Introduction
Hormone replacement therapy (HRT) may exert
beneficial effects in the bone of patients with postmenopausal
osteoporosis; however, the chronic use of HRT has been associated
with several risk factors (1). This
is particularly apparent for hormone-dependent gynecological
malignancies, and the appropriate usage of HRT has become a highly
controversial issue (1,2). Therefore, there is currently an urgent
requirement for non-estrogenic, bone protective compounds.
Phytoestrogens, which have similar although milder effects to those
of estradiol-17β benzoate, are involved in the maintenance of bone
mass in the post-menopausal period for their putative function as
osteoprotective agents (3). Numerous
traditional Chinese medicine (TCM) compounds, particularly
kidney-beneficial herbal medicines such as Zuogui Pill,
Rehmannia glutinosa, Ferula, in addition to various
extracts from food or TCM herbs such as isoflavones (e.g.
daidzein), have been suggested to prevent ovariectomy-induced bone
loss and other antiosteoporotic effects without influencing
hormones such as estrogen (3–6).
Phytoestrogenic isoflavones and their derivatives extracted from
soy have been shown to increase bone density in postmenopausal
women following high dietary intake (3–7).
The human diet may contain the phytochemicals
possessing antioxidant and anti-inflammatory properties, and
antioxidant nutrients may enhance bone formation and reduce the
production of free radicals that contribute to bone resorption
(8,9). Furthermore, the bone-protective effects
of estrogen may involve the suppression of inflammatory cytokines
such as interleukin-1β (IL-1β), IL-6 and tumor necrosis factor-α
(TNF-α) (10). The lowest incidence
of osteoporosis has been reported in the Mediterranean area within
Europe (11,12), due to the so-called Mediterranean
diet, which reportedly exerts a beneficial effect on gynecological
malignancies, such as breast and endometrial cancer (13,14).
Olive oil is one of the three most important and characteristic
components of the Mediterranean diet, which contains a series of
phenolic minor compounds such as hydroxytyrosol and oleuropein, and
is able to scavenge superoxide radicals and inhibit neutrophil
respiratory burst (15). The other
two primary components of the Mediterranean diet are fruits and
vegetables, and nuts and cereals.
To date, the oil extracts of numerous plant species
have been demonstrated to possess similar efficacy to 17β-estradiol
in suppressing bone loss due to ovariectomy and to have a marked
effect in anti-osteoporosis, for example of Korean safflower seed
oil, virgin coconut oil and garlic oil (16,17).
Furthermore, olive oil and its major phenolic compounds may exert a
marked effect in osteoporosis. A prior study by Puel et al
demonstrated that oleuropein and extra virgin olive oil were able
to elicit protective effects on bone loss in a model of ovariectomy
associated with inflammation, probably by modulating variables of
inflammation, such as α-1-acid glycoprotein (18). In another study, OVX rats were
administered oleuropein (a compound extracted from olive leaf) at
2.5, 5, 10 or 15 mg/kg body weight per day for 100 days, and plasma
fibrinogen concentration (g/l) was found to be significantly higher
in the OVX group compared with the SH group (19). A further increase was observed after
inflammation in estrogen-deficient animals, while this marker for
inflammation remained unchanged in intact rats (12,20,21).
This pattern was partially improved by polyphenol supplementation,
as fibrinogen was restored to values measured in OVX animals
without talc administration (22).
In this case, talc was used to stimulate and induce inflammation
(22). In addition, an extra virgin
olive oil total polyphenolic fraction (TPF) was isolated and its
effect on the bone loss attenuation was investigated, the result
showed that the TPF only slightly induced the uterine wet weight
(P=0.058) (23). Garia-Villalba
et al results suggest that postmenopausal women could be a
target population for the intake of olive phenolics in order to
prevent age-related and oxidative stress-related processes such as
osteoporosis (23).
Consequently, the aim of the present study was to
investigate the effects of olive oil supplementation in OVX rats
modelling post-menopausal osteoporosis. Furthermore, olive oil was
compared with E2 to determine whether olive oil has
functions at the uterine tissue level in a manner similar to
17β-estradiol.
Materials and methods
Animals and treatments
A total of 34 female Sprague-Dawley rats (weight,
190±20 g) were provided by the Division of Comparative Medicine of
Fuzhou General Hospital of Nanjing Command PLA [permission no. SCXK
(2012–0001); Fuzhou, China] were used for the present experiments.
All rats were maintained in individual cages in a
temperature-controlled room (21°C) with a 12-h light/dark cycle
with free access to water. The rats were fed a diet constructed in
the laboratory according to the normal nutritional dietary
requirement (59% carbohydrates, 7% fat, 21% protein and 13%
minerals and ash). The protocol was approved by the Ethics
committee of Fuzhou General Hospital of Nanjing Command PLA and was
conducted in accordance with current legislation on animal
experiments.
All rats were sham-operated or surgically
ovariectomized under anesthesia using chloral hydrate (4 ml/kg;
Fluka Chemie AG, Buchs, Switzerland) in 80 g/l saline solution (9 g
NaCl/l), and were randomly allocated into four groups: i)
Sham-operated control rats (sham group, n=8); ii) Ovariectomized
rats (OVX group, n=9); iii) Diethylstilbestrol-supplemented
ovariectomized rats (E2 group, n=8); and iv) olive
oil-supplemented ovariectomized rats (olive group, n=9). All rats
were orally administered with their allocated diet (1 ml/100 g) by
oral tubal feeding for 12 weeks. Sham and OVX groups were
administered daily with saline. E2 group mice received a
solution of diethylstilbestrol (0.0033 mg; Zhunzi H34021250; batch
number: 20100920; Hefei Lifeon Pharmaceutical Co., Ltd., Hefei,
China). Olive group mice received Terra Creta Kolymvari Chania
Cretei Extra Virgin Olive Oil (Terra Creta SA, Chania, Crete,
Greece), with the following component values: Acidity, <0.5%;
fatty acid composition, 79% monounsaturates and 7% polyunsaturates;
and trans fatty acid C18 composition, 1T≤0.05 (C18:2T + C18:3T;
integrated value ≤0.05). All administered substances were gavaged
at 1 ml/100 g once daily for 12 consecutive weeks. Body mass was
weighed once a week to adjust the dosage. To prevent hyperphagia
associated with ovariectomy, the OVX rats were pair-fed to the mean
intake of those in the sham group. At the end of the experiments,
animals were sacrificed using ether (Shanghai Lianshi Chemical
Reagent Co., Ltd., Shanghai, China) inhalation and
exsanguination.
Serum analyses
Blood samples were centrifuged (2,500 × g for 20
min) and the was serum stored at −20°C for further analysis. Serum
E2 levels were determined by radioimmunoassay kit (Fu
Sheng Industrial Co., Ltd., Shanghai, China), while serum IL-1β and
IL-6 levels was measured using an ELISA (Fu Sheng Industrial Co.,
Ltd.) double antibody sandwich method. The clinical laboratory of
Fuzhou General Hospital was entrusted to complete all of the above
test indexes according to the specific measurement of their
indicators by double-blind testing.
Bone mineral density (BMD)
measurements
The BMD of the lumbar spine was measured using a
Discovery dual-energy X-ray absorptiometer (DXA) (S/N 806409) bone
densitometer (Hologic, Inc., Bedford, MA, USA), equipped with
appropriate software for bone density assessment in small
laboratory animals. Unit: (g/cm2). The intra-assay and
inter-assay coefficients of variability for lumbar spine assays
were 0.22 and 0.24% respectively.
Femoral head visualization
Two left femoral heads of the proximal femur were
selected from each group rat, cut into 1×1×1 mm pieces and washed
in phosphate-buffered saline (PBS) (Fuzhou Maixin Bio, Co., Ltd.,
Fuzhou, China). The samples were then placed in 2.5% glutaric
aldehyde solution (Sinopharm Chemical Reagent Co., Ltd., Shanghai,
China) for 3 h at 4°C, decalcified in 5% ethylenediaminetetraacetic
acid (EDTA) (Fuzhou Maixin Bio, Co., Ltd.) for three weeks by
changing EDTA solution every week. Subsequently, the samples were
sent to the Department of Pathology to be fixed in 1% osmium
tetroxide, embedded and cut into thin sections with Epon812 (both
purchased from SPI Supplies, West Chester, PA, USA), double-stained
using uranyl acetate (China National Nuclear Corporation, Beijing,
China) and lead citrate (Sinopharm Chemical Reagent Co., Ltd.),
finally observed using transmission electron microscope (EM208S;
Philips, Amsterdam, Netherlands).
Uterine endothelium visualization
Samples of uterine tissues were fixed, embedded in
paraffin and cut into serial cross sections. The skin was cut
vertically in the median of the lower abdomen and the peritoneum
was opened into the abdominal cavity. The ‘Y’ type uterus was
extracted and adipose tissue removed. Wet weight was recorded and
the uterus index calculated (uterine wet weight/body weight). Then,
two embedded rat uteri were selected from each group, and made into
paraffin sections. The extent of endometrial hyperplasy was
observed under a light microscope [2506; Motic (Xiamen) Electric
Group Co., Ltd., Xiamen, China] and photographed using a microscope
with a digital camera (BX51; Olympus Corporation, Tokyo,
Japan).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical significance for data was determined using one-way
analysis of variance with post-hoc test, significance
calculated using a Least Significant Difference multiple range-test
to find inter-group significance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Comparison of serum E2
levels among groups
By analysis of variance indicated statistically
significant differences in E2 expression among groups
(F=9.934; overall significance, P=0.000). Serum E2
values in the olive group were not significantly different compared
with the sham group (P>0.05); however, they were significantly
increased compared with the OVX group (P=0.001), as shown in
Table I.
 | Table I.Serum E2 measured in
groups (mean ± standard deviation). |
Table I.
Serum E2 measured in
groups (mean ± standard deviation).
| Group | n | E2
(ng/ml) | Pa | Pb |
|---|
| Sham | 8 | 66.765±4.394 | – | 0.000 |
| OVX | 9 | 30.087±5.504 | 0.000 | – |
| E2 | 8 | 45.226±4.745 | 0.005 | 0.038 |
| Olive | 9 | 54.452±4.774 | 0.087 | 0.001 |
Comparison of serum IL-1β levels among
groups
Serum IL-1β concentration was similar in the sham,
E2 and olive groups (P>0.05). This marker was
increased in the OVX animals, but was mitigated in the olive and
E2 group rats (Table
II).
 | Table II.Serum IL-1β measured in groups (mean
± standard deviation). |
Table II.
Serum IL-1β measured in groups (mean
± standard deviation).
| Group | n | IL-1β (pg/ml) | Pa | Pb |
|---|
| Sham | 8 | 83.764±6.508 | – | 0.012 |
| OVX | 9 | 124.273±14.470 | 0.012 | – |
| E2 | 8 | 91.730±9.816 | 0.612 | 0.039 |
| Olive | 9 | 93.417±9.209 | 0.527 | 0.044 |
Comparison of serum IL-6 levels among
groups
In OVX group, IL-6 levels were significantly higher
than Sham group and E2 group, the difference was
statistically significant (P<0.01), but no statistical
significance with Olive group (P>0.05). There was not
statistically significant in E2 group compared with Sham
group (P>0.05), as shown in Table
III.
 | Table III.Serum IL-6 measured in groups (mean ±
standard deviation). |
Table III.
Serum IL-6 measured in groups (mean ±
standard deviation).
| Group | n | IL-6 (pg/ml) | Pa | Pb |
|---|
| Sham | 8 |
111.433±16.574 | – | 0.001 |
| OVX | 9 | 226.700±23.731 | 0.001 | – |
| E2 | 8 | 126.885±18.996 | 0.622 | 0.002 |
| Olive | 9 |
176.254±23.447 | 0.040 | 0.095 |
Comparison of absolutes values
(g/cm2) of BMD of the lumbar spine among groups
Lumbar spine BMD in the olive group was
significantly higher compared with the OVX group, but showed no
significant difference compared with the sham group. However,
lumbar spine BMD was not significantly different between the
E2 and OVX groups (P>0.05), as shown in Table IV.
 | Table IV.BMD of the lumbar spine among groups
(mean ± standard deviation). |
Table IV.
BMD of the lumbar spine among groups
(mean ± standard deviation).
| Group | n | BMD
(g/cm2) | Pa | Pb |
|---|
| Sham | 8 | 0.2476±0.0049 | – | 0.004 |
| OVX | 9 | 0.2262±0.0049 | 0.004 | – |
| E2 | 8 | 0.2291±0.0053 | 0.015 | 0.679 |
| Olive | 9 | 0.2431±0.0046 | 0.521 | 0.018 |
Femoral head under transmission
electron microscopy
Collagen fibers in the sham group showed light and
dark stripes, closely and neatly arranged. In addition, osteoblasts
and numerous Golgi apparatus, endoplasmic reticula and other
organelles could be seen, as shown in Fig. 1.
Collagen fibers in the OVX group were sparse,
exhibited bending and breaking, and numerous voids are visible,
also osteoclast were clearly visible, presenting pathological
features of osteoporosis, as shown in Fig. 1.
The arrangement of collagen fibers in the
E2 and olive groups improved compared with OVX group,
with organelles such as the rough endoplasmic reticulum,
mitochondria and Golgi apparatus being visible and active, as shown
in Fig. 1.
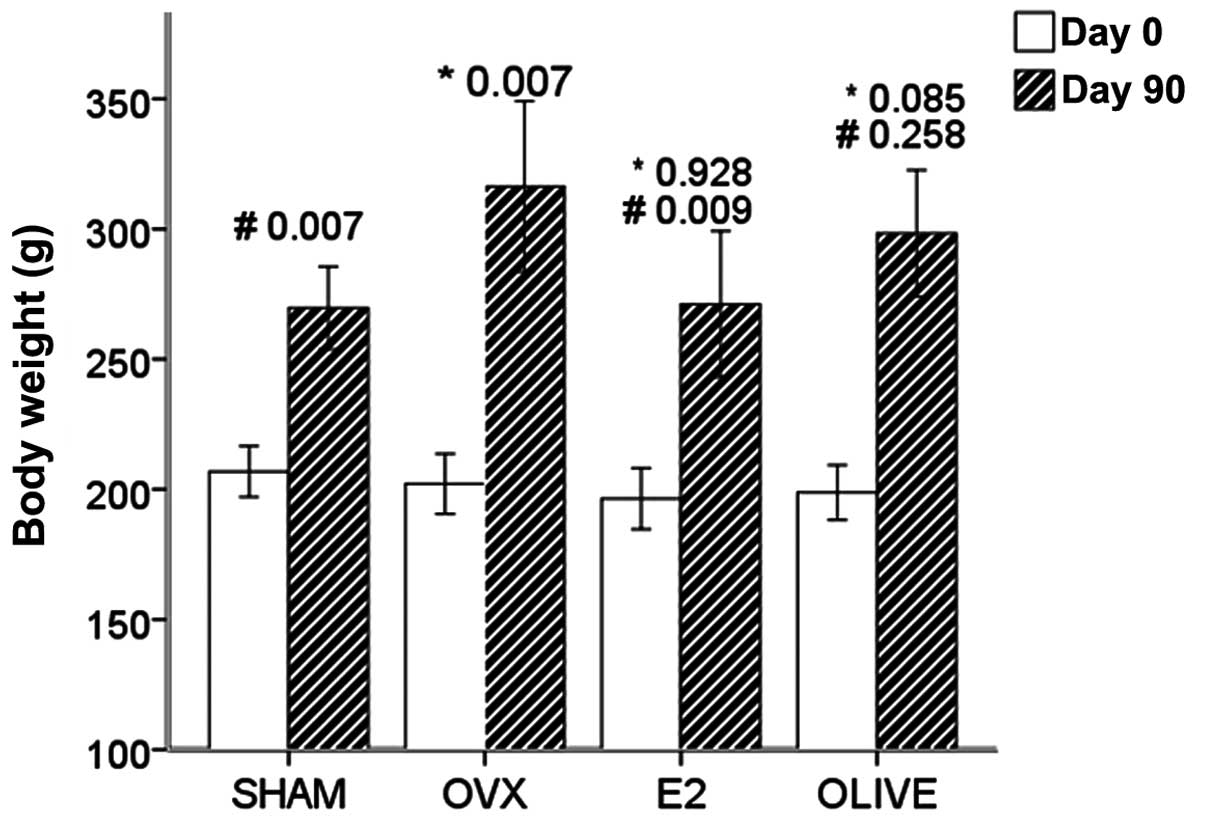
Body weight pre-treatment and
post-treatment
None of the groups exhibited a significant
difference in mean initial body weight (P>0.05). At 90 days
after operation, weights of OVX group rats were increased by
57.16%, which was increased compared with the sham group (30.95%).
The two groups showed a significant difference in body weight after
90 days (sham, 269.63±6.70 g vs. OVX, 316.33±14.20 g, P=0.007).
Weights of E2 group rats showed no significant
difference compared with the sham group rats, but were reduced
compared with the OVX group rats. Weights of the olive group rats
did not significantly differ from those of the OVX and sham groups
(P>0.05) (Fig. 2).
Uterine weight
Uterine weights and uterine index values in the OVX
and olive group rats were significantly decreased compared with the
sham group rats (P<0.001). Treatment of E2 group
significantly increased uterine weights, significantly different
with OVX group, but had no significant difference with sham group
(P>0.05). The E2 group exhibited no significant
difference compared with the OVX group (P>0.05) (Table V).
 | Table V.Uterine weight and uterine index
among groups (mean ± standard deviation). |
Table V.
Uterine weight and uterine index
among groups (mean ± standard deviation).
| Group | Uterine weight
(mg) | Uterine index
(mg/g) | Pa | Pb |
|---|
| Sham | 674.05±68.40 | 2.485±0.227 | – | 0.000 |
| OVX | 116.71±15.54 | 0.358±0.035 | 0.000 | – |
| E2 | 539.61±58.15 | 2.061±0.299 | 0.118 | 0.000 |
| Olive | 233.69±22.17 | 0.785±0.073 | 0.000 | 0.097 |
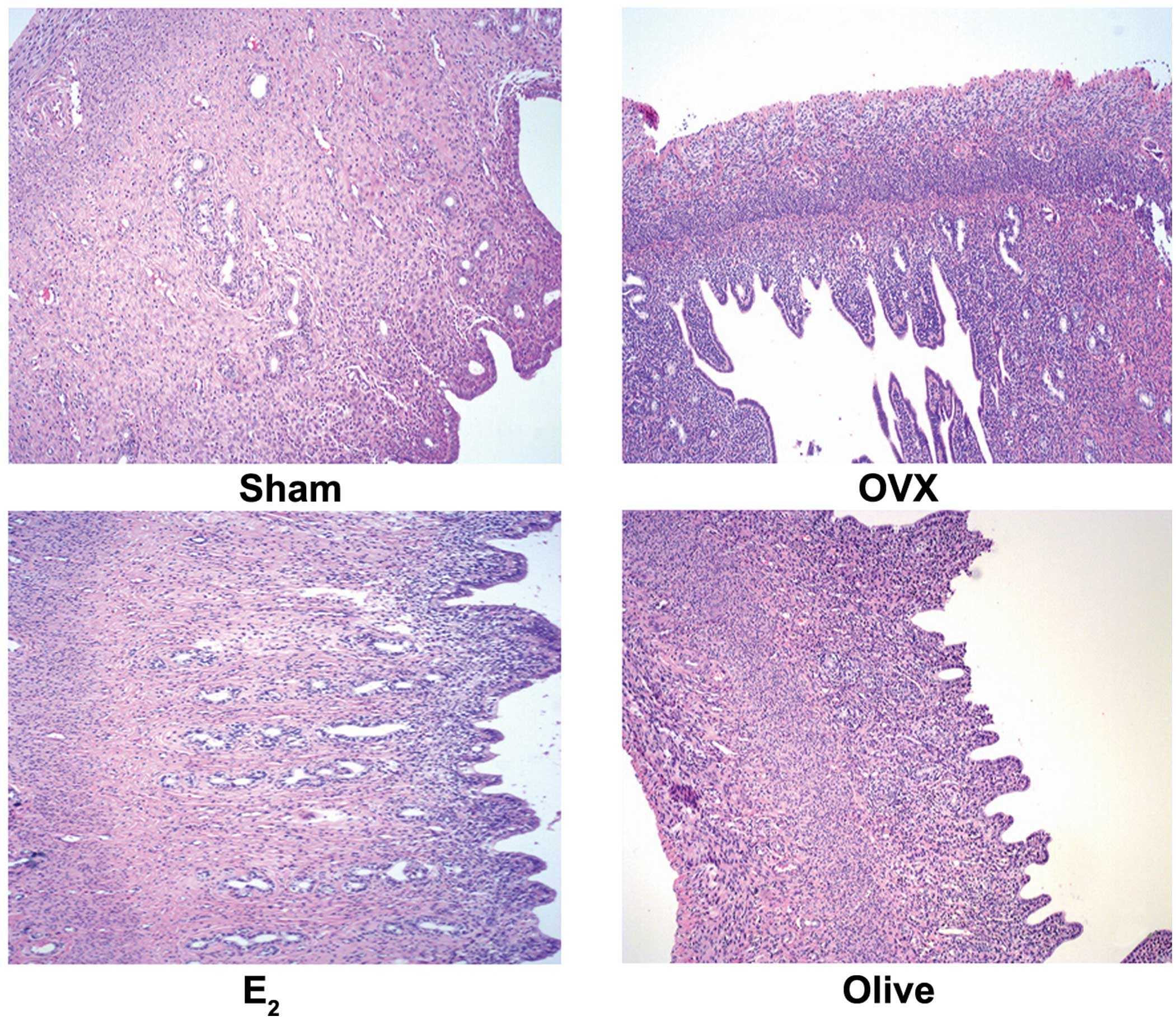
Comparison of histomorphometric
variables of uterine among groups
HE staining and visualization using light microscopy
at low magnification (magnification, ×40) revealed that the olive
and OVX groups showed thin endometria, with no proliferation.
However, in the E2 group the columnar epithelial
thickened, the number of glands increased, with evident hyperplasia
(Fig. 3).
Discussion
Ovarian, cervical and endometrial cancers are common
gynecological malignancies, whose incidence are increasing
(24–26). This is particularly evident in the
case of ovarian cancer, which poses a serious threat to female
health (25). With the continuous
development of medical diagnosis and treatment technology, the cure
rate and disease-free survival of patients has improved
significantly (27). However,
radical surgery and adjuvant chemotherapy-induced menopause
syndrome are becoming serious factors affecting the quality of life
of patients (28), particularly
osteoporosis, whose risk is significantly higher than natural
menopause, and the problems caused by its fracture in
postmenopausal women has been described as a ‘silent killer’
(29). Hormone replacement therapy
(HRT) has been publicly recognized as the first choice prevention
and treatment for postmenopausal osteoporosis, and the majority of
obstetrician-gynecologists are aware of the osteoporosis problem.
However, non-HRT users are often screened, and in a recent survey
69.4% of gynecologists speculated that HRT could increase the risk
of breast cancer, and 52.9% speculated that HRT may increase the
risk of endometrial cancer (30).
The appropriate use of HRT for the treatment of osteoporosis and
postoperative gynecological malignancies has become a highly
controversial issue, particularly in the case of hormone-dependent
gynecological malignancies (31).
Therefore, there is a requirement for treatments for osteoporosis
that have fewer undesirable side effects on the female reproductive
system as estradiol-17β benzoate.
The present results show that the administration of
olive oil to ovariectomized rats resulted in increased serum levels
of E2 compared with the OVX group, but with no
statistically significant difference compared with the sham group.
The serum IL-1β level in the olive group rats showed no
statistically significant difference compared with the sham and
E2 groups. The serum IL-6 level in the olive group rats
did not differ significantly compared with the OVX group. These
results indicate that olive oil supplementation can result in
increased serum E2 and decreased IL-1β levels. By
contrast, diethylstilbestrol supplementation of the ovariectomized
rats increased serum E2 and decrease IL-1β and IL-6
levels.
Ovariectomy induced a significant reduction in total
BMD (OVX, 0.2262±0.0049 vs. sham, 0.2476±0.0049 g/cm2;
P=0.004). However, olive oil reduced this bone loss and showed no
significant difference compared with the E2 group
(olive, 0.2431±0.0046 vs. E2 0.2291±0.0053
g/cm2; P=0.053). The arrangement of collagen fibers in
the olive group were more tightly packed and regular compared with
the OVX group, while organelles such as the rough endoplasmic
reticulum, mitochondria and Golgi apparatus were visible and
active, indicating micro proliferation of bone.
Estrogen deficiency induces a mild increase in the
production of proinflammatory cytokines, such as IL-1β, IL-6, and
TNF-α, systemically and locally, which promotes osteoclastogenesis
in the bone marrow (32,33). IL-1β and IL-6 are proinflammatory
cytokines involved in bone turnover. IL-1β is a potent stimulant of
bone resorption, acting directly on osteoclasts and osteoclast
precursors (34). Numerous studies
have investigated the associations between IL-1β gene polymorphisms
and susceptibility to osteoporosis and BMD in various populations
(35–36). A prior study enrolled 226
postmenopausal women with a diagnosed BMD T-score <-2.5 standard
deviation (SD) and 224 postmenopausal women with a BMD T-score
>-2.5 SD to investigate the associations between cytokine gene
polymorphisms (IL-1β, IL-2 and IL-6) and BMD values (37). The result suggested that among the
women with T-scores <-2.5 SD, the BMD values were significantly
lower in the carriers of the IL-6 GG genotype compared with those
with the CC and GC genotypes. Furthermore, there was a
statistically significant association between the IL-6 −174 G/C
polymorphism and osteoporosis in postmenopausal women (37). As the immune system plays such a
critical role in the pathophysiology of postmenopausal
osteoporosis, cytokines have the ability to influence each other;
just as a mild increase in the production of proinflammatory
cytokines would be found in a patient with osteoporosis, an
increase in proinflammatory cytokines may exacerbate osteoporosis
(37). Terauchi has suggested that
counteracting these interactions could be a novel strategy for
osteoporosis treatment (38). Puel
et al showed that the daily consumption of the major
phenolic compounds in olive oil for 84 days modulated bone loss in
an ovariectomy/inflammation experimental model, concluding that
polyphenol consumption of olive oil may be associated with the
mitigation of bone loss (22).
The aforementioned results suggest that olive oil is
able to increase E2 levels, and had positive effects on
proinflammatory cytokines IL-1β and bone loss, as indicated by the
collagen fibers of femoral, indicating that olive oil
supplementation in OVX-rats attenuated ovariectomy-induced
osteoporosis. A possible reason for this improvement in bone loss
could be attributed to the elevated levels of E2 and the
high content of monounsaturated fatty acid in olive oil, which has
been reported to affect BMD (39).
However, as supplementation with diethylstilbestrol was also able
to increase serum E2 and decrease IL-1β and IL-6 levels
in the ovariectomized rats, the estrogenic effects were more marked
compared with olive oil. It is possible that the total quantity of
olive oil administered in this experiment were insufficient to
equal the effects of diethylstilbestrol.
The body weights of the E2 group rats
were similar to the sham group rats, but reduced compared with the
OVX group rats. Body weights of the olive group rats showed no
significant difference compared with sham group rats (P>0.05),
suggesting that olive oil may not share the positive effect of
controlling body weight increase induced by ovariectomy as
exhibited by estrogen supplement. A diet rich in unsaturated fatty
acids from olive oil reduces waist girth, a significant indicator
of metabolic disease, in addition to reducing body mass index
(40). In addition, a study supports
the hypothesis that extra virgin olive oil phenols protect low
density lipoproteins in the plasma against oxidation (41).
Uterine wet weight and uterine index values of the
olive group rats decreased compared with sham group (P<0.01),
but showed no significant difference compared with the OVX group
(P>0.05). By contrast, the uterine weight and uterine index of
the E2 group rats significantly increased compared with
the OVX group (P<0.01), and the uterine index was not
statistically significant compared with the sham group.
Furthermore, the endometria in the olive group were atrophic,
thinner and the number of glands was reduced, as imaged using a
light microscope. The endometria exhibited hyperplasia in the
E2 group, and were significantly different compared with
the olive and OVX groups. These results suggest that olive oil had
no significant or only a mild effect on the uterus, indicating that
it may be safe to use as a form of hormone replacement therapy in
treating female genital mutilation. The results may be similar to
Keiler AM and colleagues' study, in which female Lewis rats
ovariectomized and fed a diet enriched with a total phenolic
extract of extra virgin olive oil in a concentration of 800 mg/kg
diet for 12 weeks (42). The results
showed that oleocanthal, a compound of the polyphenolic fraction,
showed a higher relative estrogen receptor binding affinity to the
estrogen receptor α (ERα) compared to the ERβ. While the total
polyphenolic fraction (TFP) only mildly increased the uterine wet
weight (490.7±53.7 vs. 432.7±23 mg/kg body weight; P=0.058), it
regulated estrogen response genes in the uterus (progesterone
receptor, antigen identified by monoclonal antibody Ki67,
complement C3). These results suggested that the administration of
extra virgin olive oil polyphenols regulated uterine estrogen
response marker genes in an E2-agonistic manner
(42).
Olive oil may be rich in phytoestrogens, similar to
other plants or their ingredient extracts. Traditional Chinese
medicinal herbs such as Rehmannia glutinosa and
Ferula, or oils extracted from various plant species, which
have similar though milder effects to that of estradiol-17β
benzoate, may be associated with the maintenance of bone mass in
the post-menopausal period due to their putative function as
osteoprotective agents (42).
Estrogen exerts certain effects by binding to
different estrogen receptors, including ERα and ERβ. ERα is
predominant in a number of tissues and is primarily involved in the
reproductive system, whereas ERβ is expressed in numerous tissues
including bone (34). ERα and ERβ
may be present in rat and human osteoblasts in cortical and
trabecular compartments of bone (36,37), and
a number of phytoestrogens, including genistein and coumestrol,
show a higher affinity to ERβ (20).
A possible explanation for olive oil exhibiting bone
protective effects but no estrogenic effects may be its
phytoestrogen effect; olive oil is structurally related to
17β-estradiol, but has much stronger affinity for ERβ than
17β-estradiol. As Annekathrin Martina Keiler do has proved the
polyphenols impact on estrogen response genes because of the
aromatic ring structurally related to 17β-estradiol binding to
estrogen receptors. Seidlova-Wuttke et al previously showed
that β-ecdysone has an antiosteoporotic effect which does not
involve the activation of ER in ovariectomized rats. Thus, olive
oil may a exert a similar bone protective effect, with no
associated estrogenic effects (21).
Furthermore, olive oil also has anti-cancer effect
as there has been reported that olive oil contains a number of
compounds which by their antioxidative action reduce the risk of
cell damage and their consequential uncontrolled growth and
division (43). Therefore, olive oil
may be more suitable to the treatment of osteoporosis associated
with postoperative gynecological malignancies, displaying desirable
estrogenic effects but with fewer undesirable side effects.
In conclusion, the present study demonstrates the
beneficial effects of olive oil on osteoporosis via serum
proinflammatory cytokines and lumbar spine BMD. However, further
research is required in order to determine the mechanism of its
effects, possibly using an ER-ligand binding assay or
phytoestrogen.
Acknowledgements
This study was supported by the Nanjing Command
Medical Science and Technology Innovation Project (no.
11MA111).
References
|
1
|
Rozenberg S, Murillo D, Gevers R and
Vandromme J: Propensity of gynaecologists towards osteoporosis
management and treatment. Maturitas. 53:483–488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barakat RR, Bundy BN, Spirtos NM, Bell J
and Mannel RS: Gynecologic Oncology Group Study: Randomized
double-blind trial of estrogen replacement therapy versus placebo
in stage I or II endometrial cancer: A Gynecologic Oncology Group
Study. J Clin Onco1. 24:587–592. 2006. View Article : Google Scholar
|
|
3
|
Lim DW and Kim YT: Dried Root of
Rehmannia glutinosa prevents bone loss in ovariectomized
rats. Molecules. 18:5804–5813. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu MJ, Li Y, Pan JH, Liu H, Wang SJ, Teng
JR, Zhao HY and Ju DH: Effects of zuogui pill (see text) on Wnt
signal transduction in rats with glucocorticoid-induced
osteoporosis. J Tradit Chin Med. 31:98–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Palumbo C, Ferretti M, Bertoni L, Cavani
F, Resca E, Casolari B, Carnevale G, Zavatti M, Montanari C,
Benelli A and Zanoli P: Influence of ferutinin on bone metabolism
in ovariectomized rats. I: Role in preventing osteoporosis. J Bone
Miner Metab. 27:538–545. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tyagi AM, Srivastava K, Sharan K, Yadav D,
Maurya R and Singh D: Daidzein prevents the increase in
CD4+CD28 null T cells and B lymphopoesis in
ovariectomized mice: A Key Mechanism for anti-osteoclastogenic
effect. PLoS One. 6:e212162011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mei J, Yeung SS and Kung AW: High dietary
phytoestrogen intake is associated with higher bone mineral density
in postmenopausal but not premenopausal women. J Clin Endocrinol
Metab. 86:5217–5221. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Puel C, Mardon J, Kati-Coulibaly S,
Davicco MJ, Lebecque P, Obled C, Rock E, Horcajada MN, Agalias A,
Shaltsounis LA and Coxam V: Black Lucques olives prevented bone
loss caused by ovariectomy and talcgranulomatosis in rats. J Brit
Nutri. 97:1012–1020. 2007. View Article : Google Scholar
|
|
9
|
Rendina E, Hembree KD, Davis MR, Marlow D,
Clarke SL, Halloran BP, Lucas EA and Smith BJ: Dried plum's unique
capacity to reverse bone loss and alter bone metabolism in
postmenopausal osteoporosis model. J PLoS One. 8:e605692013.
View Article : Google Scholar
|
|
10
|
Kim HM, An CS, Jung KY, Choo YK, Park JK
and Nam SY: Rehmannia glutinosa inhibits tumour necrosis
factor-alpha and interleukin-1 secretion from mouse astrocytes.
Pharmacol Res. 40:171–176. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanis JA: The incidence of hip fracture in
Europe. Osteoporos Int. 3(Suppl 1): S10–S15. 1993. View Article : Google Scholar
|
|
12
|
Benetou V, Orfanos P, Pettersson-Kymmer U,
Bergström U, Svensson O, Johansson I, Berrino F, Tumino R, Borch
KB, Lund E, et al: Mediterranean diet and incidence of hip
fractures in a European cohort. Osteoporos Int. 24:1587–1598. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buckland G, Travier N, Cottet V, González
CA, Luján-Barroso L, Agudo A, Trichopoulou A, Lagiou P,
Trichopoulos D, Peeters PH, et al: Adherence to the mediterranean
diet and risk of breast cancer in the European prospective
investigation into cancer and nutrition cohort study. Int J Cancer.
132:2918–2927. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dalvi TB, Canchola AJ and Horn-Ross PL:
Dietary patterns, Mediterranean diet, and endometrial cancer risk.
Cancer Causes Control. 18:957–966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Omar SH: Oleuropein in olive and its
pharmacological effects. Sci Pharm. 78:133–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mukherjee M, Das AS, Das D, Mukherjee S,
Mitra S and Mitra C: Effects of garlic oil on postmenopausal
osteoporosis using ovariectomized rats: Comparison with the effects
of lovastatin and 17beta-estradiol. Phytother Res. 20:21–27. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mukherjee M, Das AS, Mitra S and Mitra C:
Prevention of bone loss by oil extract of garlic (Allium
sativum Linn) in an ovariectomized rat model of osteoporosis.
Phytother Res. 18:389–394. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Puel C, Mardon J, Agalias A, Davicco MJ,
Lebecque P, Mazur A, Horcajada MN, Skaltsounis AL and Coxam V:
Major phenolic compounds in olive oil modulate bone loss in an
ovariectomy/inflammation experimental model. J Agric Food Chem.
56:9417–9422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Puel C, Mathey J, Agalias A,
Kati-Coulibaly S, Mardon J, Obled C, Davicco MJ, Lebecque P,
Horcajada MN, Skaltsounis AL and Coxam V: Dose-response study of
effect of oleuropein, an olive oil polyphenol, in an
ovariectomy/inflammation experimental model of bone loss in the
rat. Clin Nutr. 25:859–868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moreira AC, Silva AM, Santos MS and Sardão
VA: Phytoestrogens as alternative hormone replacement therapy in
menopause: What is real, what is unknown. J Steroid Biochem Mol
Biol. 143:61–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seidlova-Wuttke D, Christel D, Kapur P,
Nguyen BT, Jarry H and Wuttke W: Beta-ecdysone has bone protective
but no estrogenic effects in ovariectomized rats. Phytomedicine.
17:884–889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Puel C, Quintin A, Agalias A, Mathey J,
Obled C, Mazur A, Davicco MJ, Lebecque P, Skaltsounis AL and Coxam
V: Olive oil and its main phenolic micronutrient (oleuropein)
prevent inflammation-induced bone loss in the ovariectomized rat.
Br J Nutr. 92:119–127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
García-Villalba R, Larrosa M, Possemiers
S, Tomás-Barberán FA and Espín JC: Bioavailability of phenolics
from an oleuropein-rich olive (Olea europaea) leaf extract
and its acute effect on plasma antioxidant status: Comparison
between pre- and postmenopausal women. Eur J Nutr. 53:1015–1027.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dietl J: Revisiting the pathogenesis of
ovarian cancer: the central role of the fallopian tube. Arch
Gynecol Obstet. 289:241–246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Acharya UR, Sree SV, Saba L, Molinari F,
Guerriero S and Suri JS: Ovarian tumor characterization and
classification using ultrasound-a new online paradigm. J Digit
Imaging. 26:544–553. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cormio A, Cormio G, Musicco C, Sardanelli
AM, Gasparre G and Gadaleta MN: Mitochondrial changes in
endometrial carcinoma: possible role in tumor diagnosis and
prognosis. Oncol Rep. 33:1011–1018. 2015.PubMed/NCBI
|
|
27
|
Poveda A, Ray-Coquard I, Romero I,
Lopez-Guerrero JA and Colombo N: Emerging treatment strategies in
recurrent platinum-sensitive ovarian cancer: focus on trabectedin.
Cancer Treat Rev. 40:366–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nishio K, Tanabe A, Maruoka R, Nakamura K,
Takai M, Sekijima T, Tunetoh S, Terai Y and Ohmichi M: Bone mineral
loss induced by anticancer treatment for gynecological malignancies
in premenopausal women. Endocr Connect. 2:11–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang SF, Hong CM and Yang RS: The
performance of an online osteoporosis detection system a
sensitivity and specificity analysis. J Clin Nurs. 23:1803–1809.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Yang X, Li X, He X and Zhao Y:
Knowledge and personal use of menopausal hormone therapy among
Chinese Obstetrician-gynecologists: Results of a survey. Menopause.
21:1190–1196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stavraka C, Maclaran K, Gabra H, Agarwal
R, Ghaem-Maghami S, Taylor A, Dhillo WS, Panay N and Blagden SP: A
study to evaluate the cause of bone demineralization in
gynecological cancer survivors. Oncologist. 18:423–429. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Orsal E, Halici Z, Bayir Y, Cadirci E,
Bilen H, Ferah I, Aydin A, Ozkanlar S, Ayan AK, Seven B and Ozaltin
S: The role of carnitine on ovariectomy and inflammation-induced
osteoporosis in rats. Exp Biol Med. 238:1406–1412. 2013. View Article : Google Scholar
|
|
33
|
Brincat SD, Borg M, Camilleri G and
Calleja-Agius J: The role of cytokines in postmenopausal
osteoporosis. Minerva Ginecol. 66:391–407. 2014.PubMed/NCBI
|
|
34
|
Knudsen S, Harsløf T, Husted LB, Carstens
M, Stenkjaer L and Langdahl BL: The effect of interleukin-1alpha
polymorphisms on bone mineral density and the risk of vertebral
fractures. Calcif Tissue Int. 80:21–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim JG, Lim KS, Ku SY, Kim SH, Choi YM and
Moon SY: Relations between interleukin-1, its receptor antagonist
gene polymorphism and bone mineral density in postmenopausal Korean
women. J Bone Miner Metab. 24:53–57. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen HY, Chen WC, Wu MC, Tsai FJ and Lin
CC: Interleukin-1beta and interleukin-1 receptor antagonist gene
polymorphism in postmenopausal women: Correlation to bone mineral
density and susceptibility to osteoporosis. Maturitas. 44:49–54.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Czerny B, Kaminski A, Kurzawski M, Kotrych
D, Safranow K, Dziedziejko V, Bohatyrewicz A and Pawlik A: The
association of IL-1beta, IL-2 and IL-6 gene polymorphisms with bone
mineral density and osteoporosis in postmenopausal women. Eur J
Obstet Gynecol Reprod Biol. 149:82–85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Terauchi M: Role of the immune system in
the pathophysiology of postmenopausal osteoporosis. Nihon Rinsho.
69:1215–1219. 2011.(In Japanese). PubMed/NCBI
|
|
39
|
Saleh NK and Saleh HA: Olive oil
effectively mitigates ovariectomy-induced osteoporosis in rats. BMC
Complement Altern Med. 11:102011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Paniagua JA, de la Gallego Sacristana A,
Romero I, Vidal-Puig A, Latre JM, Sanchez E, Perez-Martinez P,
Lopez-Miranda J and Perez-Jimenez F: Monounsaturated fat-rich diet
prevents central body fat distribution and decreases postprandial
adiponectin expression induced by a carbohydrate-rich diet in
insulin-resistant subjects. Diabetes Care. 30:1717–1723. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Leenen R, Roodenburg AJ, Vissers MN,
Schuurbiers JA, van Putte KP, Wiseman SA and van de Put FH:
Supplementation of plasma with olive oil phenols and extracts:
Influence on LDL oxidation. J Agric Food Chem. 27:1290–1297. 2002.
View Article : Google Scholar
|
|
42
|
Keiler AM, Zierau O, Bernhardt R,
Scharnweber D, Lemonakis N, Termetzi A, Skaltsounis L, Vollmer G
and Halabalaki M: Impact of a functionalized olive oil extract on
the uterus and the bone in a model of postmenopausal osteoporosis.
Eur J Nutr. 53:1073–1081. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Keiler AM, Zierau O, Bernhardt R,
Scharnweber D, Lemonakis N, Termetzi A, Skaltsounis L, Vollmer G
and Halabalaki M: Impact of a functionalized olive oil extract on
the uterus and the bone in a model of postmenopausal osteoporosis.
Eur J Nutr. 53:1073–1081. 2014. View Article : Google Scholar : PubMed/NCBI
|