Introduction
Acute lung injury (ALI) is a life-threatening
disease that is characterized by acute onset, pulmonary
inflammation, edema due to increased vascular permeability and
severe hypoxemia (1). The incidence
of ALI in the USA is 79/100,000, with an overall mortality count
that is comparable to that of breast cancer (2). A previous study has demonstrated that
lung injury caused by direct factors, including hypoxaemia and
trauma, results in significant alveolar collapse, damage and edema
(3). Acute hypoxia is a crucial
cause of ALI, which may cause the body to produce extensive
vasoconstriction, including a series of complicated changes to the
internal environment (4).
In ALI, the inflammatory response to a primary
infection in the lungs or systemic inflammation is mediated by a
complex network of cytokines (5).
Levels of proinflammatory cytokines, such as interleukin (IL)-1β,
tumor necrosis factor (TNF)-α, IL-6 and IL-8, and anti-inflammatory
cytokines, such as IL-1 receptor antagonist, IL-10 and IL-13, are
elevated in the plasma or bronchoalveolar lavage fluid (BALF) in
cases of ALI, indicating that a balance of these mediators
determines the development of ALI (6–8).
Pulmonary surfactant consists of phospholipids and
surfactant-specific proteins that determine the ability of a
surfactant to reduce surface tension and thereby prevent alveolar
collapse. Phospholipids are released from the lamellar bodies of
alveolar type II cells and interact with surfactant proteins to
form large aggregates. When alveolar type II cells are damaged, the
synthesis and recirculation of surfactant is reduced (9).
Sevoflurane is among the most commonly used general
anesthetics in clinical practice. Schwarzkopf et al
(10) hypothesized that inhaled
anesthetics may inhibit hypoxic pulmonary vasoconstriction and
aggravate pulmonary shunt, interfere with alveolar type II
epithelial cell metabolism and reduce the production of pulmonary
surfactants. Therefore, sevoflurane may aggravate lung injury. In
recent years, thoracic epidural anesthesia (TEA) has been widely
used in major cardiac surgery (11).
Compared with general anesthesia alone, general anesthesia combined
with TEA may reduce respiratory system complications (12). However, the influence of TEA on
hypoxia-induced ALI remains unclear.
In the present study, it was hypothesized that TEA
would exert a protective effect against hypoxia-induced lung
injury. In order to investigate this hypothesis, an ALI model was
established in rabbits and the impact of TEA on a number of
inflammatory factors was evaluated, including the serum
concentrations of IL-6, IL-8 and IL-10. Furthermore, the mRNA
expression levels of IL-6, IL-8 and IL-10 in the rabbit lung
tissues were quantified. Finally, the impact of ALI and TEA on
pulmonary surfactants and the ultrastructure in the rat lung tissue
were investigated using microscopy and histological techniques.
Materials and methods
Animals and sampling
This study was conducted in strict accordance with
the recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (Eighth Edition,
2011). The animal use protocol was reviewed and approved by the
Institutional Animal Care and Use Committee of Fudan University
(Shanghai, China). A total of 56 New Zealand male white rabbits
(age, 4–5 months old; weight, 2–3 kg) were provided by the Animal
Center of Fudan University. Anesthesia was induced with a 30-mg/kg
intravenous dose of pentobarbital (P3761; Sigma-Aldrich, St. Louis,
MO, USA). Following tracheotomy and intubation, the right carotid
artery was cannulated for blood sampling. The auricular vein was
cannulated for the administration of fluids and drugs.
Epidural catheter insertion for
TEA
The first thoracic spinous process of the rabbits
was upright and rapidly located. An epidural catheter was inserted
by removing the fifth or sixth thoracic spinous process by vertical
needling and was advanced 1 cm cephalad into the epidural space.
Subsequently, 0.3 ml contrast medium (60% Conray; Shanghai Sangon
Biological Engineering Co., Ltd., Shanghai, China) was injected
into the epidural space and 16-slice computed tomography (GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA) and epidurography was
performed to confirm catheterization.
Grouping and study protocol
The rabbits were allocated at random into four
groups (n=14 per group): Control group (Group C), hypoxia group
(Group H), sevoflurane group (Group S) and the combined
sevoflurane-epidural anesthesia group (Group ES). Group ES received
3 mg/kg 2% lidocaine (070310; Shanghai Fuda Pharmaceutical Co.,
Ltd, Shanghai, China) and Groups C, H and S received an identical
volume of saline injected via the epidural catheter, followed by
the injection of an identical dose every 1 h. All animals
maintained spontaneous breathing [fraction of inspired oxygen
(FiO2), 21%] and were then mechanically ventilated using
an ALC-V8 Rodent Ventilator (Shanghai Alcott Biotech Co., Shanghai,
China). For Groups H, S and ES, the proportion of O2 in
N2 was 14%; and for Group C the FiO2 was 21%.
For all four groups, the tidal volume was 10–12 ml/kg, the
respiratory rate was 25 bpm and the inspiratory-to-expiratory time
ratio was 1:2. Sevoflurane (00784 S046F712; Baxter International
Inc., Deerfield, IL, USA) was administered to the rabbits in Groups
S and ES via inhalation at 1 minimum alveolar concentration using a
Capnomac Ultima Anesthesia Monitor (Datex Ohmeda Oy, Helsinki,
Finland). Anesthesia was maintained with 0.6 mg/kg/h vecuronium
(250393; Organon Pharmaceutical Co. Ltd., Nanjing, China) and 10
ml/kg/h saline.
Establishment of the ALI model
The ALI model was considered to have been
successfully induced after an arterial oxygen partial pressure
(PaO2)/FiO2 value of <300 was obtained.
Blood gas analysis was performed every 5 min using the i-STAT blood
gas analysis system (Baxter Healthcare Corporation, Bloomington,
IN, USA) following hypoxia until ALI was induced. At 3 h after
reaching the ALI standard, the experiment was terminated. Blood gas
analysis was performed during spontaneous breathing (T0,
baseline) and at 15, 30, 60, 120 and 180 min following ALI
induction (T1–5, respectively) in Groups H, S and ES,
and at the corresponding time of mechanical ventilation in Group
C.
Detection of inflammatory factors and
mRNA expression
At T0 and T5, 5 ml blood
samples were obtained and centrifuged for 10 min at 700 × g. The
serum was stored at −70°C for subsequent evaluation of the levels
of IL-6, IL-8 and IL-10. After completing these steps, blood was
extracted from the right carotid arterial catheter, whilst
anaesthetized, until the animals died. The chest wall was then
opened and the lungs were excised. Pieces of lung (1×1 cm) were
extracted and homogenized in order to measure the mRNA expression
levels of IL-6, IL-8 and IL-10 using reverse
transcription-quantitative polymerase chain reaction analysis, as
previously described (13) using an
ABI 7300 Real-Time PCR system (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Primers were synthesized by Shanghai Sangon
Biological Engineering Co., Ltd., as follows: IL-6, upstream
5′-GAGAAAAGAGTTGTGCAATGGC-3′ and downstream
5′-ACTAGGTTTGCCGAGTAGACC-3′; IL-8, upstream
5′-TTTGGAGCAGAGAGGAGGCAATG-3′ and downstream
5′-ACCACAATTCTGTCTTTCACGGGG-3′; and IL-10, upstream
5′-CATGCCTGGCTCAGCACTGC-3′ and downstream
5′-GGGAACTGAGGTATCAGAGG-3′. Thermal cycling was performed as
follows, 95°C for 15 min followed by 40 cycles of 94°C for 15 s and
60°C for 60 s. Rabbit enzyme-linked immunosorbent assay (ELISA)
kits were used to determine the serum concentrations of IL-6
(YS25008) IL-8 (YS25011) and IL-10 (YS25012; R&D Systems, Inc.,
Minneapolis, MN, USA).
Measurement of lung dry/wet weight
(D/W) ratio and histological examination
Wet weights of some lung samples (0.5×0.5 cm) were
measured and the samples were then placed in a thermostatic
incubator at 60°C for 48 h for measurement of the dry weight
(PYX-DHS-500; Shanghai Yuejin Medical Instruments Co., Ltd.,
Shanghai, China). Other samples were fixed in glutaraldehyde
(Sigma-Aldrich) and cut into 6 µm sections prior to examination
using a DVM6 optical microscope (Leica Microsystems GmbH, Wetzlar,
Germany) and H-600 transmission electron microscope (Hitachi, Ltd.,
Tokyo, Japan).
Evaluation of BALF
Bronchoalveolar lavage was performed twice
consecutively with 50 ml 0.9% NaCl each time. The fluid was
instilled via the trachea into the lungs and immediately withdrawn.
Following centrifugation in a cytocentrifuge for 10 min at 500 × g
(80–2B; Shanghai Anting Scientific Instrument Factory, Shanghai,
China), the supernatant fluid (BALF) was frozen and stored at −70°C
in a deep cryogenic refrigerator (Sanyo Electric Co., Ltd., Tokyo
Japan) for further analyses. Total phospholipid (TPL), saturated
phosphatidylcholine (SatPC) and total protein (TP) levels in the
BALF were measured using Bartlett's (14), Mason's (15) and Lowry's (16) methods, respectively. The SatPC/TPL
and SatPC/TP ratios represented the activity of pulmonary
surfactant.
Statistical analysis
SPSS software, version 11.5 (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. Results are expressed
as the mean ± standard deviation. Data were compared using one-way
analysis of variance and the t-test (Student-Newman-Keuls method).
Non-normally distributed data were tested using Kruskal-Wallis
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Animal model establishment
Rabbit gender and weight were not significantly
different between the groups (P>0.05). ALI models were induced
at 15 min after hypoxia in Groups H, S and ES and was maintained at
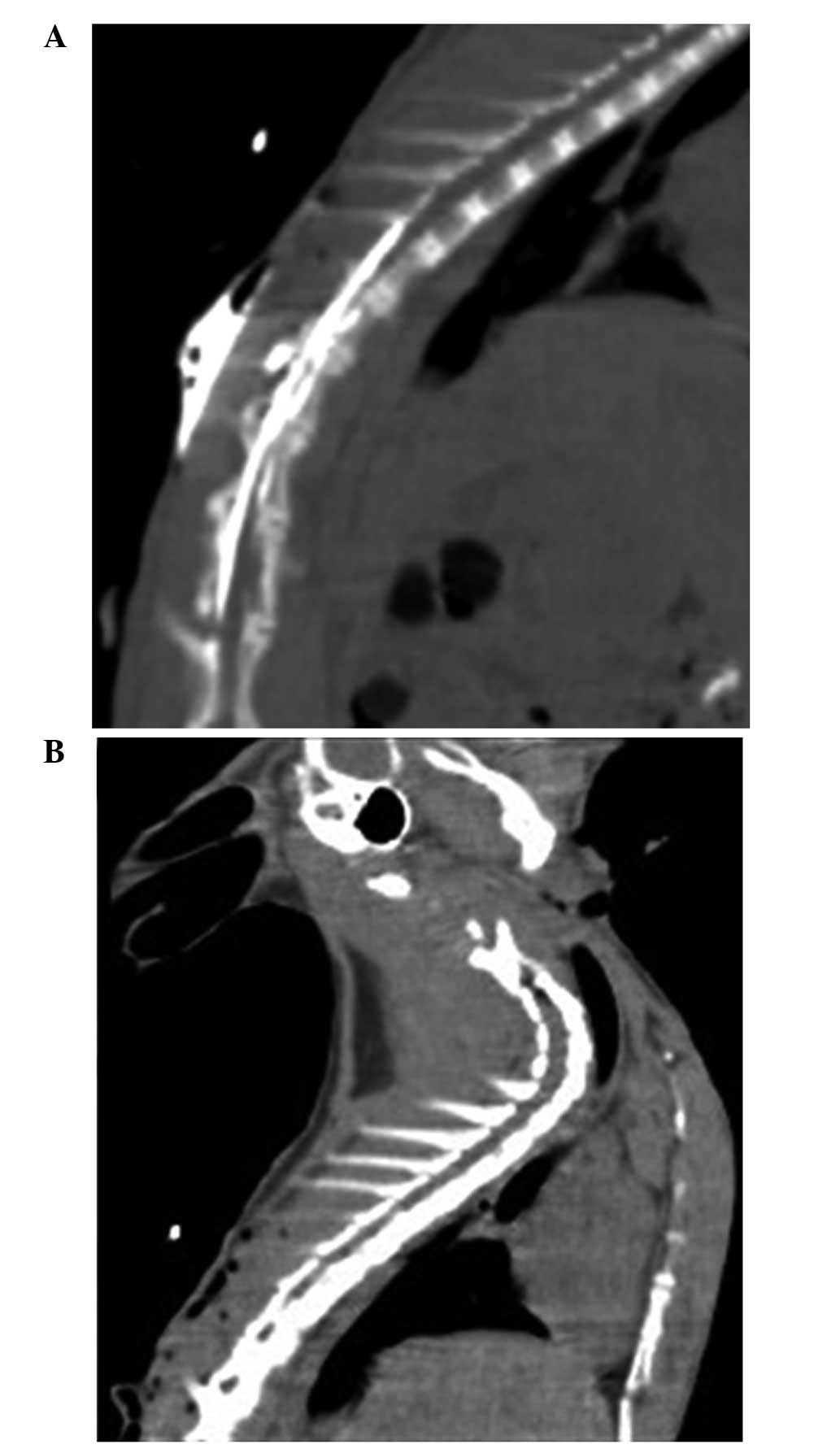
the standard of ALI during the entire process of hypoxia (Table I). The successful establishment of
the TEA model with an epidural catheter placed into the fifth or
sixth thoracic spinous process was confirmed by epidurography
(Fig. 1).
 | Table I.Changes in PaO2 in the four
groups (mmHg). |
Table I.
Changes in PaO2 in the four
groups (mmHg).
| Group | T0 | T1 | T2 | T3 | T4 | T5 |
|---|
| C |
115.4±39.7 |
84.5±18.8 |
101.2±20.7 |
106.3±19.9 |
121.5±9.2 |
122.7±30.1 |
| H |
115.7±29.2 |
29.5±4.4 |
27.6±8.6 |
30.5±8.9 |
30.4±9.4 |
32.8±4.1 |
| S |
94.1±11.6 |
25±3.3 |
29.4±1.5 |
34.2±4.1 |
34.1±2.9 |
32.3±3.5 |
| ES |
86.1±6.1 |
33.3±6.7 |
34.5±6.32 |
33.2±4.9 |
38.7±5.2 |
39.2±3.5 |
Serum cytokine concentrations and mRNA
expression following hypoxia
The serum IL-6 and IL-8 levels were significantly
higher in Groups H and S than in Group C while the IL-10 level in
the two groups was significantly decreased at 3-h after the
induction of ALI. Furthermore, there was significantly lower level
of IL-6 in Group ES than in Groups H and S at this time (Table II).
 | Table II.Serum cytokine concentration changes
following ALI induction. |
Table II.
Serum cytokine concentration changes
following ALI induction.
| Group | Time | IL-6 | IL-8 | IL-10 |
|---|
| C | T0 |
49.4±17.6 |
43.1±14.2 |
39.5±18.7 |
|
| T5 |
49.1±11.1 |
52.5±20.8 |
30.2±10.6 |
| H | T0 |
33.8±5.7 |
29.4±6.1 |
57.7±13.3 |
|
| T5 |
94.1±15.1a,b |
59.5±14.9a |
24.9±7.6a |
| S | T0 |
39.7±11.6 |
37.3±13.4 |
49.2±23.9 |
|
| T5 |
90.2±17.3a,b |
53.9±8.7a |
25.2±4.9a |
| ES | T0 |
54.1±8.2 |
42.9±13.9 |
29.3±5.1 |
|
| T5 |
56.2±19.9c,d |
49.7±18.7 |
30.5±13.5 |
Group H showed significantly higher IL-6 mRNA
expression levels compared with the other three groups, and Group S
showed significantly higher IL-6 mRNA expression compared with
Groups C and ES (P<0.05). Groups H and S showed significantly
higher IL-8 mRNA expression compared with the other two groups
(P<0.05); however, there was no significant difference between
Groups H and S (P>0.05). Group ES showed significantly higher
IL-10 mRNA expression than Groups H and S (P<0.05). Compared
with Group C, Groups H and S showed significantly reduced IL-10
mRNA expression (P<0.05); however, no significant difference was
detected between Groups H and S (P>0.05; Table III).
 | Table III.Relative mRNA expression levels of
IL-6, IL-8 and IL-10 in the lung tissue (95% confidence
interval). |
Table III.
Relative mRNA expression levels of
IL-6, IL-8 and IL-10 in the lung tissue (95% confidence
interval).
| Group | IL-6 | IL-8 | IL-10 |
|---|
| C | 1.000
(0.671–1.671)a,b | 1.000
(0.370–1.370)a,b | 1.000
(0.263–1.263)a,b |
| H | 6.063
(1.848–7.911) | 4.925
(0.764–5.689) | 0.253
(0.075–0.328) |
| S | 3.605
(1.589–5.203)a | 5.401
(2.328–7.730) | 0.287
(0.092–0.379) |
| ES | 1.258
(0.110–1.368)a,b | 1.217
(0.361–1.577)a,b | 0.880
(0.452–1.332)a,b |
Pulmonary surfactants and the D/W
ratio following hypoxia
The SatPC/TPL and SatPC/TP ratios were decreased in
Groups H, S and ES compared with Group C. The SatPC/TP ratio in
Group S was significantly reduced compared with those in Groups H
and ES, while the SatPC/TPL ratio in Group S was reduced compared
with that in Group ES (P<0.05). Group H was the only group in
which the D/W ratio was significantly reduced compared with that of
Group C (P<0.05; Table IV).
 | Table IV.Pulmonary surfactant and D/W ratio
changes after ALI induction. |
Table IV.
Pulmonary surfactant and D/W ratio
changes after ALI induction.
| Group | SatPC/TPL | SatPC/TP | D/W |
|---|
| C |
0.62±0.08 |
0.67±0.29 |
0.21±0.01 |
| H |
0.44±0.14a |
0.28±0.11a |
0.17±0.02a |
| S |
0.31±0.13a |
0.14±0.04a,b |
0.18±0.01 |
| ES |
0.49±0.08a,c |
0.29±0.16a,c |
0.20±0.02 |
Light microscopy and transmission
electron microscopy observations
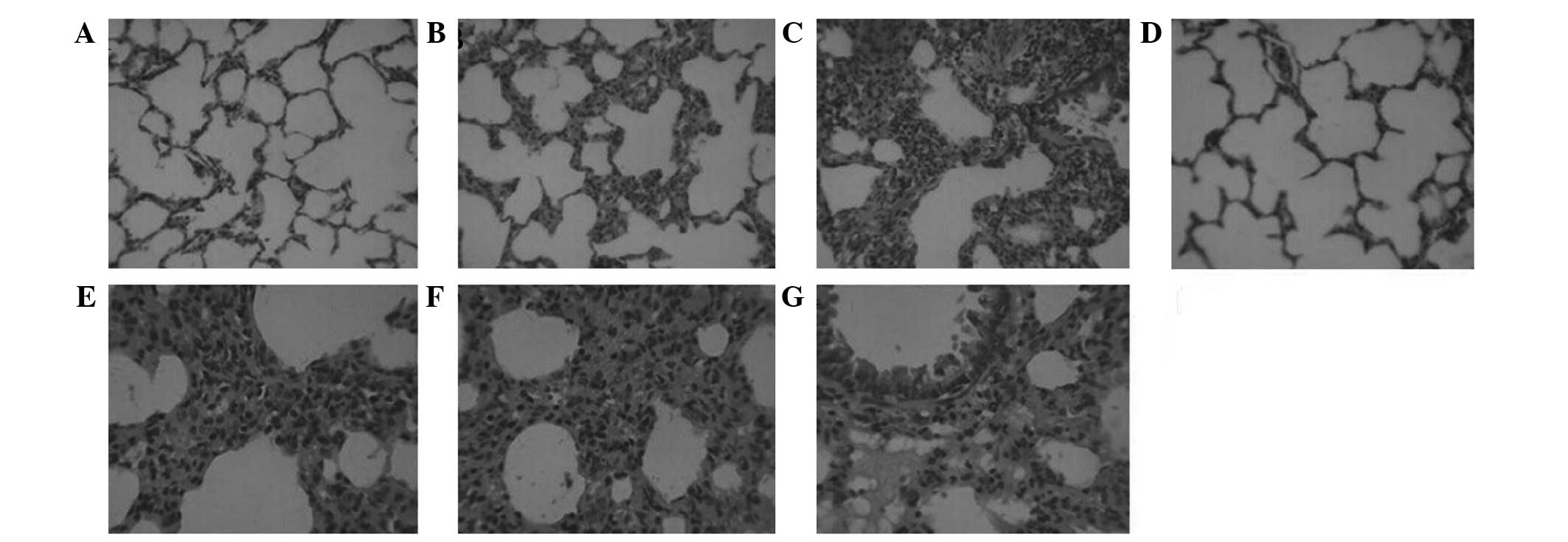
The histopathological examination revealed severe
hyperemia, exudate in a number of alveolar spaces, and obvious
vascular expansion of the alveolar walls in Groups H and S.
Bronchial epithelial cells appeared to be undergoing detachment,
and pulmonary interstitial edema was evident, with a large quantity
of neutrophil infiltration. The alveolar interval was increased and
numerous alveoli were atelectatic. Reduced neutrophil infiltration
and fewer collapsed alveoli were observed in Group ES compared with
Groups H and S (Fig. 2). The
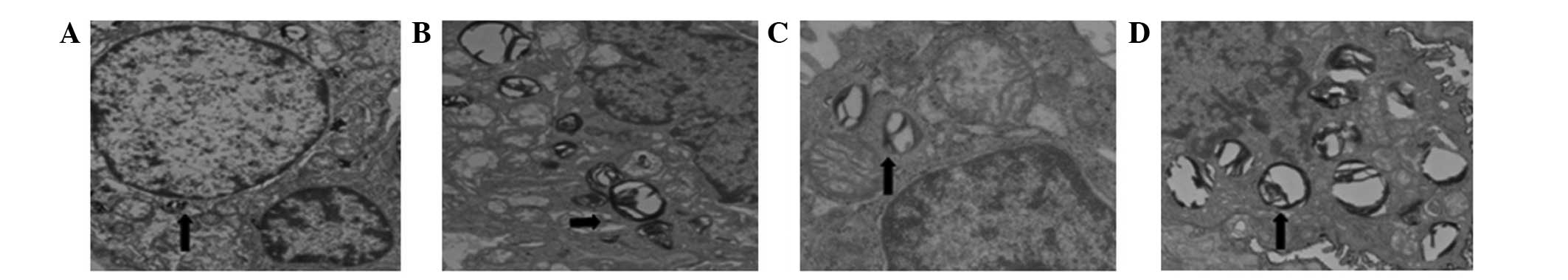
alveolar type II cells in all groups contained numerous lamellar
bodies (surfactant). In Groups H and S the alveolar type II cells
were swollen, and the numbers of lamellar bodies and the amount of
pulmonary surfactants within them were reduced compared with the
other groups. The ultrastructure of the alveolar type II cells and
of the capillary endothelium were comparable in Groups C and ES
(Fig. 3).
Discussion
The present study investigated the potential effect
of TEA on hypoxia-induced acute lung injury (ALI) using a rabbit
model of ALI induced by exposure to acute hypoxia (14%
O2 in N2) at 15 min after intubation and
mechanical ventilation.
ALI is a local pulmonary manifestation of the
mononuclear macrophage systemic inflammatory response, which is
caused by severe infection or trauma (17). It has previously been demonstrated
that the mechanism of ALI involves the abnormal release of
inflammatory mediators and a deficiency in the release of
endogenous anti-inflammatory mediators (18). The imbalance between inflammatory and
anti-inflammatory mediators aggravates the inflammatory response
and promotes the development of ALI (19). In the present study, IL-6, IL-8 and
IL-10 were selected for investigation, and the results showed that
the levels of the inflammatory mediators IL-6 and IL-8 and the
anti-inflammatory mediator IL-10 changed in all groups following
ALI induction.
IL-6 is produced rapidly following tissue damage and
is the primary proinflammatory cytokine responsible for inducing
the systemic changes known as the acute-phase response. The IL-6
level provides an accurate indication of the severity of systemic
inflammatory response syndrome (20). It has previously been demonstrated
that systemic and local lung tissue levels of IL-6 are increased
during invasive mechanical ventilation (21). The present study showed that the IL-6
levels in Groups H and S were significantly higher compared with
those in Groups ES and C, and that IL-6 levels increased compared
with baseline following ALI induction. These results indicate that
IL-6 functions as a mediator of the inflammatory cascade reaction
in ALI, and that TEA is able to effectively mitigate the increase
in the IL-6 level, which may be associated with sympathetic nerve
blockade and the reduction of nociceptive transmission. The present
results are consistent with the findings of a previous study
(22).
IL-8 is an inflammatory chemokine that serves a
crucial function in the regulation of inflammation and immunity. A
clinical trial including 27 patients that underwent an
esophagectomy showed that the level of IL-8 in the peripheral blood
and chest drainage liquid in 11 patients with ALI was significantly
increased compared with that in the 16 patients without ALI
(23). In the present study, acute
hypoxia led to an clear increase in the IL-8 level in Group S.
According to previous studies (24),
IL-8 participates in the development of ALI by influencing the
biological activity of granulocytes via polymorphonuclear
leukocytes (25).
IL-10 is produced by various inflammatory cells,
especially macrophages, and exhibits multifaceted anti-inflammatory
properties, including the inhibition of matrix degrading
metalloproteinase production, leading to suppressed cytokine
production, reduction of tissue factor expression and inhibition of
the apoptosis of macrophages and monocytes after infection. These
inflammatory mechanisms have been demonstrated to contribute to
atherosclerotic lesion development and progression, indicating a
potential regulatory role of IL-10 (26). Sevoflurane is an inhalational
anesthetic that is widely used in clinical practice. Goto et
al (27) showed that sevoflurane
had no influence on neutrophil apoptosis, nor on IL-6 or IL-8
concentrations. Furthermore, El Azab et al (28) showed that sevoflurane did not
significantly affect inflammatory cells. In the present study,
compared with baseline, the serum IL-6 and IL-8 levels in Groups H
and S significantly increased, while the IL-10 level significantly
decreased following ALI. The imbalance between proinflammatory and
anti-inflammatory factors may underlie the ALI-associated tissue
damage. However, the lack of a significant difference between
Groups H and S, suggests that sevoflurane has no clear influence on
inflammation during acute hypoxia. This may be due to the relative
brevity of the hypoxic period. Furthermore, the serum IL-6 level in
Group ES was significantly reduced compared those in Groups H and
S, suggesting that epidural anesthesia inhibited the systemic
inflammatory response to a certain extent.
ALI affects the systemic inflammatory response in
addition to the local inflammation of lung tissue. The present
study observed that the mRNA expression levels of IL-6, IL-8 and
IL-10 changed in the four groups following acute hypoxia, and the
changes were consistent with a previous study by Kalenka et
al (20), suggesting that
epidural anesthesia exerted a protective effect. Although epidural
anesthesia cannot completely inhibit systemic and local
inflammation, it may be able to decrease the severity of
reactions.
Pulmonary surfactants have unique physiological
effects in maintaining the normal breathing function. A previous
study indicated that inhalational anesthetics have a direct impact
on pulmonary surfactants; the inhalation of fat-soluble anesthetics
increased alveolar surface tension, which was unchanged following
the inhalation of non-fat-soluble anesthetics (29). The higher surface tension increased
the release and synthesis of the primary active components, such as
phosphatidylcholine (30). It has
been demonstrated that phosphatidylglycerol is able to maintain the
structural integrity of lipid-protein complexes, promote membrane
activity and stability and increase membrane fluidity (31). Furthermore, it has been reported that
purine metabolites in alveolar lavage fluid may be used as an index
to judge the severity of lung injury (32). In order to correct the deviation
caused by the dilution difference between alveolar lavage and
phosphatidylcholine, SatPC/TPL and SatPC/TP ratios were used in the
present study as indices of phosphatidylcholine activity. The
results showed that the SatPC/TPL and SatPC/TP ratios in Groups H,
S and ES were significantly decreased compared with those in Group
C, which additionally demonstrated the successful induction of the
ALI model. The SatPC/TP ratio in Group S was decreased compared
with those in Groups H and ES, while the SatPC/TPL ratio was lower
compared with that in Group ES, suggesting that sevoflurane reduced
the activity of the pulmonary surfactants. The electron microscopy
results were consistent with this phenomenon.
Animal experiments have shown that following the
administration of inhalation anesthetics, the main change in
alveolar type II cell ultrastructure is that involving the lamellar
bodies (33). Lamellar bodies
constitute the storage pool of pulmonary surfactants. Changes in
the lamellar body number and volume indicate the transportation of
pulmonary surfactants. Following the administration of inhalational
non-fat-soluble anesthetics, lamellar body exocytosis increases.
However, the volume density and number density remain unchanged,
suggesting that the synthesis and secretory function of
surface-active substances by alveolar type II cells are not
obviously influenced (34).
Following the inhalation of fat-soluble anesthetics, exocytosis of
lamellar bodies is rare; however, the volume density and number
density are significantly increased, suggesting that the secretory
function of alveolar type II cells is disturbed while synthesis is
not influenced (35). Therefore, the
surface-active substance becomes isolated within the cell. In the
present study, the morphological observations were consistent with
the quantitative test results.
In the present study, the alveolar type II cells
appeared round, oval or polygonal under transmission electron
microscopy. The membranes contained microvilli and features of the
nucleus, mitochondria and lamellar bodies were observable inside
the cells. Certain cells were rich in endoplasmic reticulum.
Surface-active substances were adherent to the alveolar cavity
wall, rendering a slightly higher electron density at the
interface. The alveolar cavity contained macrophagocytes, and
occasionally exhibited a paracrystalline appearance, which
represented the absence of surface-active substance movement.
Furthermore, the alveolar type II epithelial cells were swollen in
Groups H and S. Microvilli were shortened and reduced in number. No
obvious changes were observed in Group ES. There were fewer
lamellar bodies in Group S, and they demonstrated a large quantity
of vacuolation; however, the lamellar bodies were not clearly
reduced in Group ES, suggesting that the primary effect of
sevoflurane on alveolar type II cells was on the lamellar
bodies.
The present study had a limited sample size, and so
further extended analysis with a larger sample size or an
independent replication study is required to confirm the positive
association of TEA with ALI. ALI is a complex pathophysiological
process; therefore, the mechanism underlying the impact of epidural
anesthesia on ALI remains unclear and requires further
investigation.
In conclusion, the present study investigated the
effects of TEA on function and morphology in rabbits exposed to
hypoxia-induced lung injury. The results indicated that TEA serves
a crucial function in reducing systemic and local inflammation,
decreasing the interference of inhaled anesthetics with pulmonary
surfactants and improving the alveolar ultrastructure.
References
|
1
|
Reiss LK, Uhlig U and Uhlig S: Models and
mechanisms of acute lung injury caused by direct insults. Eur J
Cell Biol. 91:590–601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jain S and Bellingan G: Basic science of
acute lung injury. Surgery. 25:112–116. 2007.
|
|
4
|
Rassler B, Marx G, Reissig C, Rohling MA,
Tannapfel A, Wenger RH and Zimmer HG: Time course of
hypoxia-induced lung injury in rats. Respir Physiol Neurobiol.
159:45–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhargava M and Wendt CH: Biomarkers in
acute lung injury. Transl Res. 159:205–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cross LJ and Matthay MA: Biomarkers in
acute lung injury: Insights into the pathogenesis of acute lung
injury. Crit Care Clin. 27:355–377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dombrowsky H, Barrenschee M, Kunze M and
Uhlig S: Conserved responses to trichostatin A in rodent lungs
exposed to endotoxin or stretch. Pulm Pharmacol Ther. 22:593–602.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calfee CS, Eisner MD, Ware LB, Thompson
BT, Parsons PE, Wheeler AP, Korpak A and Matthay MA: Acute
Respiratory Distress Syndrome Network, National Heart, Lung, and
Blood Institute: Trauma-associated lung injury differs clinically
and biologically from acute lung injury due to other clinical
disorders. Crit Care Med. 35:2243–2250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reyal Y and Bellingan G: The basic science
of acute lung injury. Surgery. 22:iii–vii. 2004.
|
|
10
|
Schwarzkopf K, Schreiber T, Bauer R,
Schubert H, Preussler NP, Gaser E, Klein U and Karzai W: The
effects of increasing concentrations of isoflurane and desflurane
on pulmonary perfusion and systemic oxygenation during one-lung
ventilation in pigs. Anesth Analg. 93:1434–1438. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Onan IS, Onan B, Korkmaz AA, Oklu L,
Kilickan L, Gonca S, Dalcik H and Sanisoglu I: Effects of thoracic
epidural anesthesia on flow and endothelium of internal thoracic
artery in coronary artery bypass graft surgery. J Cardiothorac Vasc
Anesth. 25:1063–1070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abdelrahman RS: Effects of thoracic
epidural anesthesia on pulmonary venous admixture and oxygenation
with isoflurane or propofol anesthesia during one lung ventilation.
Egyptian J Chest Dis Tuberc. 61:477–483. 2012. View Article : Google Scholar
|
|
13
|
Kaneda H, Waddell TK, de Perrot M, Bai XH,
Gutierrez C, Arenovich T, Chaparro C, Liu M and Keshavjee S:
Pre-implantation multiple cytokine mRNA expression analysis of
donor lung grafts predicts survival after lung transplantation in
humans. Am J Transplant. 6:544–551. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bartlett GR: Phosphorous assay in column
chromatography. J Biol Chem. 234:466–468. 1959.PubMed/NCBI
|
|
15
|
Mason RJ, Nellenbogen J and Clements JA:
Isolation of disaturated phosphatidylcholine with osmium tetroxide.
J Lipid Res. 17:281–284. 1976.PubMed/NCBI
|
|
16
|
Magnusson OT, Toyama H, Saeki M,
Schwarzenbacher R and Klinman JP: The structure of a biosynthetic
intermediate of pyrroloquinoline quinine (PQQ) and elucidation of
the final step of PQQ biosynthesis. J Am Chem Soc. 126:5342–5343.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bein T, Zimmermann M, Schiewe-Langgartner
F, Strobel R, Hackner K, Schlitt HJ, Nerlich MN, Zeman F, Graf BM
and Gruber M: Continuous lateral rotational therapy and systemic
inflammatory response in posttraumatic acute lung injury: Results
from a prospective randomised study. Injury. 43:1892–1897. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goodman RB, Pugin J, Lee JS and Matthay
MA: Cytokine-mediated inflammation in acute lung injury. Cytokine
Growth Factor Rev. 14:523–535. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu PK, Yang CY, Tsai HT and Hsieh CL:
Moutan cortex radicis improves lipopolysaccharide-induced acute
lung injury in rats through anti-inflammation. Phytomedicine.
19:1206–1215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kalenka A, Feldmann RE Jr, Otero K, Maurer
MH, Waschke KF and Fiedler F: Changes in the serum proteome of
patients with sepsis and septic shock. Anesth Analg. 103:1522–1526.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Van Wessem KJ, Hennus MP, Heeres M,
Koenderman L and Leenen LP: Mechanical ventilation is the
determining factor in inducing an inflammatory response in a
hemorrhagic shock model. J Surg Res. 180:125–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yokoyama M, Itano Y, Katayama H, Morimatsu
H, Takeda Y, Takahashi T, Nagano O and Morita K: The effects of
continuous epidural anesthesia and analgesia on stress response and
immune function in patients undergoing radical esophagectomy.
Anesth Analg. 101:1521–1527. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morita M, Yoshida R, Ikeda K, Egashira A,
Oki E, Sadanaga N, Kakeji Y, Ichiki Y, Sugio K, Yasumoto K and
Maehara Y: Acute lung injury following an esophagectomy for
esophageal cancer, with special reference to the clinical factors
and cytokine levels of peripheral blood and pleural drainage fluid.
Dis Esophagus. 21:30–36. 2008.PubMed/NCBI
|
|
24
|
Callister ME, Burke-Gaffney A, Quinlan GJ,
Nicholson AG, Florio R, Nakamura H, Yodoi J and Evans TW:
Extracellular thioredoxin levels are increased in patients with
acute lung injury. Thorax. 61:521–527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Misumi T, Tanaka T, Mikawa K, Nishina K,
Morikawa O and Obara H: Effects of sivelestat, a new elastase
inhibitor, on IL-8 and MCP-1 production from stimulated human
alveolar epithelial type II cells. J Anesth. 20:159–165. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li JJ, Guo YL and Yang YJ: Enhancing
anti-inflammatory cytokine IL-10 may be beneficial for acute
coronary syndrome. Med Hypotheses. 65:103–106. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goto Y, Ho SL, McAdoo J, Fanning NF, Wang
J, Redmond HP and Shorten GD: General versus regional anaesthesia
for cataract surgery: Effects on neutrophil apoptosis and the
postoperative pro-inflammatory state. Eur J Anaesthesiol.
17:474–480. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
El Azab SR, Rosseel PM, De Lange JJ, van
Wijk EM, van Strik R and Scheffer GJ: Effect of VIMA with
sevoflurane versus TIVA with propofol or midazolam-sufentanil on
the cytokine response during CABG surgery. Eur J Anaesthesiol.
19:276–282. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Southorn P, Rehder K and Hyatt RE:
Halothane anesthesia and respiratory mechanics in dogs lying
supine. J Appl Physiol Respir Environ Exerc Physiol. 49:300–305.
1980.PubMed/NCBI
|
|
30
|
Zuo YY, Veldhuizen RA, Neumann AW,
Petersen NO and Possmayer F: Current perspectives in pulmonary
surfactant-inhibition, enhancement and evaluation. Biochim Biophys
Acta. 1778:1947–1977. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Agassandian M and Mallampalli RK:
Surfactant phospholipid metabolism. Biochim Biophys Acta.
1831:612–625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Verbrugge SJ, de Jong JW, Keijzer E, de
Anda Vazquez G and Lachmann B: Purine in bronchoalveolar lavage
fluid as a marker of ventilation-induced lung injury. Crit Care
Med. 27:779–783. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Southorn P, Rehder K and Hyatt RE:
Halothane anesthesia and respiratory mechanics in dogs lying
supine. J Appl Physiol Respir Environ Exerc Physiol. 49:300–305.
1980.PubMed/NCBI
|
|
34
|
Evans JA, Hamilton RW Jr, Kuenzig MC and
Peltier LF: Effects of anesthetic agents on surface properties of
dipalmitoyllecithin: Lung surfactant model. Anesth Analg.
45:285–289. 2002.
|
|
35
|
Song J, Lin G and Guo S: Effects of
inhalational anesthetic agents on ultrastructure of the type II
alveolar cell. Acta Academiae Medicinae Nanjing. 22:497–499.
2004.(In Chinese).
|