Introduction
Multiple organ dysfunction syndrome (MODS) is a
systemic inflammation resulting from infectious or non-infectious
stimuli, leading to the dysfunction of two or more organs and the
failure of multiple systems (1,2). MODS
remains the most common cause of mortality in intensive care units,
and may be triggered by a variety of stimuli, including severe
trauma, infection, shock, cardiopulmonary resuscitation and surgery
(3). The mechanism underlying MODS
is complicated; however, it has been widely accepted that
uncontrolled inflammatory response contributes to the rapidly
progressive development of MODS (4).
In 1995, Sakaguchi et al identified a
subpopulation of CD4+ T lymphocytes with high cell
surface expression of interleukin (IL)-2 receptor α chain (CD25);
namely, CD4+CD25+ regulatory T (Treg) cells
(5). These cells are crucially
involved in the regulation of autoimmune diseases, transplant
tolerance, and infectious and anti-tumor immune responses (6–9). Using
the CD4+ CD45RB high T cell mice transfer model of
inflammatory bowel disease, Mottet et al (10) showed that
CD4+CD25+ cells, but not
CD4+CD25− CD45RB low T cells, were able to
cure intestinal inflammation, indicating the critical role played
by CD4+CD25+ Treg cells in the prevention of
the T cell-mediated immune response. Furthermore, the forkhead
family transcriptional regulator Foxp3, a lineage-specific marker
of CD4+CD25+ Treg cells, has been recognized
to be critical for the development and function of Treg cells
(11). Notably, FOXP3 expression
regulates the activity of the Treg cells in vitro and in
vivo (11). Depletion of
CD4+CD25+ Treg cells from melanoma patients
resulted in enhanced immune responses and substantial development
of antigen-specific CD8+ T cells in peptide-vaccinated
individuals (12). In addition, the
presence of CD4+CD25+ Treg cells in patients
with active rheumatoid suppresses the proliferation of autologous T
cells from synovial and peripheral blood by promoting the
production of IL-10 and transforming growth factor-β (TGF-β)
secreted by Treg cells (13).
In patients with MODS, proinflammatory and
anti-inflammatory cytokine levels are imbalanced and abnormal
cytokine secretion may lead to exaggerated inflammation (14). Application of monoclonal antibodies
for tumor necrosis factor-α (TNF-α) or soluble TNF-α receptor
suppresses the elevated expression of IL-1 and IL-6 via the
reduction of the release of TNF-α, and thus protects against MODS
(3). Additionally, specific IL-1
receptor antagonists have been shown to reduce the mortality of
endotoxin-induced shock in rabbits (15). However, such anti-inflammatory
therapies may act as a double-edged sword, as they may suppress the
damage caused by excessively-activated inflammation while
simultaneously eliminating the benefits of the inflammatory
response. Therefore, efficient therapy for the regulation of
inflammation is required.
Due to its powerful effect in suppressing immune
responses in various human diseases, the application of
CD4+CD25+Foxp3+ Treg cells may
provide a possible therapy for the treatment of MODS. However, the
potential role for these cells in MODS is not well understood. In
the present study, the role of
CD4+CD25+Foxp3+ Treg cells in MODS
was investigated.
Materials and methods
Reagents
Ficoll was purchased from Tianjin Hao Yang
Biological Manufacture Co., Ltd. (Tianjin, China).
Phosphate-buffered saline (PBS) was obtained from Beijing Chemical
Works (Beijing, China).
Patients
A total of 42 patients (age range, 15–82 years) were
selected from the Intensive Care Unit of the First Hospital of
Jilin University (Changchun, China) between January 2009 and
February 2010. All patients met MODS diagnostic criteria (16) and 27 patients remained in the
hospital for over one week. These 42 patients were assigned into 2
groups; Survival >15 days (n=15) and Survival <15 days
(n=27), as 15 patients survived and 27 patients succumbed to MODS
in the hospital between days 1 and 5 following admission. In
addition, MODS was complicated by various disease states, as
follows: Five cases had septic shock; 11 cases received
cardiopulmonary resuscitation; 11 cases had surgical palliation for
gastric cancer, gallbladder carcinoma, colon or rectum; 7 cases had
multiple injuries; 3 cases had allergic shock; 3 cases had severe
pancreatitis; 1 case had cesarean section; and 1 case had ectopic
pregnancy. All patients had injuries in between 2 to 6 organs. Ten
healthy physical examinees aged 20–28 years were selected as
control subjects. Informed consent was provided by all participants
who met eligibility criteria. Ethical approval for this study was
provided by the Ethics Committee at First Hospital of Jilin
University.
Peripheral blood collection
Blood samples (2 ml) were collected from study
cohorts at indicated time points and were allowed to clot for 2 h
at room temperature. Serum was isolated by centrifugation (1,100 ×
g for 30 min at 20°C) and stored at −20°C until further use.
Antibodies and flow cytometric
analysis
Mouse anti-human IgG (PAB9307), fluorescein
isothiocyanate (FITC)-conjugated anti-CD25 mAb (ANC-174-040),
anti-CD4 mAb (ANC-148-040), phycoerythrin (PE)-conjugated anti-CD8
mAb (ANC-154-070), anti-Foxp3 mAb (AG-20A-0025-C050), PerCP5
conjugated anti-CD4 (CYT-4C3), anti-CD3 (CYT-3C4), and isotype
control antibodies were purchased from Caltag Laboratories
(Carlsbad, CA, USA). Peripheral blood samples (2 ml) were collected
for flow cytometric analysis, and the levels of
CD4+CD25+Foxp3+, CD3+,
CD4+ and CD8+ were determined as described
previously (9,11).
Percentage of Tregs in gated lymphocytes was
measured based on a method previously described by Liu et al
(17). Briefly, lymphocytes were
washed and resuspended in PBS with 1% bovine serum albumin (BSA;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
incubated with PerCP-conjugated anti-CD4 (550631), PerCP-conjugated
anti-CD25 (558689) and FITC-conjugated anti-CD25 (124774) (all
dilution 1:50; all purchased from BD Biosciences, San Jose, CA,
USA) for 20 min at 4°C. Cells were fixed and permeabilized with
Fix/Perm buffer (005523; eBioscience, San Diego, CA, USA) followed
by washing and blocking with normal rat serum (eBioscience). Cells
were then stained with PE-conjugated anti-Foxp3 (Foxp3 Staining
Set, clone PCH101; eBioscience) for 60 min, and analyzed using a
FACSCalibur flow cytometer (BD Biosciences). The data were analyzed
using FlowJo software (version 7.6.1; Tree Star, Inc., San Carlos,
CA, USA).
Enzyme-linked immunosorbent assay
(ELISA)
ELISA kits were used to determine the concentration
levels of TNF-α (EK0986), IL-2 (EK0397), IL-4 (EK0404), IL-6
(EK0410), IL-8 (EK0413), IL-10 (EK0416) and IL-1β (EK0392),
measured in triplicate according to the manufacturer's instructions
(Wuhan Boster Biological Technology, Ltd., Wuhan, China). The
optical density values were detected using an ELISA reader (Anthos
2010; Biochrom Ltd., Cambourne, UK) at a 450-nm wavelength and
calculated in the linear part of the curve.
Statistical analyses
Data were analyzed using SPSS software, version 11.5
(SPSS, Inc., Chicago, IL, USA) and are presented as the mean ±
standard deviation. Comparisons between the two groups of subjects
were made using Student t-test or χ2 test. P<0.05 was
considered to indicate a statistically significantly
difference.
Results
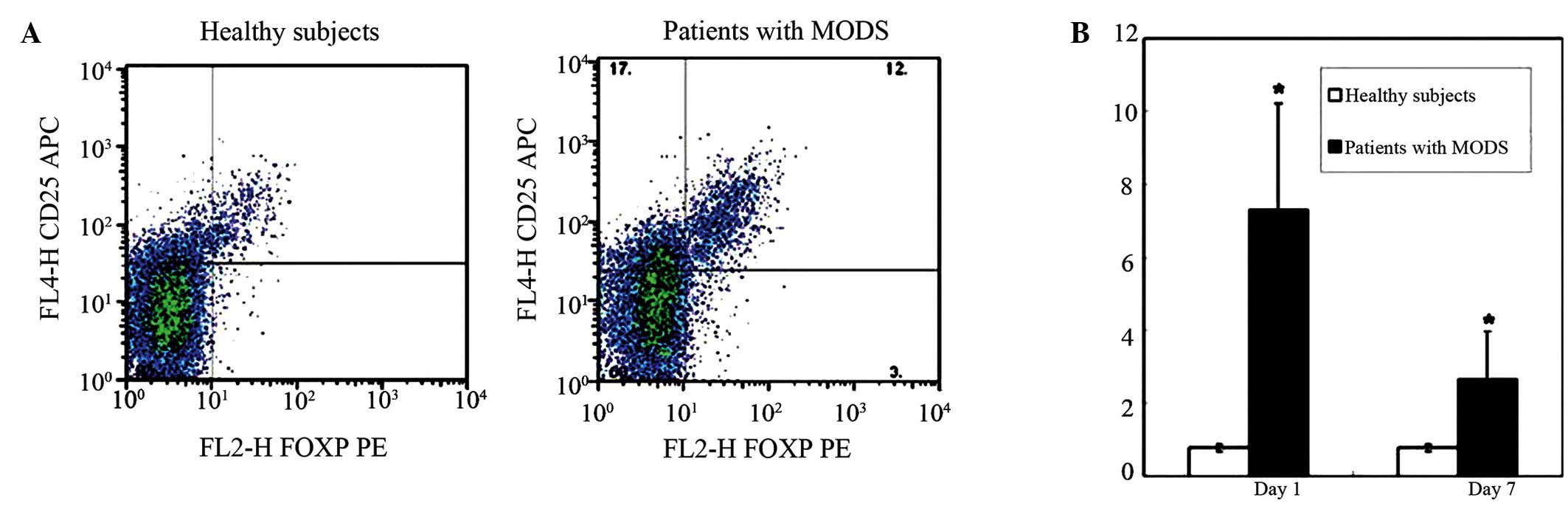
Increased percentage of
CD4+CD25+FOXP3+ Treg cells in
patients with MODS
To investigate the potential role of
CD4+CD25+Foxp3+ Treg cells in
patients with MODS, a total of 27 patients that fulfilled the MODS
diagnostic criteria were recruited and the percentages of
CD4+CD25+Foxp3+ Treg cells in
total lymphocytes in the patients' peripheral blood were evaluated
using flow cytometry on Days 1 and 7 following administration.
Meanwhile, ten healthy subjects were selected as controls. As shown
in Fig. 1, a markedly elevated
CD4+CD25+Foxp3+ Treg cell number
was detected in patients with MODS on Day 1 compared to the healthy
control group (P<0.05). Notably, although it was reduced on Day
7 as compared with Day 1, the percentage of
CD4+CD25+Foxp3+ Treg cells in MODS
patients remained higher than the control group on Day 7
(P<0.05; Fig. 1B). These results
suggest that CD4+CD25+Foxp3+ Treg
cells may play a functional role during the disease progression of
MODS.
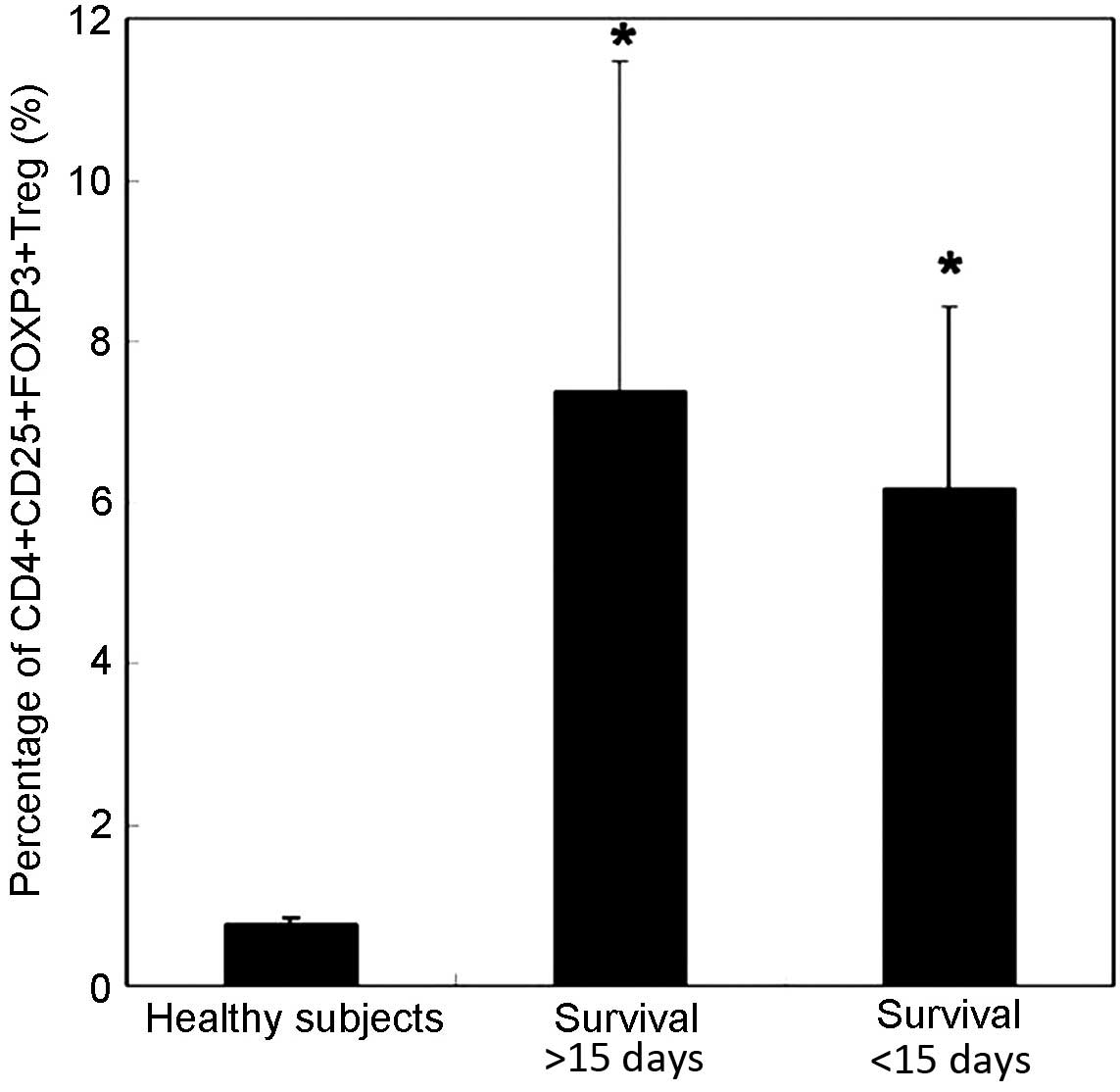
Prognostic value of percentage of
CD4+CD25+Foxp3+ Treg cells in MODS
To investigated the possible prognostic value of
CD4+CD25+Foxp3+ Treg cells in
MODS, a total of 42 patients with MODS were divided into two
groups: Survival >15 days, including patients that survived over
15 days in hospital (n=15); and Survival <15 days, including
patients that succumbed to MODS within 15 days (n=27). Consistent
with our previous results, the percentage of
CD4+CD25+Foxp3+ Treg cells was
very low in healthy subjects (0.77±0.09%) (Fig. 2). An increased percentage of
CD4+CD25+Foxp3+ Treg cells in
total lymphocytes was observed in patients with MODS on Day 1. In
addition, the percentage of CD4+CD25+Foxp3+ Treg cells was lower in
the Survival <15 days group (6.16±2.25%) compared with the
Survival >15 days group (7.37±4.08%); however, the difference
did not reach significance (P>0.05). These results indicate that
Treg frequency may have no prognostic value in patients with
MODS.
T-lymphocyte subsets CD4+
and CD8+ and the CD4+/CD8+
ratio
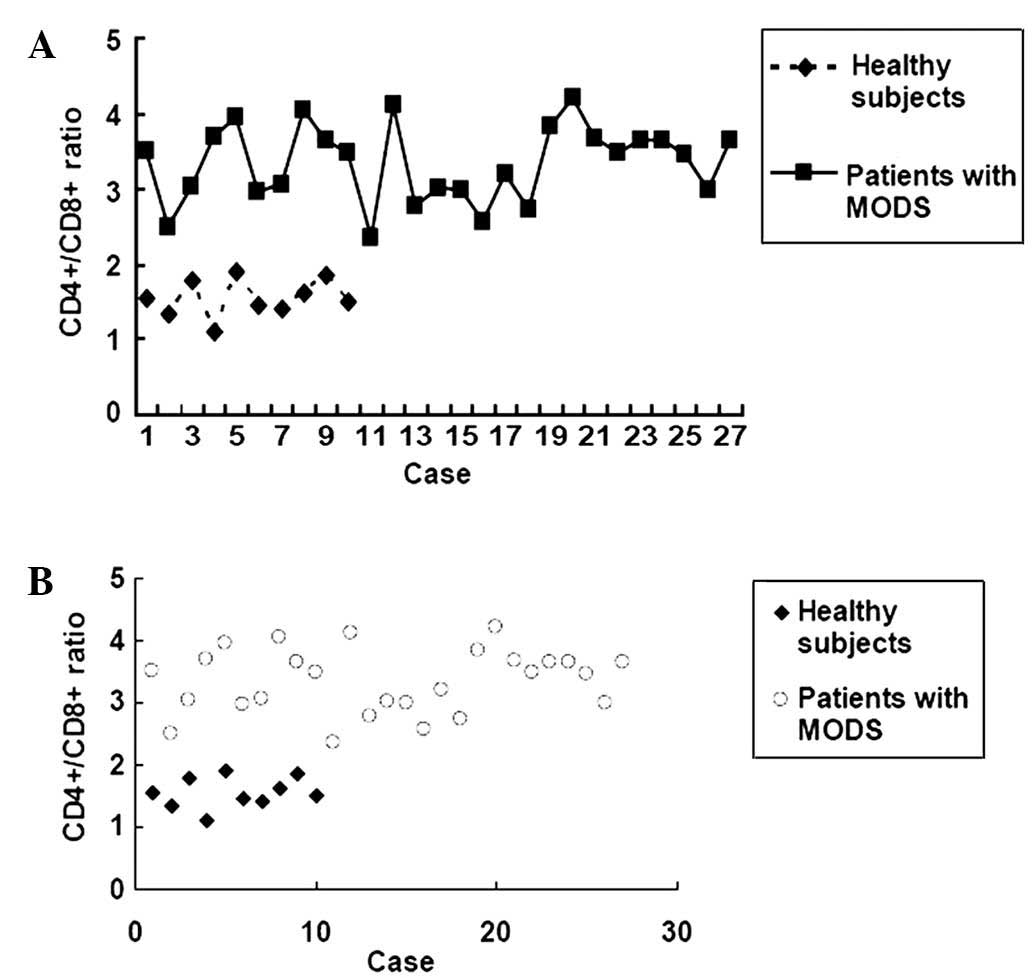
The CD4+/CD8+ ratio is a key
indicator of immune function. Therefore, the levels of the
T-lymphocyte subsets CD4+ and CD8+ in
peripheral blood from patients with MODS were evaluated at day 1 of
administration. As shown in Table I,
the percentage of CD4+ was significantly increased,
whereas the percentage of CD8+ was decreased in MODS
patients (n=27) as compared with healthy control subjects (n=10)
(P<0.05). Furthermore, the CD4+/CD8+ ratio
was calculated, and an elevated CD4+/CD8+
ratio was observed in patients with MODS (3.33±3.54) as compared
with healthy subjects (1.55±0.81) (Table
I and Fig. 3), indicating the
elevated immunoactivity in patients with MODS. No significant
difference was detected in the CD4+/CD8+
ratio between the survival >15 days group and the survival
<15 days group (P>0.05).
 | Table I.Ratio of
CD4+/CD8+ in the various study cohorts. |
Table I.
Ratio of
CD4+/CD8+ in the various study cohorts.
|
|
| Percentage of total
gated lymphocytes (%) |
|
|---|
|
|
|
|
|
|---|
| Group | n |
CD3+CD4+ |
CD3+CD8+ |
CD4+/CD8+ |
|---|
| Healthy
subjects | 10 | 37.61±10.56 | 24.64±6.54 | 1.55±0.81 |
| Patients with
MODS | 27 |
52.43±14.78a |
22.50±8.62a |
3.33±3.54a |
Cytokines in the blood of patients
with MODS
Serum blood concentrations of cytokines, including
TNF-α, IL-1β, IL-2, IL-4, IL-6, IL-8 and IL-10, were determined in
the various patient groups. As shown in Table II, compared with the healthy
subjects, the levels of all seven cytokines were elevated in
patients with MODS on Day 1. On Day 7, levels of TNF-α, IL-2, IL-4
and IL-10 were gradually reduced to similar levels as the healthy
controls, while IL-6, IL-8 and IL-1β levels were remained at
significantly higher levels compared with the healthy controls
(P<0.001) (Table I). No
significant difference was detection in the levels of these
cytokines between the survived and deceased patients
(P>0.05).
 | Table II.Levels of cytokines in patients with
MODS as compared with healthy subjects. |
Table II.
Levels of cytokines in patients with
MODS as compared with healthy subjects.
| Group | Day | TNF-α (ng/l) | IL-2 (ng/l) | IL-4 (ng/l) | IL-6 (ng/l) | IL-8 (ng/l) | IL-10 (ng/l) | IL-1β (ng/l) |
|---|
| Healthy
subjects | 1 |
28.63±4.51 |
28.63±4.51 |
18.90±4.11 |
46.97±8.16 |
37.83±5.61 |
17.14±7.76 |
38.13±5.14 |
| Patients with
MODS | 1 |
108.47±6.34a |
246.38±19.29a |
78.29±3.78a |
774±87.41a |
2654.89±34.56a |
62.62±6.34a |
72.93±8.50a |
| Patients with
MODS | 7 |
24.21±3.45b |
55.00±5.88b |
18.38±5.67b |
206.42±18.86a,b |
559.83±56.21a,b |
16.55±4.56b |
59.9±7.23a,b |
Discussion
MODS has been recognized as a severe human disease,
with massive inflammation and a high mortality rate (1,2). The
cellular mechanisms underlying the development and progression of
MODS are complicated, and pathological processes including
uncontrolled inflammation, systemic inflammation, imbalanced immune
activity, tissue hypoxia, dysregulated apoptosis, microvascular
coagulopathy and endothelial activation have been suggested to lead
to the clinical manifestations of this disease (18). Among all these processes, an
uncontrolled inflammatory response is known to contribute to the
rapidly progressive development of MODS (3). The dynamic balance between pro- and
anti-inflammatory cytokines is critical to maintaining normal
function of the immune system (19).
However, during the progression of MODS, an imbalanced generation
of anti-inflammatory cytokines (including IL-4, IL-10, IL-13, TGF-β
and soluble TNF-α receptor) and proinflammatory cytokines
(including IL-1β, IL-6, IL-8 and TNF-α) has become the key event
(19).
Previous studies indicated that the naturally
arising CD4+CD25+Foxp3+ Treg
cells, the majority of which are produced by the normal thymus as a
functionally mature T-cell subpopulation, are crucially involved in
the maintenance of physiological and pathological immune responses
(20,21). In this study, a marked elevation in
CD4+CD25+Foxp3+ Treg cells was
detected in patients with MODS on Day 1 of admission as compared
with the healthy subjects (Fig. 1),
demonstrating that CD4+CD25+Foxp3+
Treg cells may be involved in the development of MODS.
Furthermore, alterations in the levels of various
pro- and anti-inflammatory cytokines were observed in the present
study. Increased concentrations of proinflammatory cytokines, such
as IL-2, TNF-α, IL-1β, IL-6 and IL-8, were found in the early
stages of this disease (Table II),
suggesting that inflammation plays a dominant role in the
progression of MODS. Similarly, the levels of the anti-inflammatory
cytokines IL-4 and IL-10 in patients with MODS were increased on
Day 1 of admission, which was parallel to the increase in
CD4+CD25+Foxp3+ Treg cells. It is
possible that CD4+CD25+FOXP3+ Treg
cells protect against MODS via the secretion of anti-inflammatory
cytokines, thereby inhibiting the exaggerated inflammation. The
present results demonstrated that during the early stages of MODS,
the production of proinflammatory and anti-inflammatory cytokines
was elevated, and these cytokines interact with each other, playing
pivotal roles in the regulation of the disease process.
The CD4+/CD8+ ratio is widely
accepted as an indicator of immune activity (22), and also provides valuable prognostic
information for patients with certain type of tumor, such as renal
cell carcinoma (23) and metastatic
melanoma (24). In the present
study, an increased CD4+/CD8+ ratio was
detected in patients with MODS, indicating elevated immunoactivity
in these patients.
The results suggest that the percentage of
CD4+CD25+Foxp3+ Treg cells and the
levels of pro- and anti-inflammatory cytokines may be used as a
biomarker for evaluating the status of disease, in addition to
prognosis. Furthermore, the suppression of the secretion of
proinflammatory cytokines, such as TNF-α, IL-2, IL-6 and IL-8, may
provide a valuable tool for the clinical therapy of MODS.
Acknowledgements
The authors would like to thank Medjaden Bioscience
Limited for assisting in the preparation of the manuscript. This
study was supported by Changchun City Science and Technology Agency
(grant no. 20081020).
References
|
1
|
Beal AL and Cerra FB: Multiple organ
failure syndrome in the 1990s. Systemic inflammatory response and
organ dysfunction. JAMA. 271:226–233. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bone RC, Balk RA, Cerra FB, Dellinger RP,
Fein AM, Knaus WA, Schein RM and Sibbald WJ: ACCP/SCCM Consensus
Conference Committee: Definitions for sepsis and organ failure and
guidelines for the use of innovative therapies in sepsis. The
ACCP/SCCM consensus conference committee. American college of chest
Physicians/Society of critical care medicine. 1992. Chest.
136(Suppl 5): e282009.
|
|
3
|
Wang Y: Multiple organs dysfunction
syndrome. Emergency Medicine. 6:Higher Education Press. (Beijing,
China). 162–168. 2006.
|
|
4
|
Abraham E, Wunderink R, Silverman H, Perl
TM, Nasraway S, Levy H, Bone R, Wenzel RP, Balk R, Allred R, et al:
Efficacy and safety of monoclonal antibody to human tumor necrosis
factor alpha in patients with sepsis syndrome. A randomized,
controlled, double-blind, multicenter clinical trial. TNF-alpha MAb
Sepsis Study Group. JAMA. 273:934–941. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sakaguchi S, Sakaguchi N, Asano M, Itoh M
and Toda M: Immunologic self-tolerance maintained by activated T
cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a
single mechanism of self-tolerance causes various autoimmune
diseases. J Immunol. 155:1151–1164. 1995.PubMed/NCBI
|
|
6
|
Gallimore A and Sakaguchi S: Regulation of
tumour immunity by CD25+ T cells. Immunology. 107:5–9. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shevach EM: Regulatory T cells in
autoimmmunity*. Annu Rev Immunol. 18:423–449. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suvas S, Azkur AK, Kim BS, Kumaraguru U
and Rouse BT: CD4+CD25+ regulatory T cells control the severity of
viral immunoinflammatory lesions. J Immunol. 172:4123–4132. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wood KJ and Sakaguchi S: Regulatory T
cells in transplantation tolerance. Nat Rev Immunol. 3:199–210.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mottet C, Uhlig HH and Powrie F: Cutting
edge: Cure of colitis by CD4+CD25+ regulatory T cells. J Immunol.
170:3939–3943. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ziegler SF: FOXP3: Not just for regulatory
T cells anymore. Eur J Immunol. 37:21–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mahnke K, Schönfeld K, Fondel S, Ring S,
Karakhanova S, Wiedemeyer K, Bedke T, Johnson TS, Storn V,
Schallenberg S and Enk AH: Depletion of CD4+CD25+ human regulatory
T cells in vivo: Kinetics of treg depletion and alterations in
immune functions in vivo and in vitro. Int J Cancer. 120:2723–2733.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao D, Malmström V, Baecher-Allan C,
Hafler D, Klareskog L and Trollmo C: Isolation and functional
characterization of regulatory CD25brightCD4+ T cells from the
target organ of patients with rheumatoid arthritis. Eur J Immunol.
33:215–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aikawa N: Cytokine storm in the
pathogenesis of multiple organ dysfunction syndrome associated with
surgical insults. Nihon Geka Gakkai Zasshi. 97:771–777. 1996.(In
Japanese). PubMed/NCBI
|
|
15
|
Ohlsson K, Björk P, Bergenfeldt M, Hageman
R and Thompson RC: Interleukin-1 receptor antagonist reduces
mortality from endotoxin shock. Nature. 348:550–552. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marshall JC, Cook DJ, Christou NV, Bernard
GR and Sibbald Sprung WJ: Multiple organ dysfunction score: A
reliable descriptor of a complex clinical outcome. Crit Care Med.
23:1638–1652. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee
MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St Groth
Fazekas B, et al: CD127 expression inversely correlates with FoxP3
and suppressive function of human CD4+ T reg cells. J Exp Med.
203:1701–1711. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marshall JC: Inflammation, coagulopathy,
and the pathogenesis of multiple organ dysfunction syndrome. Crit
Care Med. 29(Suppl 7): S99–S106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yi L: Effects of gene types on
proinflammatory cytokines and anti-inflammatory cytokines in
patients with multiple organ dysfunction syndrome. Zhong Guo Wei
Zhong Bing Ji Jiu Yi. 15:962003.
|
|
20
|
Sakaguchi S, Ono M, Setoguchi R, Yagi H,
Hori S, Fehervari Z, Shimizu J, Takahashi T and Nomura T: Foxp3+
CD25+ CD4+ natural regulatory T cells in dominant self-tolerance
and autoimmune disease. Immunol Rev. 212:8–27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakaguchi S, Setoguchi R, Yagi H and
Nomura T: Naturally arising Foxp3-expressing CD25+CD4+ regulatory T
cells in self-tolerance and autoimmune disease. Curr Top Microbiol
Immunol. 305:51–66. 2006.PubMed/NCBI
|
|
22
|
Mansilla-Roselló A, Ferrón-Orihuela JA,
Ruiz-Cabello F, Garrote-Lara D, Delgado-Carrasco S and Tamayo-Pozo
F: Interleukin-1beta and ibuprofen effects on CD4/CD8 cells after
endotoxic challenge. J Surg Res. 65:82–86. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hernberg M, Muhonen T, Turunen JP,
Hahka-Kemppinen M and Pyrhonen S: The CD4+/CD8+ ratio as a
prognostic factor in patients with metastatic melanoma receiving
chemoimmunotherapy. J Clin Oncol. 14:1690–1696. 1996.PubMed/NCBI
|
|
24
|
Hernberg M, Muhonen T and Pyrhönen S: Can
the CD4+/CD8+ ratio predict the outcome of interferon-alpha therapy
for renal cell carcinoma? Ann Oncol. 8:71–77. 1997. View Article : Google Scholar : PubMed/NCBI
|