclerostin stimulates osteocyte
support of osteoclast activity by a RANKL-dependent pathway. PLoS
One. 6:e259002011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang
J, Li Y, Feng G, Gao X and He L: Sclerostin mediates bone response
to mechanical unloading through antagonizing Wnt/beta-catenin
signaling. J Bone Miner Res. 24:1651–1661. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Naveh GR, Lev-Tov CN, Zaslansky P, Shahar
R and Weiner S: Tooth-PDL-bone complex: Response to compressive
loads encountered during mastication-a review. Arch Oral Biol.
57:1575–1584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gerritsen AE, Allen PF, Witter DJ,
Bronkhorst EM and Creugers NH: Tooth loss and oral health-related
quality of life: A systematic review and meta-analysis. Health Qual
Life Outcomes. 8:1262010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schropp L, Wenzel A, Kostopoulos L and
Karring T: Bone healing and soft tissue contour changes following
single-tooth extraction: A clinical and radiographic 12-month
prospective study. Int J Periodontics Restorative Dent. 23:313–323.
2003.PubMed/NCBI
|
|
24
|
Tan WL, Wong TL, Wong MC and Lang NP: A
systematic review of post-extractional alveolar hard and soft
tissue dimensional changes in humans. Clin Oral Implants Res.
23(Suppl 5): S1–S21. 2012. View Article : Google Scholar
|
|
25
|
Sennerby L, Carsson GE, Bergman B and
Warfvinge J: Mandibular bone resorption in patients treated with
tissue-integrated prostheses and in complete-denture wearers. Acta
Odontol Scand. 46:135–140. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bodic F, Hamel L, Lerouxel E, Baslé MF and
Chappard D: Bone loss and teeth. Joint Bone Spine. 72:215–221.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Klein-Nulend J, Bakker AD, Bacabac RG,
Vatsa A and Weinbaum S: Mechanosensation and transduction in
osteocytes. Bone. 54:182–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bonewald LF: The amazing osteocyte. J Bone
Miner Res. 26:229–238. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bonewald LF: Mechanosensation and
transduction in osteocytes. Bonekey Osteovision. 3:7–15. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Robling AG, Niziolek PJ, Baldridge LA,
Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido
TM, Harris SE and Turner CH: Mechanical stimulation of bone in vivo
reduces osteocyte expression of sost/sclerostin. J Biol Chem.
283:5866–5875. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ellies DL, Viviano B, McCarthy J, Rey JP,
Itasaki N, Saunders S and Krumlauf R: Bone density ligand,
sclerostin, directly interacts with LRP5 but not LRP5G171V to
modulate Wnt activity. J Bone Miner Res. 21:1738–1749. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tu X, Rhee Y, Condon KW, Bivi N, Allen MR,
Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI and Bellido
T: Sost downregulation and local Wnt signaling are required for the
osteogenic response to mechanical loading. Bone. 50:209–217. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lewiecki EM: Role of sclerostin in bone
and cartilage and its potential as a therapeutic target in bone
diseases. Ther Adv Musculoskelet Dis. 6:48–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Virdi AS, Irish J, Sena K, Liu M, Ke HZ,
McNulty MA and Sumner DR: Sclerostin antibody treatment improves
implant fixation in a model of severe osteoporosis. J Bone Joint
Surg Am. 97:133–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moe SM, Chen NX, Newman CL, Organ JM,
Kneissel M, Kramer I, Gattone VH 2nd and Allen MR: Anti-sclerostin
antibody treatment in a rat model of progressive renal
osteodystrophy. J Bone Miner Res. 30:499–509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Costa AG, Bilezikian JP and Lewiecki EM:
The potential use of antisclerostin therapy in chronic kidney
disease-mineral and bone disorder. Curr Opin Nephrol Hypertens.
24:324–329. 2015.PubMed/NCBI
|
|
37
|
Tanaka Y, Nakayamada S and Okada Y:
Osteoblasts and osteoclasts in bone remodeling and inflammation.
Curr Drug Targets Inflamm Allergy. 4:325–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakashima T, Hayashi M, Fukunaga T, Kurata
K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, et
al: Evidence for osteocyte regulation of bone homeostasis through
RANKL expression. Nat Med. 17:1231–1234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Semënov M, Tamai K and He X: SOST is a
ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem.
280:26770–26775. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang
J, Harris SE and Wu D: Sclerostin binds to LRP5/6 and antagonizes
canonical Wnt signaling. J Biol Chem. 280:19883–19887. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Muramoto T, Takano Y and Soma K:
Time-related changes in periodontal mechanoreceptors in rat molars
after the loss of occlusal stimuli. Arch Histol Cytol. 63:369–380.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lekic P and McCulloch CA: Periodontal
ligament cell population: The central role of fibroblasts in
creating a unique tissue. Anat Rec. 245:327–341. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ke HZ, Richards WG, Li X and Ominsky MS:
Sclerostin and dickkopf-1 as therapeutic targets in bone diseases.
Endocr Rev. 33:747–783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shahnazari M, Wronski T, Chu V, Williams
A, Leeper A, Stolina M, Ke HZ and Halloran B: Early response of
bone marrow osteoprogenitors to skeletal unloading and sclerostin
antibody. Calcif Tissue Int. 91:50–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Spatz JM, Ellman R, Cloutier AM, Louis L,
van Vliet M, Suva LJ, Dwyer D, Stolina M, Ke HZ and Bouxsein ML:
Sclerostin antibody inhibits skeletal deterioration due to reduced
mechanical loading. J Bone Miner Res. 28:865–874. 2013. View Article : Google Scholar : PubMed/NCBI
|