Introduction
At present, the severity of a traumatic brain injury
(TBI) and the clinical outcome of acute TBI are predicted based on
patient history, clinical manifestations, a clinical examination
[Glasgow Coma Scale (GCS)] and cranial computed tomography findings
(1). However, these commonly used
techniques have significant limitations, including their ability to
predict outcomes or detect subtle damage (2,3). Rapid,
definitive diagnostic tests for TBI that enable physicians to
determine the seriousness of an injury on the basis of quantifiable
neurochemical markers, and to guide the implementation of the
appropriate triage and medical management, are required.
Oncotic necrosis is characterized by cell and
organelle swelling, leading to nuclear degradation, disruption of
the cell membrane and cell lysis (4,5).
Typically, when oncotic necrosis occurs, activation of members of
the calpain family is upregulated (6). Conversely, apoptosis is characterized
by chromatin condensation, internucleosomal DNA fragmentation, cell
shrinkage and cell dismantlement into membrane-enclosed vesicles
(7). In addition, it typically
involves the activation of a specific family of proteases termed
caspases (8). Generally, the oncotic
necrosis of neurons is observed during the acute period following
TBI, in particular within contused or hemorrhaging regions, whereas
apoptotic neurons are detected a few days or weeks post-trauma
(9,10). Importantly, the calpains and caspases
produced during these processes may be evaluated as neurochemical
markers of brain injury.
α-II-spectrin is a cytoskeletal protein that is a
substrate for the calcium-activated cysteine proteases calpain and
caspase-3. Calpain and caspase-3 cleave α-II-spectrin to generate
α-II-spectrin breakdown products (SBDPs), including SBDP145,
SBDP150 and SBDP120. Following acute neuronal injury, increased
levels of SBDPs have been detected in several models of neuronal
injury (11–13). In addition, following TBI, increased
levels of calcium and calpain have been shown to correlate well
with the magnitude of injury in rat models (14,15).
Therefore, the present study aimed to investigate the potential use
of SBDPs in the cerebrospinal fluid (CSF) as a biomarker of
TBI.
Materials and methods
Patients
A total of 17 patients (5 women and 12 men) with an
isolated head injury who had been admitted to the Emergency
Department and Neurosurgical Intensive Care Unit of the Nanjing
First Hospital (Nanjing, China), between June 2009 and December
2010, were included in this prospective study. The inclusion
criteria were as follows: The patient had to have sustained a
severe head injury with a GCS score of ≤8 and the patient required
ventricular intracranial pressure monitoring. The patients were
followed for 30 days following study entry. The age of the patients
ranged from 24–68 years (mean age, 51.8±12.9 years). All patients
were admitted to the hospital <6 h after sustaining the head
injury. Patient consent was obtained within 24 h of study
enrollment. The present study was approved by the Institutional
Review Board of the Third Clinical Medical College of Nanjing
Medical University.
Clinical variables that were potentially associated
with outcome were recorded at 30 days post-injury, and included the
GCS score on admission, as well as the patient's age, gender and
Glasgow Outcome Score (GOS) (16).
The GCS scores were assigned by a single neurosurgeon, who was
blinded to a patient's SBDP levels. Intubation and sedation
procedures were performed following the examination. Patients were
classified according to a commonly used dichotomization of the GCS
score (17): Most severe brain
injury, GCS score 3–5; severe brain injury, GCS score 6–8.
The clinical outcome at 30 days after injury was
considered the short-term outcome, which was assessed using the
GOS. The GOS at 30 days after injury was determined by a designated
member of the research team. Contact by telephone was made either
to the patient (when appropriate) or to his/her relatives (who were
identified at the time of admission and agreed to be contacted in
the future for the purpose of outcome assessment) to assess the
clinical outcome. Patients who continued to receive clinical care
for rehabilitation at the time of the 30-days GOS were assessed by
discussion with the patient's health care professional. For
statistical analyses, outcome was divided into a good prognosis
(GOS, 4–5) and a poor prognosis (GOS, 1–3) (18).
A total of 10 control patients without TBI, who
required lumbar anesthesia for other medical reasons and exhibited
normal pressure hydrocephalus, were enrolled from the Nanjing First
Hospital. CSF samples were obtained from each patient prior to
anesthesia. All patient identifiers remained confidential.
The exclusion criteria for the present study were as
follows: Concomitant extracranial injuries such as pelvic or
extremity fractures; intra-abdominal, intrapleural, or
retroperitoneal hemorrhages; hepatic or splenic injuries; thorax or
spinal cord damage; and major health problems such as diabetes
mellitus; renal or cardiac failure; central nervous system
diseases; bleeding disorders prior to the trauma, and alcohol
intoxication. Patients with a history of neurological and
neuropsychiatric disorders, history of alcohol, drug, or substance
abuse, or a history of previous TBI were also excluded from this
study.
CSF sample collection
Ventriculostomy catheters were placed under routine
medical care for patients with severe TBI in the study institution.
Therefore, CSF samples from patients with severe TBI were directly
collected from the ventriculostomy catheter at 24, 48 and 72 h
after injury. At each sampling point, 3–4 ml CSF was collected from
each patient. The samples were immediately centrifuged for 10 min
at 2,130 × g to separate the CSF from blood cells, and were then
frozen and stored at −80°C until examination.
Immunoblotting assay
The protein content of the CSF was assayed using a
micro bicinchoninic acid assay kit (KGPBCA; KeyGen Biotech Co.,
Ltd., Nanjing, China) with albumin standards. For each sample, 40
µg CSF protein was added to 5X loading buffer (KGP101; KeyGen
Biotech Co., Ltd.) and then heated at 100°C for 5 min. Samples were
loaded onto a 6.5% stacking acrylamide gel and electrophoresed in a
vertical electrophoresis chamber for 100 min at 130 V. Separated
proteins were horizontally transferred to Immobilon-P
polyvinylidene fluoride membrane (KeyGen Biotech Co., Ltd.) and
semi-dried at 15 V for 75 min at room temperature. All gels were
stained with Coomassie blue (Bioss, Beijing, China) to confirm
equal loading of proteins on the gel. After three 10-min rinses in
Tris-buffered saline (TBS), blots were blocked for 2 h in 5%
non-fat dry milk in TBS with 1% Tween 20 (TBST). Following
incubation overnight with primary anti-α-spectrin monoclonal
antibody (1:5,000 dilution; MA1-91103; Thermo Fisher Scientific,
Rockford, USA) and 5% non-fat milk/TBST at 4°C, and three 10-min
rinses in TBST, the blots were incubated with horseradish
peroxidase-conjugated goat anti-mouse secondary antibody (1:5,000
dilution; bs-0296G-HRP; Bioss) for 1 h at room temperature. Blots
were then rinsed again three times for 10 min in TBS at room
temperature. Enhanced chemiluminescence reagents (ECL and ECL-Plus;
KGP1125; KeyGen Biotech, Co., Ltd.) and Kodak BioMax Light Film
(Eastman Kodak; Rochester, NY, USA) were used to visualize the
immunolabeling. Semi-quantitative evaluation of protein levels was
performed using computer-assisted one-dimensional densitometric
scanning (Image J, Version 1.29x; National Institutes of Health,
Bethesda, MD, USA). Data were acquired as integrated densitometric
values from similarly exposed films.
Statistical analysis
Statistical analyses were performed using SPSS
software, version 17.03 for Windows (SPSS, Inc., Chicago, IL, USA).
Counting variables were analyzed using Fisher's exact test. Results
are presented as the mean ± standard deviation (SD). Normally
distributed variables were compared using Student's t-test, and
non-normally distributed variables were analyzed using the
Mann-Whiney U test. Analysis of variance was used to determine the
statistical significance. A value of P<0.05 was considered
statistically significant.
Results
General information
The present study included 17 patients with severe
TBI (GCS score, 3–8) and 10 control patients with normal pressure
hydrocephalus. Six patients had severe brain injury (GCS score,
6–8), and 11 patients had most severe brain injury (GCS score,
3–5). There were no significant differences in patient age and
gender between the two groups (P>0.05; Table I). A total of 12 patients were
considered to have a poor outcome (GOS score, 1–3), whereas the
remaining five patients had a good outcome (GOS score, 4–5).
 | Table I.General characteristics of patients
with severe traumatic brain injury. |
Table I.
General characteristics of patients
with severe traumatic brain injury.
| Brain injury
severity | Age (years) | Gender (M/F) |
|---|
| Severe (GCS score
6–8) | 55.8±6.0 | 3/3 |
| Most severe (GCS
score 3–5) | 49.6±15.3 | 9/2 |
| Statistical
value | t=1.833 |
χ2=1.76 |
| P-value | >0.05 | >0.05 |
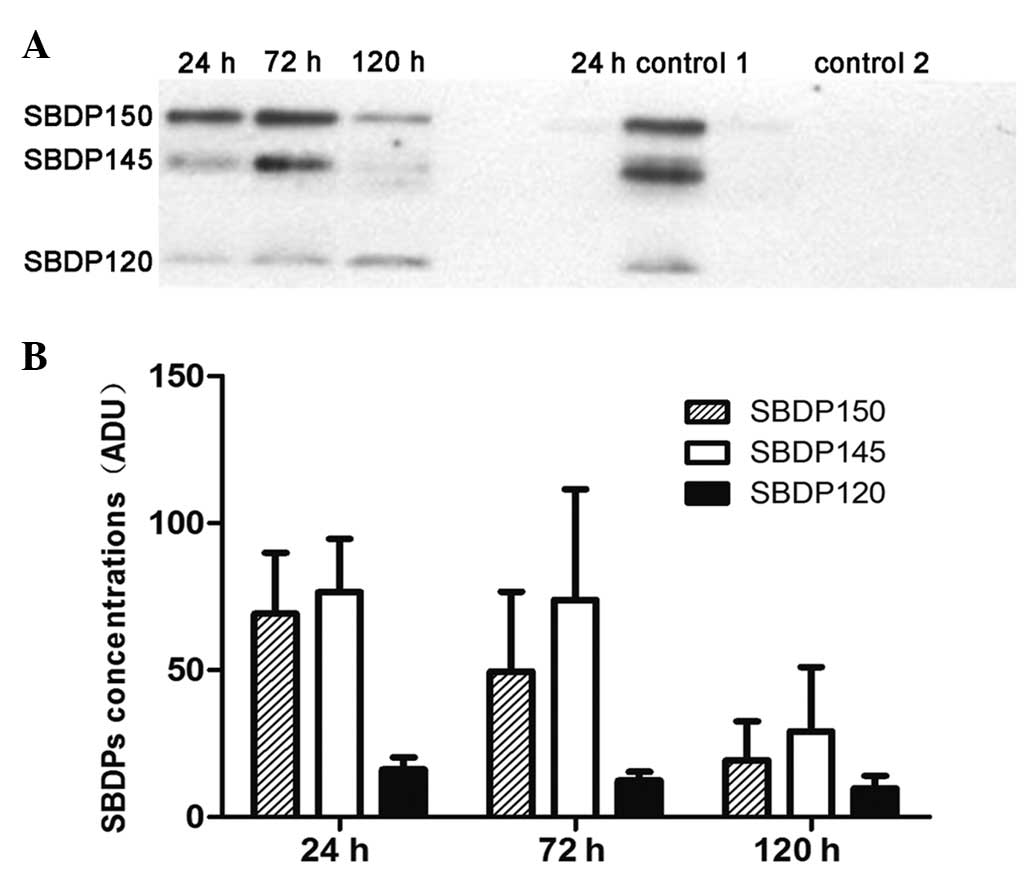
Concentrations of CSF α-II-SBDP over 5
days after injury in patients with severe TBI
SBDPs were not detectable in the CSF of the patients
from the control group. Therefore, the SBDP concentrations of the
control group were considered to be ~0, and further data for the
control group are not presented. The mean concentrations of SBDP150
and SBDP145 peaked at 24 h after injury and then showed a downward
trend. No significant changes in the mean concentrations of SBDP120
were observed during the study (Fig.
1).
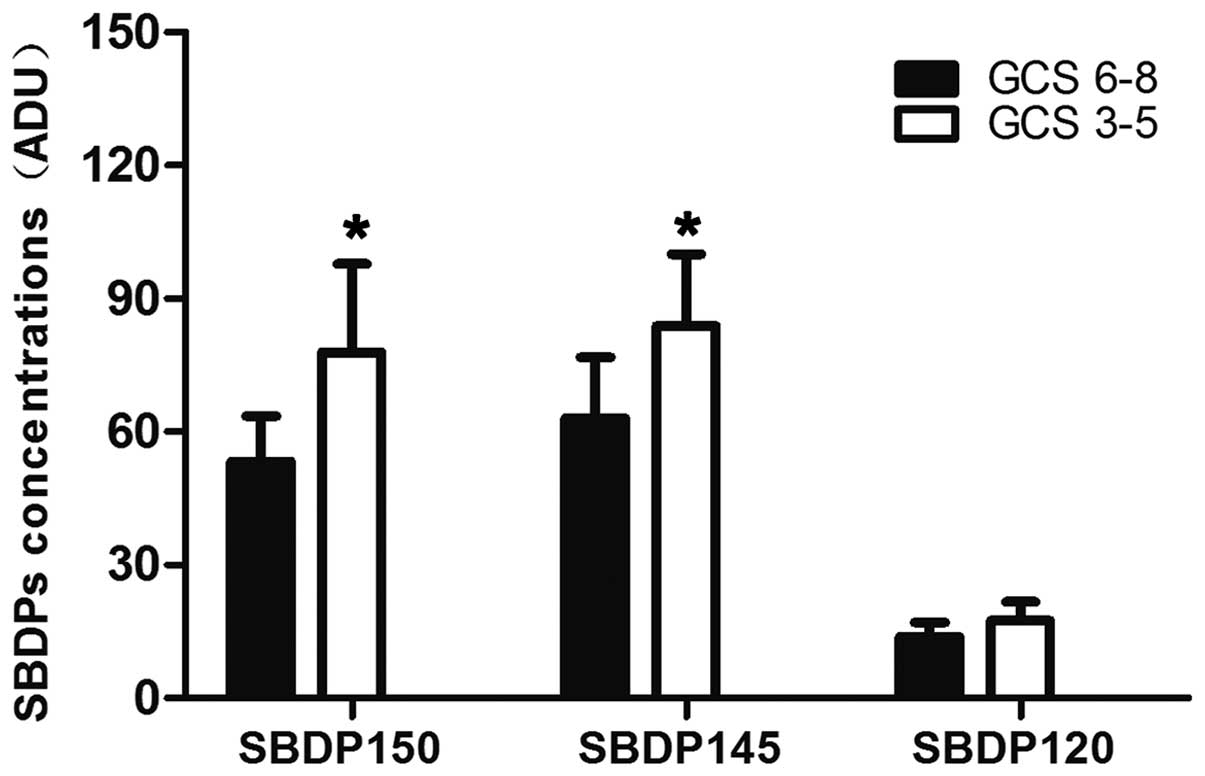
Association between the levels of
SBDPs and GCS score
Within the first 24 h after TBI, the SBDP145 and
SBDP150 levels, but not the SBDP120 levels, were significantly
lower in the patients with GCS scores of 6–8 than in patients with
lower admission GCS scores of 3–5 (P<0.05; Fig. 2).
Association between the levels of
SBDPs and clinical outcome
Higher levels of CSF SBDP145 and SBDP150 were found
in patients who succumbed, remained vegetative, or were severely
disabled (GOS score l-3) than in patients who showed a good
prognosis (GOS score 4–5) at all time points (P<0.05). The
levels of CSF SBDP120 of the two outcome groups peaked at 24 h
after injury and then showed a downward trend; it decreased more
slowly in the poor prognosis group than in the good prognosis
group. The levels of CSF SBDP120 at 24 h after trauma showed no
significant differences between the two groups (P>0.05).
However, the CSF SBDP120 levels were significantly higher at 72 h
(P<0.05) and at 120 h (P<0.05) in the poor prognosis group
(Table II).
 | Table II.Levels of CSF SBDPs between groups at
all time points. |
Table II.
Levels of CSF SBDPs between groups at
all time points.
|
|
| CSF SBDP levels |
|---|
|
|
|
|
|---|
| Biomarkers | Prognosis on the
basis of GOS | 24 h | 72 h | 120 h |
|---|
| SBDP150 | Good |
52.69±10.01 |
30.36±5.92 |
8.75±2.03 |
|
| Poor |
76.59±18.37a |
58.01±26.76a |
22.98±13.08a |
| SBDP145 | Good |
62.25±13.9 |
36.73±10.25 |
10.90±3.33 |
|
| Poor |
83.42±17.21a |
76.12±22.1a |
36.99±20.70a |
| SBDP120 | Good |
13.40±2.74 |
9.56±1.12 |
6.18±1.06 |
|
| Poor |
17.21±4.51 |
13.77±2.89a |
11.48±4.18a |
Discussion
SBDPs are metabolic decomposition products of
α-II-spectrin, which is a major substrate for both calpain and
caspase-3 cysteine proteases (12).
α-II-spectrin (280 kDa) is the major structural component of the
cortical membrane cytoskeleton, and it is abundantly present in
axons and presynaptic terminals (19). TBI results in altered Ca2+
homeostasis and activates several Ca2+-dependent
enzymes, including calpain and caspase-3 (20). Calpain proteolysis is primarily
associated with oncotic necrosis, while caspase-3 proteolysis is
primarily associated with apoptosis (6). Calpain cleaves α-II-spectrin to
generate SBDP145 and SBDP150. Caspase-3 cleaves α-II-spectrin to
generate SBDP120 and SBDP150. The SBDPs transfer from the damaged
brain cells into the CSF. Hence, the levels of SBDPs increase in
the CSF following TBI. Zhang et al (21) used three chemical agents to induce
necrosis and apoptosis in PC12 neuronal like cells, and
demonstrated that the multiple α-II-SBDPs could be used as
biomarkers to distinguish between calpain- and caspase-dominant
necrotic and apoptotic cell deaths. Using a mouse model of cortical
injury, Pike et al (22)
demonstrated that the levels of SBDP150 and SBDP145 were
predominantly elevated in the first 24–72 h post injury, with
SBDP145 levels being greatly increased. In a study conducted by
Cardali et al (23), the
levels of SBDPs in CSF were measured for adult patients with severe
TBI from 6 to 96 h after injury, and it was found that the SBDPs
levels in CSF were significantly increased, as compared with those
in control patients at all time points examined. In patients with a
better outcome, CSF SBDPs levels were significantly decreased from
6 to 96 h post injury. Patients whose SBDP levels remained elevated
or failed to decline had a poor outcome (23). In the present study, the CSF levels
of SBDP150, SBDP145 and SBDP120 were significantly elevated in
patients with severe TBI, as compared with those in patients with
hydrocephalus or a subarachnoid hemorrhage. Patients with the most
severe injury (day 1 GCS score, 3–5) had significantly higher mean
levels of SBDP150 and SBDP145 in the first 24 h post-injury, as
compared with patients with a less severe injury (day 1 GCS score,
6–8); however, the difference was not significant for SBDP120.
These results suggested that the levels of SBDPs in the CSF may be
considered useful biomarkers for predicting the severity of injury
in the early period of severe TBI.
In the present study, it was found that the CSF
levels of SBDPs were significantly elevated in patients with severe
TBI. However, SBDPs were not detected in the CSF of the control
patients. SBDPs appeared rapidly in the CSF following severe TBI;
SBDP150 and SBDP145 peaked at 6 h post-TBI and remained
significantly elevated for 24 and 72 h post-injury; conversely, the
levels of SBDP120 did not have a clear trend of variation post-TBI
(24). Notably, a previous study
found that SBDP150 and SBDP145 levels peaked at 48–72 h post-injury
(25). In the present study, the
levels of SBDPs in the CSF were predominantly elevated at 24 h
post-TBI and then declined gradually over time. The CSF levels of
SBDP145 and SBDP150 remained significantly elevated up to 72 h
post-TBI and were significantly declined at 120 h post-injury,
whereas the CSF levels of the SBDP120 showed a slow gradual
decline. Generally, oncotic necrosis of neurons occurs in the acute
posttraumatic period, whereas apoptosis occurs a few days or weeks
following the trauma (9). SBDP150
and SBDP145 are predominantly produced by calpain activity, which
is primarily associated with oncotic necrosis, whereas SBDP120 is a
signature product of caspase-3 activity, which is primarily
associated with apoptosis (26).
Therefore, it can be hypothesized that the levels of SBDP150 and
SBDP145 in the CSF would likely peak in the acute posttraumatic
period, whereas the levels of SBDP120 would peak at ≥1 week
post-injury. In the present study, within the first 24 h after
injury, the CSF levels of SBDP145 and SBDP150 were significantly
higher in patients with hospital admission GCS scores of 3–5, as
compared within patients with hospital admission GCS scores of 6–8.
Although differences were observed in the CSF levels of SBDP120,
the differences were not statistically significant. These results
indicated that patients with severe brain injury had the highest
levels of SBDP145 and SBDP150 in the first 24 h. The levels of CSF
SBDP120 at 24 h post-trauma were not associated with hospital
admission GCS scores, which may be due to the more delayed
processes of apoptotic cell death.
In the present study, patients were categorized into
two groups based on their clinical outcomes. The outcome analysis
revealed a statistically significant association between the
clinical outcome and the SBDP150 and SBDP145 levels at all time
points. This indicates that the higher the levels of CSF SBDP145
and SBDP150, the greater the probability of a poor prognosis.
Moreover, a slower decline of CSF SBDP145 and SBDP150 levels may
also predict a poor prognosis. Comparative analysis of SBDP120 and
clinical outcome did not reveal a statistically significant
association at 24 h post injury. The levels of CSF SBDP120 were
higher in patients who showed a poor prognosis (GOS score, l-3)
than in patients who showed a good prognosis (GOS score, 4–5) at 72
h and 120 h after injury. Previous study results showed that CSF
SBDP120 levels exhibited no significant differences between poor
and good prognosis groups at 24 h after severe TBI (27). However, Pike et al (22) demonstrated that the levels of CSF
SBDP120 at 24 h and 7 days after severe TBI were significantly
correlated with GOS scores determined 3 months after injury. On
consideration of these results, it can be concluded that there is
some uncertainty concerning the trend in the changes of the levels
of CSF SBDP120, and further studies are required for elucidation.
The results of the present study provide evidence that the CSF
SBDPs can be a reliable marker of central nervous system injury.
CSF SBDP145 and SBDP150 are potentially useful biomarkers of severe
TBI in humans. The change tendency of these biomarkers measured
early after injury can be used in the evaluation of the severity of
injury and clinical outcome in patients with severe TBI. The
association between the CSF SBDP120 and the severity of injury and
clinical outcome after TBI still requires further research.
Since α-II-spectrin is not found in erythrocytes,
the influence of hemolysis can be avoided (28). The present study has several
limitations. Firstly, rapidly changing clinical conditions of
patients with severe TBI may lead to the CSF sample collection at
all time points and long-term monitoring becoming very difficult.
Thus, all available samples were included in the analyses in the
present study to maximize the sample size. Secondly, the biomarkers
were only assessed in patients experiencing severe TBI. Assessment
of these biomarkers in patients experiencing different severities
of head injury (for example, patients with mild or moderate head
injuries) in future studies is suggested. Thirdly, the levels of
SBDPs were determined using a semi-quantitative western blot
analysis. More advanced detection methods are required in future
studies. The low number of enrolled patients also impacted the
credibility of the results. However, the present study data provide
an initial assessment of relationships between CSF SBDPs and the
severity and clinical outcome of severe TBI. Many unaddressed
problems remain that require investigation in further studies, for
example, by detecting SBDPs in the serum of injured experimental
animals and humans and investigating the secondary injury effect on
CSF SBDP levels.
In conclusion, the present pilot study indicates
that the trends in CSF SBDP levels measured early after injury can
be used in the evaluation of the severity of injury and clinical
outcome of patients with severe TBI. They are potentially useful
biomarkers of severe TBI. However, a series of uncertainties remain
that require further investigation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81171786).
References
|
1
|
Perrin PB, Niemeier JP, Mougeot JL, Vannoy
CH, Hirsch MA, Watts JA, Rossman W, Grafton LM, Guerrier TD,
Pershad R, et al: Measures of injury severity and prediction of
acute traumatic brain injury outcomes. J Head Trauma Rehabil.
30:136–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brenner DJ and Hall EJ: Computed
tomography-an increasing source of radiation exposure. N Engl J
Med. 357:2277–2284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kmietowicz Z: Better safe than sorry? BMJ.
335:1182–1184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Majno G and Joris I: Apoptosis, oncosis,
and necrosis. An overview of cell death. Am J Pathol. 146:3–15.
1995.PubMed/NCBI
|
|
5
|
Van Cruchten S and Van Den Broeck W:
Morphological and biochemical aspects of apoptosis, oncosis and
necrosis. Anat Histol Embryol. 31:214–223. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang KK: Calpain and caspase: Can you tell
the difference? Trends Neurosci. 23:20–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dressler J, Hanisch U, Kuhlisch E and
Geiger KD: Neuronal and glial apoptosis in human traumatic brain
injury. Int J Legal Med. 121:365–375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castillo MR and Babson JR:
Ca(2+)-dependent mechanisms of cell injury in cultured cortical
neurons. Neuroscience. 86:1133–1144. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rathmell JC and Thompson CB: The central
effectors of cell death in the immune system. Annu Rev Immunol.
17:781–828. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raghupathi R: Cell death mechanisms
following traumatic brain injury. Brain Pathol. 14:215–222. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Newcomb JK, Kampfl A, Posmantur RM, Zhao
X, Pike BR, Liu SJ, Clifton GL and Hayes RL: Immunohistochemical
study of calpain-mediated breakdown products to alpha-spectrin
following controlled cortical impact injury in the rat. J
Neurotrauma. 14:369–383. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang KK, Posmantur R, Nath R, McGinnis K,
Whitton M, Talanian RV, Glantz SB and Morrow JS: Simultaneous
degradation of alphaII- and betaII-spectrin by caspase 3 (CPP32) in
apoptotic cells. J Biol Chem. 273:22490–22497. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valiyaveettil M, Alamneh YA, Wang Y, Arun
P, Oguntayo S, Wei Y, Long JB and Nambiar MP: Cytoskeletal protein
α-II spectrin degradation in the brain of repeated blast exposed
mice. Brain Res. 1549:32–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pike BR, Flint J, Dave JR, Lu XC, Wang KK,
Tortella FC and Hayes RL: Accumulation of calpain and caspase-3
proteolytic fragments of brain-derived alphaII-spectrin in cerebral
spinal fluid after middle cerebral artery occlusion in rats. J
Cereb Blood Flow Metab. 24:98–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ringger NC, Tolentino PJ, McKinsey DM,
Pike BR, Wang KK and Hayes RL: Effects of injury severity on
regional and temporal mRNA expression levels of calpains and
caspases after traumatic brain injury in rats. J Neurotrauma.
21:829–841. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wilson JT, Pettigrew LE and Teasdale GM:
Structured interviews for the Glasgow Outcome Scale and the
extended Glasgow Outcome Scale: Guidelines for their use. J
Neurotrauma. 15:573–585. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Narayan RK, Greenberg RP, Miller JD, Enas
GG, Choi SC, Kishore PR, Selhorst JB, Lutz HA 3rd and Becker DP:
Improved confidence of outcome prediction in severe head injury. A
comparative analysis of the clinical examination, multimodality
evoked potentials, CT scanning, and intracranial pressure. J
Neurosurg. 54:751–762. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi SC, Clifton GL, Marmarou A and Miller
ER: Misclassification and treatment effect on primary outcome
measures in clinical trials of severe neurotrauma. J Neurotrauma.
19:17–22. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reeves TM, Greer JE, Vanderveer AS and
Phillips LL: Proteolysis of submembrane cytoskeletal proteins
ankyrin-G and αII-spectrin following diffuse brain injury: A role
in white matter vulnerability at nodes of ranvier. Brain Pathol.
20:1055–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schoch KM, von Reyn CR, Bian J, Telling
GC, Meaney DF and Saatman KE: Brain injury-induced proteolysis is
reduced in a novel calpastatin-overexpressing transgenic mouse. J
Neurochem. 125:909–920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Z, Larner SF, Liu MC, Zheng W, Hayes
RL and Wang KK: Multiple alphaII-spectrin breakdown products
distinguish calpain and caspase dominated necrotic and apoptotic
cell death pathways. Apoptosis. 14:1289–1298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pike BR, Flint J, Dutta S, Johnson E, Wang
KK and Hayes RL: Accumulation of non-erythroid alpha II-spectrin
and calpain- cleaved alpha II-spectrin breakdown products in
cerebrospinal fluid after traumatic brain injury in rats. J
Neurochem. 78:1297–1306. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cardali S and Maugeri R: Detection of
alphaII-spectrin and breakdown products in humans after severe
traumatic brain injury. J Neurosurg Sci. 50:25–31. 2006.PubMed/NCBI
|
|
24
|
Pineda JA, Lewis SB, Valadka AB, Papa L,
Hannay HJ, Heaton SC, Demery JA, Liu MC, Aikman JM, Akle V, et al:
Clinical significance of alphaII-spectrin breakdown products in
cerebrospinal fluid after severe traumatic brain injury. J
Neurotrauma. 24:354–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Farkas O, Polgár B, Szekeres-Barthó J,
Dóczi T, Povlishock JT and Büki A: Spectrin breakdown products in
the cerebrospinal fluid in severe head injury-preliminary
observations. Acta Neurochir (Wien). 147:855–861. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schober ME, Requena DF, Davis LJ, Metzger
RR, Bennett KS, Morita D, Niedzwecki C, Yang Z and Wang KK: Alpha
II Spectrin breakdown products in immature Sprague Dawley rat
hippocampus and cortex after traumatic brain injury. Brain Res.
1574:105–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brophy GM, Pineda JA, Papa L, Lewis SB,
Valadka AB, Hannay HJ, Heaton SC, Demery JA, Liu MC, Tepas JJ III,
et al: alphaII-Spectrin breakdown product cerebrospinal fluid
exposure metrics suggest differences in cellular injury mechanisms
after severe traumatic brain injury. J Neurotrauma. 26:471–479.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pike BR, Flint J, Dutta S, Johnson E, Wang
KK and Hayes RL: Accumulation of non-erythroid alpha II-spectrin
and calpain-cleaved alpha II-spectrin breakdown products in
cerebrospinal fluid after traumatic brain injury in rats. J
Neurochem. 78:1297–1306. 2001. View Article : Google Scholar : PubMed/NCBI
|