Introduction
Chronic hypertension induces vascular and cardiac
remodeling (1–4). Remodeling involves the hypertrophy of
cells and the excessive deposition of extracellular matrix (ECM)
within tissues, leading to arterial wall hypertrophy, abnormal
myocardial stiffness and an impairment of left ventricular (LV)
function (2,5,6). A major
component of the myocardial ECM is collagen (1). In hypertension, the key mediators of
increases in the levels of collagen include angiotensin II (Ang II)
and transforming growth factor-β (TGF-β) (1,7); the
hypertrophic effects of Ang II are exerted via increased levels of
reactive oxygen species (ROS) and TGF-β (7–10), which
promote matrix metalloproteinase (MMP) activation. Notably, the
overexpression of MMP-2 induces severe ventricular remodeling and
systolic dysfunction in the absence of superimposed injury, which
may be due to MMP-2 activity affecting intracellular targets and
potentially impairing myocardial contractility (11–13).
Numerous treatments for hypertension have been
investigated, with a focus on reducing the risk of
hypertension-induced cardiovascular diseases. In the present study,
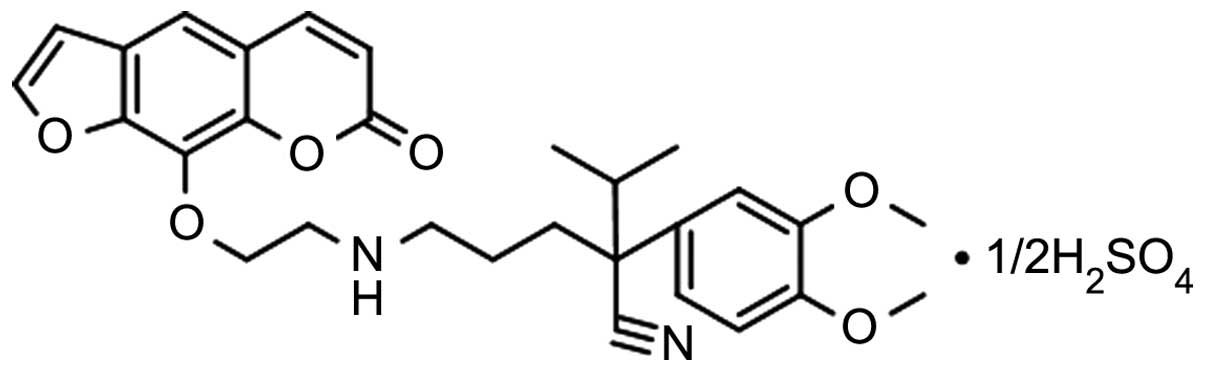
a novel imperatorin derivative,
2-(3,4-dimethoxyphenyl)-2-isopropyl-5-[(2-[(7-oxo-7H-furo[3,2-g]
chromen-4-yl)-oxy]ethyl)amino]pentanenitrile (OW1; Fig. 1), which previously exhibited
hypotensive effects in two-kidney, one-clip (2K1C) renovascular
hypertensive rats (14), was further
investigated. In the previous study, OW1 decreased the generation
of Ang II, inhibited vascular remodeling of the thoracic aorta and
improved the kidney function affected by hypertension (14). In the 2K1C model of hypertension,
activation of the renin-angiotensin-aldosterone system (RAAS)
occurs, which leads to increased cardiac myocyte thickness and is
associated with imbalanced MMP activity (15). The present study aimed to explore the
effect of OW1 on the cardiac remodeling induced by
hypertension.
Materials and methods
Experimental rats
A total of 55 male Sprague-Dawley (SD) rats (age,
6–8 weeks; body weight, 200–230 g) and two male SD rats (age, 3–4
weeks) were obtained from the Animal Center of Xi'an Jiaotong
University (Xi'an, China). The rats were maintained at room
temperature under a 12-h light/dark cycle with ad libitum
access to tap water and standard rat feed. The rats were cared for
in accordance with the principles of the National Institutes of
Health Guide for the Care and Use of Laboratory Animals (16). All protocols were approved by the
Institutional Animal Investigation Committee of Xi'an Jiaotong
University.
Preparation of OW1
OW1 was prepared in the Natural Drug Research and
Engineering Center of Xi'an Jiaotong University using xanthotoxin
(Meryer Chemical Technology Co., Ltd., Shanghai, China) as the
starting material. OW1 was synthesized via a previously reported
method comprising six reaction steps, with characterization of the
chemical structures (17).
Measurement of the vascular effects of
OW1 in rat coronary artery (CA) rings
A total of 10 male SD rats aged 6–8 weeks were
anesthetized by intraperitoneal injection with 10% chloral hydrate
(350 mg/kg body weight; Shanghai Jinjinle Industry Co., Ltd.,
Shanghai, China) and sacrificed by decapitation. The superior CAs
(2 mm) were dissected free of fat and connective tissue and mounted
in a Multi Wire Myograph System (Danish Myo Technology A/S, Inc.,
Skejbyparken, Denmark). The vessels were maintained at 37°C in
physiological Krebs-Henseleit solution (pH 7.4) through which a
gaseous mixture of 95% O2 and 5% CO2 was
bubbled. Following a 30 min equilibration period, the CA segments
were equilibrated for 1.5 h with a resting tension of 3 mN prior to
the initiation of testing. The contractile capacity of each CA
segment was tested by exposure to a K+-rich
Krebs-Henseleit solution (containing 4.45 g/l KCl), in which NaCl
was exchanged for an equimolar concentration of KCl. When two
reproducible contractions were achieved the CA segments were
further evaluated. Following equilibration, the segments were
pre-contracted with KCl (60 mM). Once the sustained tension was
obtained, OW1 (0.1 µM-0.1 mM) was added at increasing
concentrations to the baths, and concentration-response curves were
constructed.
Proliferation assay
The SD rats aged 3–4 weeks were sacrificed by
decapitation and the thoracic aortas were removed. Vascular smooth
muscle cells (VSMCs) were isolated from the thoracic aorta as
previously described (18). The
cells were grown at 37°C in a 95% O2 and 5%
CO2 atmosphere in Dulbecco's modified Eagle's medium
(Beijing Skywing Technology Co., Ltd., Beijing, China) containing
10% fetal bovine serum, 2% penicillin (100 U/ml; North China
Pharmaceutical Group Corporation, Shijiazhuang, China), and 1%
L-glutamine. Cells at passages 4–8 were used in experiments. The
VSMCs were exposed to Ang II (1 µM; Sigma-Aldrich, St. Louis, MO,
USA) for 48 h in the presence or absence of OW1 (5, 10 or 20 µM).
Cell proliferation was analyzed by the methyl thiazolyl tetrazolium
assay (MTT) method (19).
Induction of the 2K1C model of
hypertension
A total of 45 male SD rats aged 6–8 weeks were used
to establish the 2K1C rat model of hypertension. Surgical
procedures were performed under 10% chloral hydrate anesthesia (350
mg/kg body weight, intraperitoneally). 2K1C modeling was conducted
by separating the left renal artery through an abdominal approach
and placing it in a 0.3 mm silver clip. Rats in the sham-operated
group underwent the same procedure, with the exception that the
renal artery was not clipped. The rats were placed into single
cages and their airways were kept open until they regained
consciousness (20). After 3 days,
the rats were injected once daily with penicillin G (8 million
units; North China Pharmaceutical Group Co., Ltd., Shijiazhuang,
China) for 5 days. After 4 weeks, blood pressure was measured by
the tail-cuff method (21), using a
Coda-Volume Pressure Recording (VPR) system (Kent Scientific
Corporation, Torrington, CT, USA).
On week 6 after the surgery, rats with a systolic
blood pressure (SBP) ≥140 mmHg were considered to be hypertensive.
At the end of week 6, the SBP was very stable and rats with a SBP
>160 mmHg were randomly divided into four groups: i)
Hypertension group, comprising rats intragastrically treated with
vehicle (ultrapure water); ii) nifedipine group (30 mg/kg/day);
iii) OW1 high dose group (OW1-H; 80 mg/kg/day); iv) OW1 low dose
group (OW1-L; 40 mg/kg/day). A sham-operated group was also
established. Drugs or vehicle were administered by gavage once a
day for 5 weeks. The blood pressure of the rats in each group was
measured by the tail-cuff method.
Plasma samples
When the treatment period was complete, the rats
were sacrificed with 20% urethane (1 g/kg, intraperitoneally;
Yangzhou Xinhua Chemical Industry Co., Ltd., Yangzhou, China) in
the morning after overnight fasting, and blood samples were then
collected in vacuum tubes with ethylenediamine tetraacetic acid as
an anticoagulant. The blood samples were centrifuged at 1,000 × g
for 15 min at 4°C to separate the plasma, which was then frozen at
−80°C until required for testing. Levels of aldosterone (ALD) in
plasma were detected using a radioimmunoassay kit (Beijing Sino-UK
Institute of Biological Technology, Beijing, China). Levels of
TGF-β1 in the plasma were quantified using an enzyme-linked
immunosorbent assay kit (ML-Elisa-0014; Shanghai Fuzhong Bio Tech
Co., Ltd., Shanghai, China).
Harvesting of the hearts
When the treatment period was complete, the rats
were anesthetized with urethane as described above. The thoracic
cavity was opened to expose the heart, which continued to beat. The
heart was rapidly removed, rinsed in ice-cold 0.9% saline solution,
blotted and weighed. The two ventricles from the heart were
isolated, cut into two fragments by a mid-ventricular coronal
section and stored in phosphate-buffered 10% formalin (pH 7.3) for
histological examination. The remainder of the heart was frozen and
stored at −80°C until required for biochemical examination. The
levels of Ang II in the heart were detected using a
radioimmunoassay kit (Beijing Sino-UK Institute of Biological
Technology).
Histology
The thoracic aorta and heart were stained with
hematoxylin and eosin (H&E) and Masson's trichrome
respectively. Images were viewed and captured using the Leica
Q550CW Image Analysis System (Leica Microsystems GmbH, Wetzlar,
Germany) and analyzed using ImagePro Plus version 3.0 (Media
Cybernetics,. Rockville, MD, USA) to determine the aortic
cross-sectional area (CSA) and media to lumen (M/L) ratio.
Western blot analysis
VSMCs exposed to Ang II in the presence or absence
of OW1 (5, 10 or 20 µM) as in the proliferation assay, or thoracic
aortas from individual rats in each group were lysed in
radioimmunoprecipitation assay lysis buffer containing 0.1 M
phenylmethylsulfonyl fluoride and centrifuged at 13,400 × g for 20
min. The protein concentrations of the supernatants were determined
by bicinchoninic acid (BCA) Protein Assay (Applygen Technologies,
Inc., Beijing, China). Extracts were boiled in a 1:1 ratio with
loading buffer containing Tris (125 mmol/l, pH 6.8), 4% w/v sodium
dodecylsulfate, 10% v/v glycerol, 4% v/v 2-mercaptoethanol, and 2
mg/ml bromophenol blue. Equal amounts of protein (30–60 µg) were
resolved by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE; 10%) and transferred to nitrocellulose
membranes (GE Healthcare Life Sciences, Logan, UT, USA). The
membranes were incubated overnight at 4°C with mouse anti-α-smooth
muscle actin polyclonal antibody (α-SMA; 1:200; BM0002; Wuhan
Boster Biological Technology, Ltd., Wuhan, China), rabbit
anti-MMP-2 polyclonal antibody (1:500; ab37150; Abcam, Hangzhou,
China), rabbit anti-collagen I polyclonal antibody (1:100;
bs-10423R; Beijing Biosynthesis Biotechnology Co., Ltd., Beijing,
China), and rabbit anti-glyceraldehyde-3-phosphate dehydrogenase
polyclonal antibody (GAPDH; 1:500; AB-P-R 00; Hangzhou Xianzhi Bio
Tech Co., Ltd., Hangzhou, China) as an internal control. After
wshing the membranes three times with phosphate-buffered saline
containing Tween-20, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (1:2,000; 31460; Shaanxi
Xianfeng Biotechnology Co., Ltd., Shaanxi, China) for 45 min at
37°C, followed by incubation with Western Blotting Luminol Reagent
(Shanghai Tuoran Technology Co., Ltd., Shanghai, China). Images of
the membranes were captured using an image acquisition and analysis
system (ChampGel™ 6000; Beijing Sage Creation Science Co., Ltd.,
Beijing, China), and the relative expression levels were determined
using ImageJ software, version 1.46r (National Institutes of
Health, Bethesda, MA, USA).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. One-way analysis of variance followed by a Tukey's
multiple comparison test was used to test the significance between
three or more groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
OW1 induces vasodilatation of rat
coronary arteries and inhibits Ang II-induced VSMC proliferation
in vitro
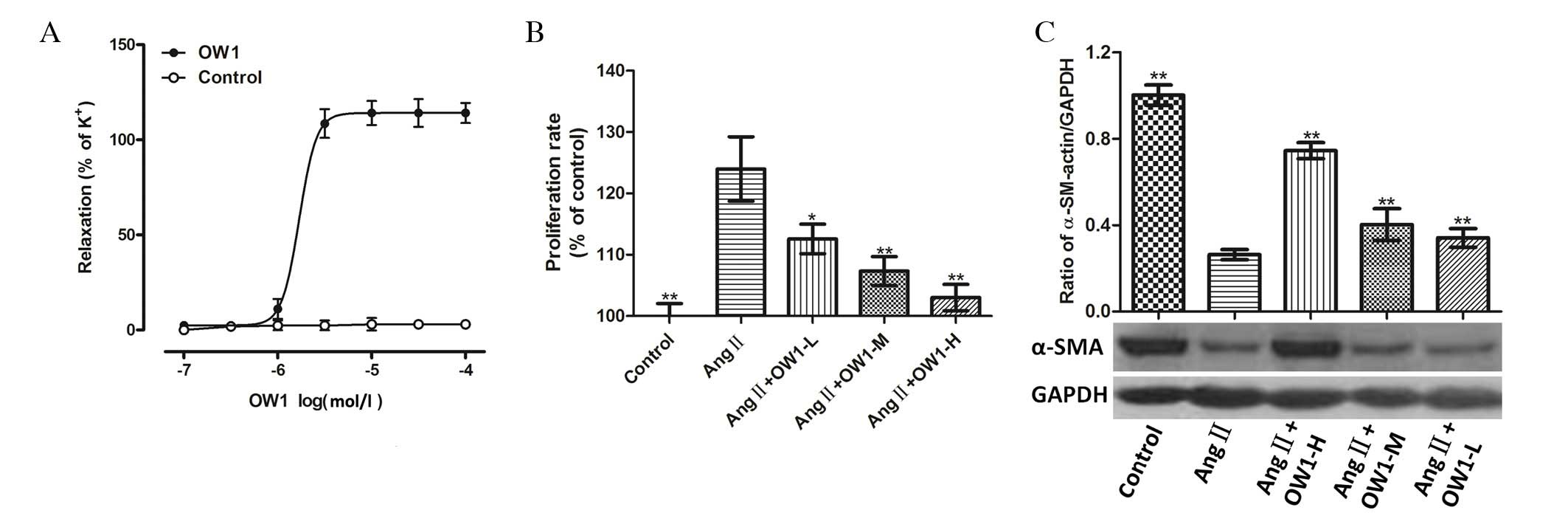
OW1 (0.1 µM-0.1 mM) relaxed the CA segments that
were pre-contracted with KCl in a concentration-dependent manner.
The maximum relaxation effect (Emax) of OW1 on the CA segments was
114.1±2.3% and the median effective concentration (EC50)
was 1.00±0.05 µM (Fig. 2A).
The MTT assay showed that OW1 (5 µM) inhibited Ang
II-induced VSMC proliferation (Fig.
2B). Higher concentrations of OW1 (10 or 20 µM) exhibited a
stronger inhibitory effect (P<0.01 vs. Ang II; Fig. 2B). The examination of the expression
levels of α-SMA by western blot analysis showed that the α-SMA
protein levels were lower in the VSMCs treated with Ang II alone.
The OW1 treatment (5, 10 and 20 µM) attenuated the Ang-II-mediated
inhibition of α-SMA levels in a concentration-dependent manner
(Fig. 2C).
OW1 reduces blood pressure in
2K1C-induced hypertension
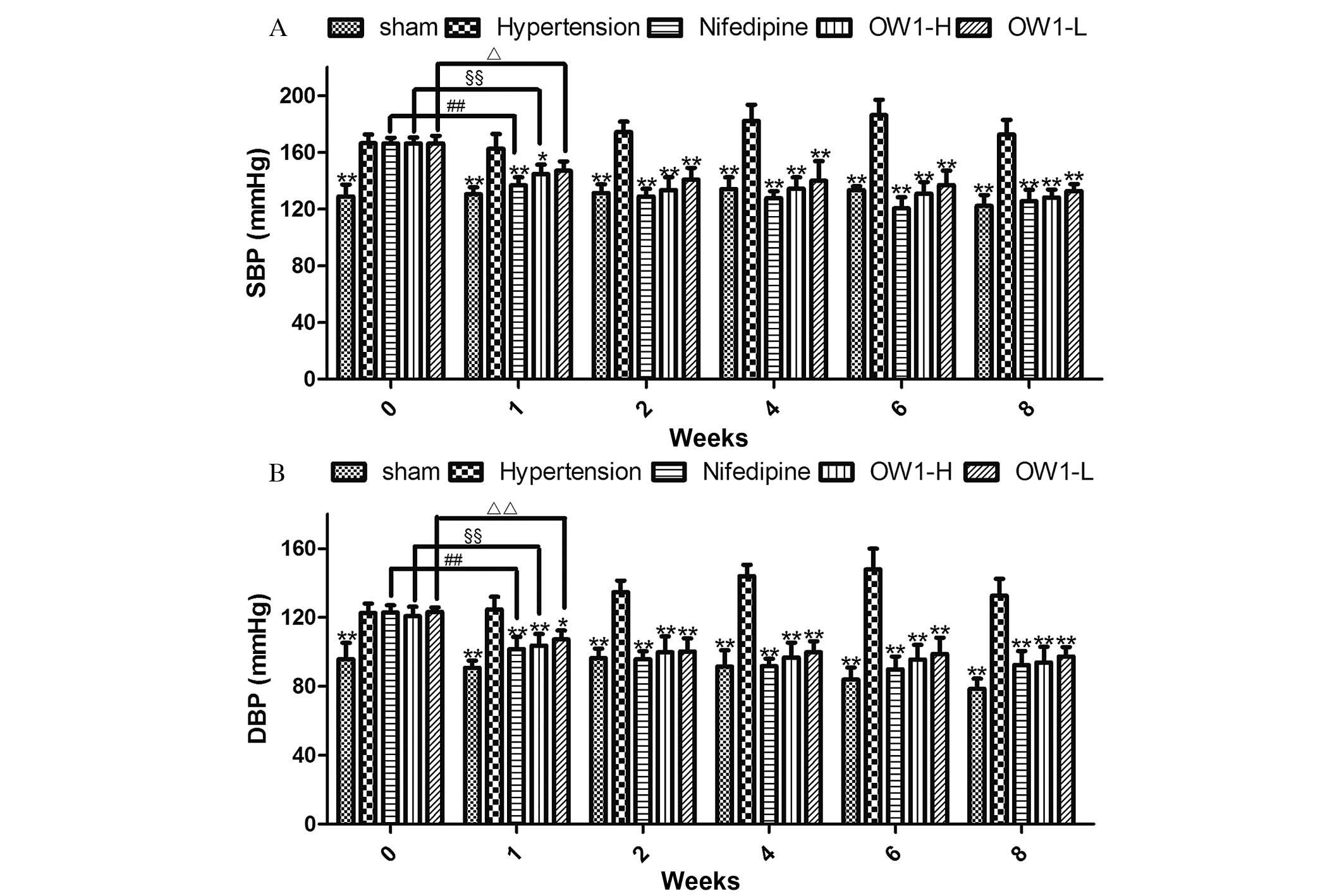
The baseline blood pressure values among the groups
were not observed to differ significantly, with the exception that
the sham-operated group had a lower SBP and diastolic blood
pressure (DBP) compared with the groups subjected to the 2K1C
modeling procedure (P<0.01; Fig.
3). During week 1 of treatment, the SBP and DBP were markedly
reduced in the nifedipine, OW1-H and OW1-L groups compared with
those in the hypertension group (P<0.05) or the baseline value
of the respective group (P<0.05).
OW1 reduces cardiac weight, and Ang
II, ALD and TGF-β1 concentration
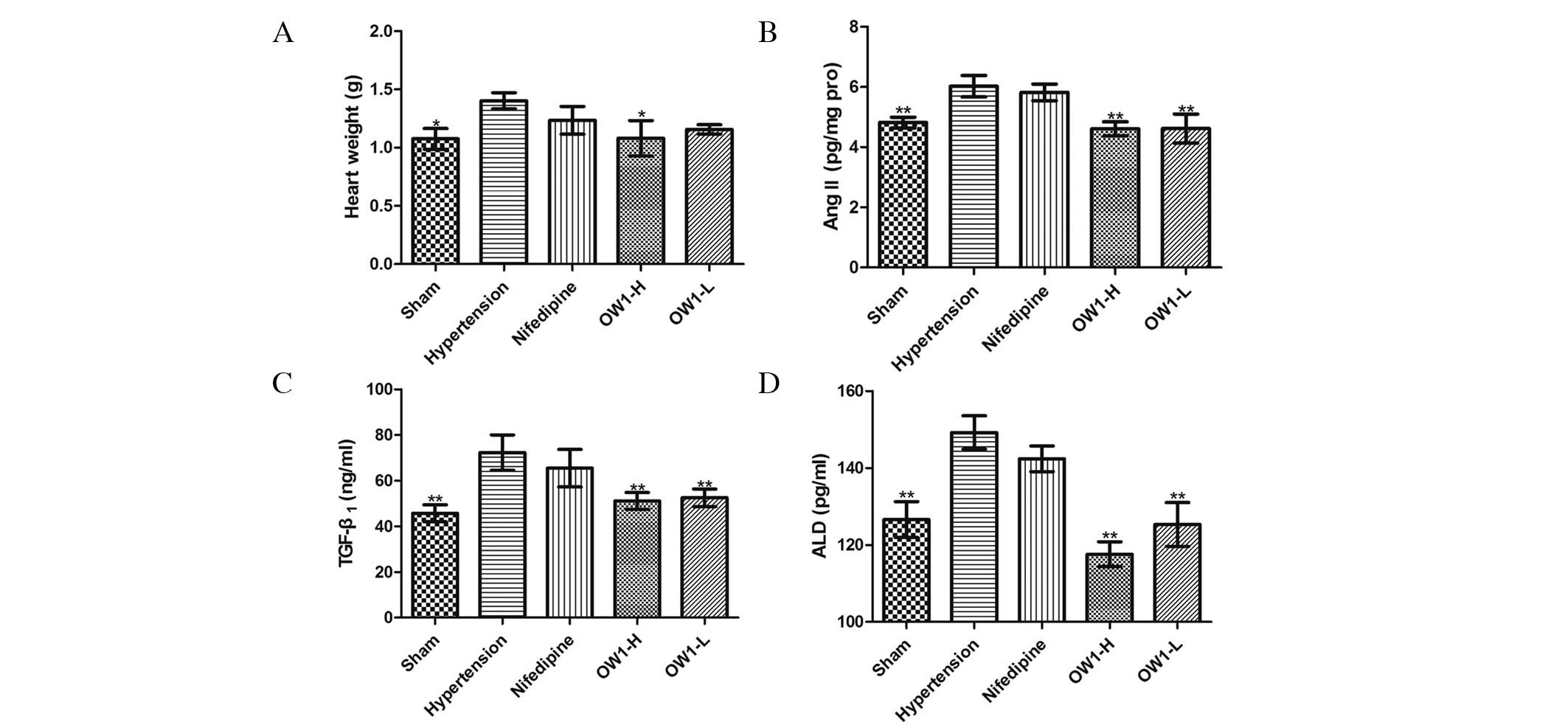
The mean heart weight was higher in the hypertension
group compared with the sham group (P<0.05). Compared with the
hypertension group, the heart weight of the OW1-H group was
significantly decreased (P<0.05; Fig.
4A). However, the mean heart weights in the nifedipine and
OW1-L groups were not significantly different from that in the
hypertension group. The cardiac concentration of Ang II was lower
in the OW1 groups compared with the hypertension group (P<0.05;
Fig. 4B), but no difference was
observed between the nifedipine and hypertension groups. Plasma
levels of ALD and TGF-β1 were elevated in the hypertension group
(P<0.01 vs. sham group) and were reduced in the two OW1 groups
but not in the nifedipine group (P<0.01 vs. hypertension group;
Fig. 4C and D).
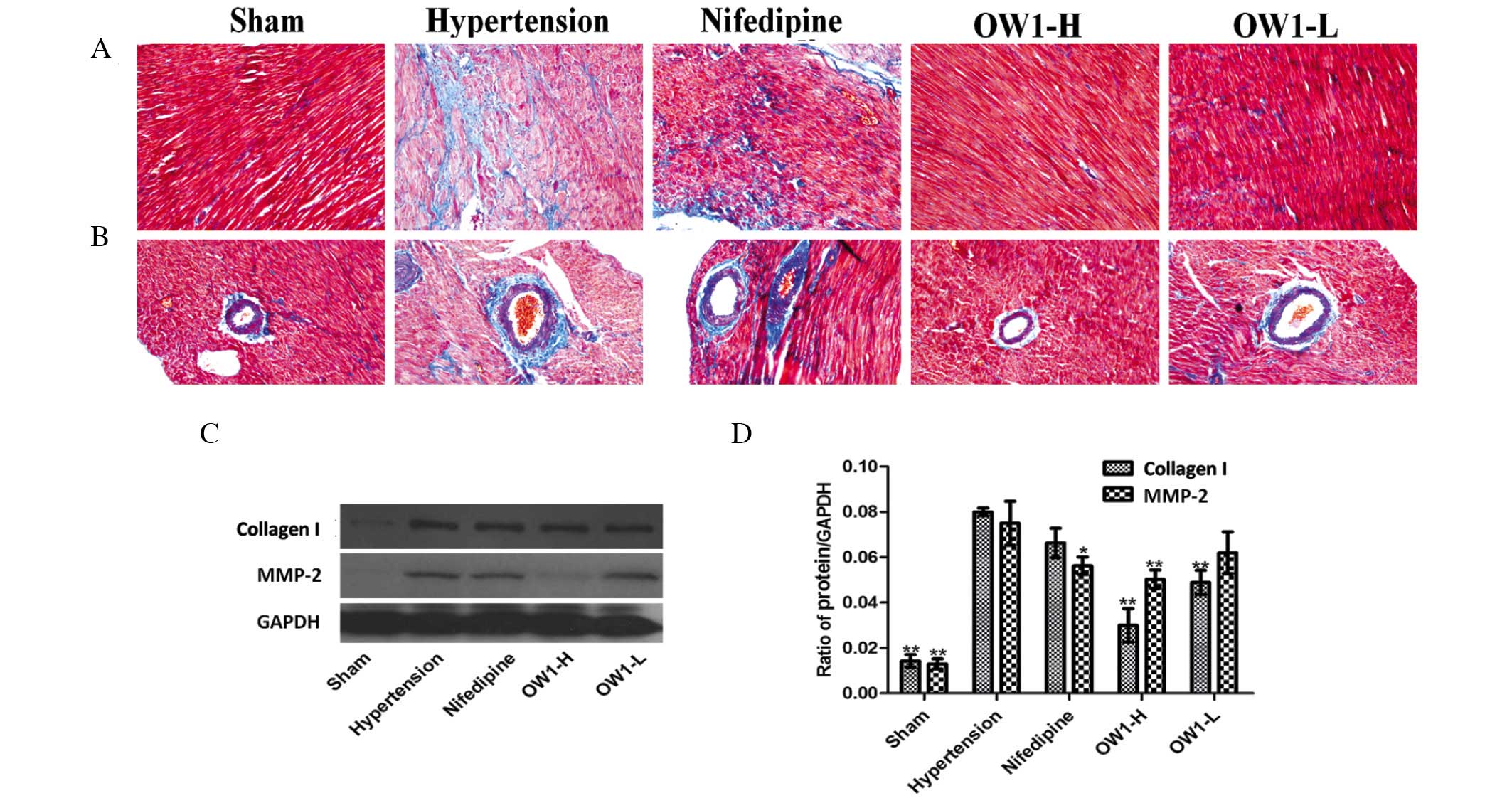
OW1 attenuates cardiac remodeling
Cardiac remodeling was observed in the hypertension
groups, with significant myocardial fibrosis (Fig. 5A). In particular, the area around the
microvasculature exhibited serious fibrosis (Fig. 5B). OW1 reduced the fibrosis to a
greater extent than nifedipine, even though the nifedipine group
had only a slight amount of myocardial fibrosis. Fibrosis in the
myocardium or around the microvasculature was difficult to identify
in the OW1 groups. Western blot analysis verified the above
results. The expression of collagen I in the heart was higher in
the hypertension group compared with the sham group (P<0.01).
The OW1 groups showed lower expression levels of collagen I
compared with that in the hypertension group (P<0.01). However,
the nifedipine group exhibited no difference from the hypertension
group. The MMP-2 level was also elevated in the hypertension group
(P<0.01 vs. sham group). OW1-H and nifedipine reduced the MMP-2
level, but no reduction was observed in the OW1-L group (Fig. 5C and D).
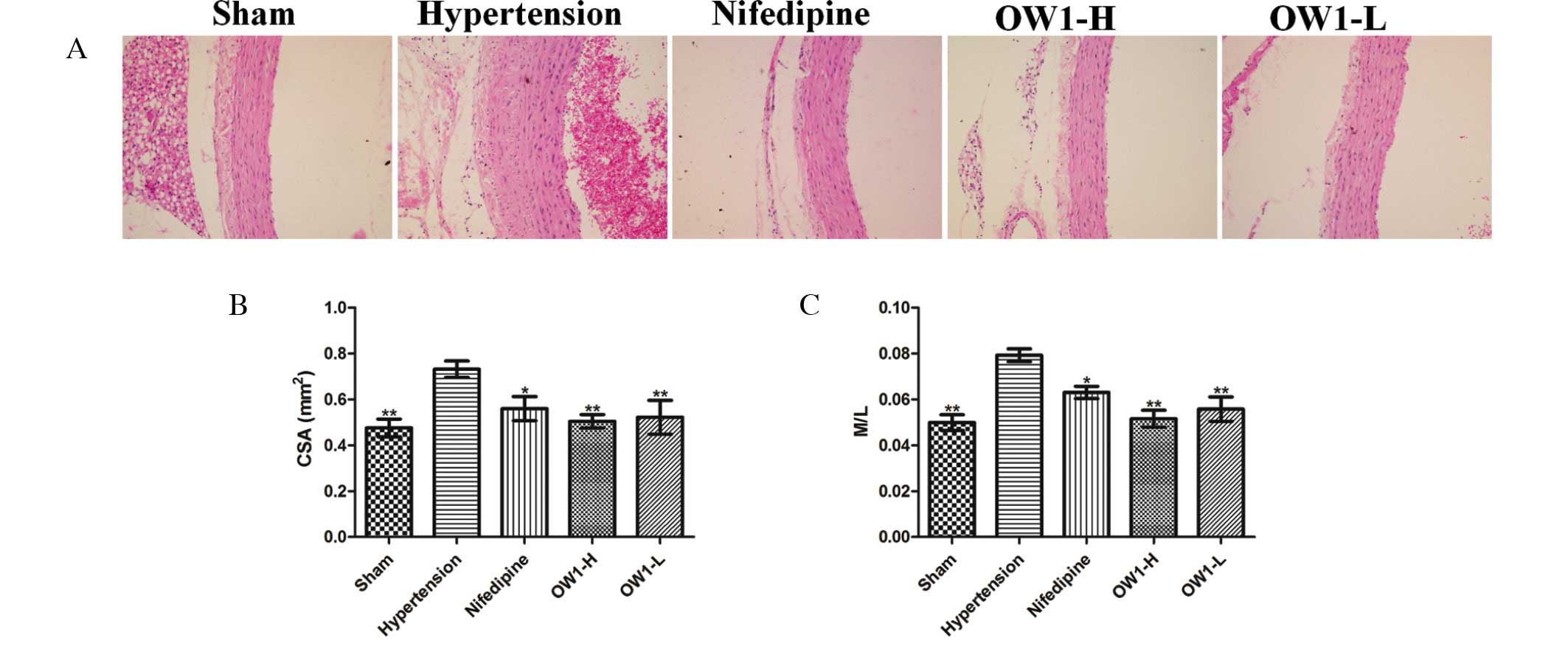
OW1 attenuates vascular
remodeling
Serious arterial wall hypertrophy was observed in
the hypertension group (Fig. 4). The
vascular wall was thickened markedly with significant increases in
VSMC numbers. The aortic CSA and M/L ratio in the hypertension
group were higher than those in the sham group (P<0.01).
Correspondingly, reduced CSA and M/L ratios were observed in the
OW1 and nifedipine groups (P<0.01 vs. hypertension group;
Fig. 6).
Discussion
The 2K1C renovascular hypertensive rat is a widely
used model of chronic hypertension; it resembles human renovascular
hypertension, with activation of the RAAS and increased Ang II
levels, which promotes vascular hypertrophy and cardiac remodeling
(22). The initial cardiac
hypertrophic response to hypertension in the 2K1C model involves an
increase in collagen deposition, which may be due to imbalanced
MMP-2 and tissue inhibitor of metalloproteinase-4 levels, resulting
in increased gelatinolytic activity, and elevated TGF-β and ROS
levels that persist until later phases of hypertension (23).
In the present study, the blood pressure in the
hypertensive rats was markedly reduced by OW1, and the
antihypertensive effects of high-dose OW1 were comparable with
those of nifedipine. The effect of OW1 on VSMC proliferation was
investigated in vitro. OW1 (5, 10 and 20 µM) inhibited Ang
II-induced VSMC proliferation and the phenotypic modulation of
VSMCs induced by Ang II, indicated by the finding that OW1
increased α-SMA levels. Moreover, OW1 inhibited VSMC proliferation
in 2K1C renovascular hypertensive rats in vivo. The
histology results illustrated that OW1 decreased the number of
VSMCs in the vessel wall and reduced vascular hypertrophy, with low
arterial CSA and M/L ratios in the OW1 groups. A particularly
notable observation in the present study was that OW1 reduced
cardiac remodeling. It has been reported that cardiac weight
increases from 15 to 75 days after arterial clipping in 2K1C
hypertensive rats (23). Increased
cardiac weight is associated with cardiomyocyte hypertrophy and
increased collagen deposition in hypertensive animals (8). In the present study, OW1 decreased the
cardiac weight and fibrosis in the myocardium or around the
microvasculature was barely visible in the OW1 groups. Western
blotting results also documented that OW1 decreased collagen I
expression in the cardiac muscle compared with that in the
hypertension group. Activation of the RAAS may induce cardiac
alterations, with increased Ang II formation causing TGF-β
activation resulting in cardiac hypertrophy and fibrosis (24,25). Ang
II, in addition to constricting blood vessels and stimulating the
adrenal cortex to secrete ALD, increase blood volume and elevate
blood pressure, also has a role in cardiovascular remodeling, heart
failure, arterial atherosclerosis and glomerular sclerosis
(26–28). Ang II exerts hypertrophic effects via
the formation of ROS (29,30), which increase TGF-β levels, a
requisite for the development of Ang II-induced cardiac hypertrophy
(31). TGF-β indirectly activates
MMPs by involvement in the transition of fibroblasts to
myofibroblasts, which produce MMPs (1). MMPs contribute to the progression of
cardiac hypertrophy to heart failure (32–35);
they degrade fibrillar collagen and contribute to cardiac
dysfunction and remodeling of the left ventricle in hypertension
(2,36). Notably, the overexpression of MMP-2
has been found to induce profound ventricular remodeling and
systolic dysfunction in the absence of superimposed injury
(37), which may be a result of
MMP-2 acting on intracellular targets and potentially impairing
myocardial contractility (38).
Increased cardiac levels of MMP-2 have been found to be
co-localized with gelatinolytic activity in the heart tissue during
all phases of 2K1C-induced hypertension (23). The results of the present study
showed that OW1 decreased the TGF-β levels in plasma, and the
levels of Ang II and MMP-2 in heart tissue, which clarify the
inhibitory effect of OW1 on cardiovascular remodeling.
The results of the present study suggested that OW1
had antihypertensive and inhibitory effects on vascular and
cardiovascular remodeling. Therefore, OW1 may reduce the risk of
hypertension-induced cardiovascular diseases, which has potential
clinical implications.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81230079, 81227802
and 81202494).
References
|
1
|
Berk BC, Fujiwara K and Lehoux S: ECM
remodeling in hypertensive heart disease. J Clin Invest.
117:568–575. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Opie LH, Commerford PJ, Gersh BJ and
Pfeffer MA: Controversies in ventricular remodelling. Lancet.
367:356–367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arribas SM, Hinek A and González MC:
Elastic fibres and vascular structure in hypertension. Pharmacol
Ther. 111:771–791. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Humphrey JD: Mechanisms of arterial
remodeling in hypertension: Coupled roles of wall shear and
intramural stress. Hypertension. 52:195–200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rossi MA: Pathologic fibrosis and
connective tissue matrix in left ventricular hypertrophy due to
chronic arterial hypertension in humans. J Hypertens. 16:1031–1041.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rossi MA and Peres LC: Effect of captopril
on the prevention and regression of myocardial cell hypertrophy and
interstitial fibrosis in pressure overload cardiac hypertrophy. Am
Heart J. 124:700–709. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosenkranz S: TGF-beta1 and angiotensin
networking in cardiac remodeling. Cardiovasc Res. 63:423–432. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rizzi E, Castro MM, Ceron CS, Neto-Neves
EM, Prado CM, Rossi MA, Tanus-Santos JE and Gerlach RF: Tempol
inhibits TGF-β and MMPs upregulation and prevents cardiac
hypertensive changes. Int J Cardiol. 165:165–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sadoshima J and Izumo S: Molecular
characterization of angiotensin II-induced hypertrophy of cardiac
myocytes and hyperplasia of cardiac fibroblasts. Critical role of
the AT1 receptor subtype. Circ Res. 73:413–423. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao W, Zhao T, Chen Y, Ahokas RA and Sun
Y: Oxidative stress mediates cardiac fibrosis by enhancing
transforming growth factor-beta1 in hypertensive rats. Mol Cell
Biochem. 317:43–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bergman MR, Teerlink JR, Mahimkar R, Li L,
Zhu BQ, Nguyen A, Dahi S, Karliner JS and Lovett DH: Cardiac matrix
metalloproteinase-2 expression independently induces marked
ventricular remodeling and systolic dysfunction. Am J Physiol Heart
Circ Physiol. 292:H1847–H1860. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sawicki G, Leon H, Sawicka J,
Sariahmetoglu M, Schulze CJ, Scott PG, Szczesna-Cordary D and
Schulz R: Degradation of myosin light chain in isolated rat hearts
subjected to ischemia-reperfusion injury: A new intracellular
target for matrix metalloproteinase-2. Circulation. 112:544–552.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang W, Schulze CJ, Suarez-Pinzon WL,
Sariahmetoglu M, Schulze CJ, Scott PG, Szczesna-Cordary D and
Schulz R: Intracellular action of matrix metalloproteinase-2
accounts for acute myocardial ischemia and reperfusion injury.
Circulation. 106:1543–1549. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou N, Wang T, Song J, He H, He J and He
L: Antihypertensive and vascular remodelling effects of the
imperatorin derivative OW1 in renovascular hypertension rats. Clin
Exp Pharmacol Physiol. 41:571–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Doggrell SA and Brown L: Rat models of
hypertension, cardiac hypertrophy and failure. Cardiovasc Res.
39:89–105. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Committee for the Update of the Guide for
the Care and Use of Laboratory Animals; National Research Council:
Guide for the Care and Use of Laboratory Animals (8th). National
Academies Press. Washington, DC: 2011.
|
|
17
|
Wang C, Wang T, Zhou N, Pan XY and He HZ:
Design, synthesis and evaluation of
9-hydroxy-7H-furo[3,2-g]chromen-7-one derivatives as new potential
vasodilatory agents. J Asian Nat Prod Res. 16:304–311. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gordon D, Mohai LG and Schwartz SM:
Induction of polyploidy in cultures of neonatal rat aortic smooth
muscle cells. Circ Res. 59:633–644. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marshall NJ, Goodwin CJ and Holt SJ: A
critical assessment of the use of microculture tetrazolium assays
to measure cell growth and function. Growth Regul. 5:69–84.
1995.PubMed/NCBI
|
|
20
|
Maliszewska-Scislo M, Chen H, Augustyniak
RA, Seth D and Rossi NF: Subfornical organ differentially modulates
baroreflex function in normotensive and two-kidney, one-clip
hypertensive rats. Am J Physiol Regul Integr Comp Physiol.
295:R741–R750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fritz M and Rinaldi G: Influence of nitric
oxide-mediated vasodilation on the blood pressure measured with the
tail-cuff method in the rat. J Biomed Sci. 14:757–765. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ceron CS, Rizzi E, Guimaraes DA,
Martins-Oliveira A, Cau SB, Ramos J, Gerlach RF and Tanus-Santos
JE: Time course involvement of matrix metalloproteinases in the
vascular alterations of renovascular hypertension. Matrix Biol.
31:261–270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rizzi E, Ceron CS, Guimaraes DA, Prado CM,
Rossi MA, Gerlach RF and Tanus-Santos JE: Temporal changes in
cardiac matrix metalloproteinase activity, oxidative stress, and
TGF-β in renovascular hypertension-induced cardiac hypertrophy. Exp
Mol Pathol. 94:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schlumberger W, Thie M, Rauterberg J and
Robenek H: Collagen synthesis in cultured aortic smooth muscle
cells. Modulation by collagen lattice culture, transforming growth
factor-beta 1 and epidermal growth factor. Arterioscler Thromb.
11:1660–1666. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang LF, Ding WH, Shi LB, Li K, Haom YJ,
Ke YN and Tang ZS: Effects of exogenous urotensin II on vascular
remodelling after balloon injury. Clin Exp Pharmacol Physiol.
37:477–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Browatzki M, Larsen D, Pfeiffer CA, Gehrke
SG, Schmidt J, Kranzhofer A, Katus HA and Kranzhofer R: Angiotensin
II stimulates matrix metalloproteinase secretion in human vascular
smooth muscle cells via nuclear factor-kappaB and activator protein
1 in a redox-sensitive manner. J Vasc Res. 42:415–423. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kranzhöfer R, Schmidt J, Pfeiffer CA, Hagl
S, Libby P and Kübler W: Angiotensin induces inflammatory
activation of human vascular smooth muscle cells. Arterioscler
Thromb Vasc Biol. 19:1623–1629. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Leeuwen RT, Kol A, Andreotti F, Kluft
C, Maseri A and Sperti G: Angiotensin II increases plasminogen
activator inhibitor type 1 and tissue-type plasminogen activator
messenger RNA in cultured rat aortic smooth muscle cells.
Circulation. 90:362–368. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baker KM and Aceto JF: Angiotensin II
stimulation of protein synthesis and cell growth in chick heart
cells. Am J Physiol. 259:H610–H618. 1990.PubMed/NCBI
|
|
30
|
Sorescu D and Griendling KK: Reactive
oxygen species, mitochondria and NAD(P)H oxidases in the
development and progression of heart failure. Congest Heart Fail.
8:132–140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jel Schultz J, Witt SA, Glascock BJ,
Nieman ML, Reiser PJ, Nix SL, Kimball TR and Doetschman T:
TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by
angiotensin II. J Clin Invest. 109:787–796. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fert-Bober J, Leon H, Sawicka J, Basran
RS, Devon RM, Schulz R and Sawicki G: Inhibiting matrix
metalloproteinase-2 reduces protein release into coronary effluent
from isolated rat hearts during ischemia-reperfusion. Basic Res
Cardiol. 103:431–443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang GY, Bergman MR, Nguyen AP, Turcato S,
Swigart PM, Rodrigo MC, Simpson PC, Karliner JS, Lovett DH and
Baker AJ: Cardiac transgenic matrix metalloproteinase-2 expression
directly induces impaired contractility. Cardiovasc Res.
69:688–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iwanaga Y, Aoyama T, Kihara Y, Onozawa Y,
Yoneda T and Sasayama S: Excessive activation of matrix
metalloproteinases coincides with left ventricular remodeling
during transition from hypertrophy to heart failure in hypertensive
rats. J Am Coll Cardiol. 39:1384–1391. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Spinale FG: Myocardial matrix remodeling
and the matrix metalloproteinases: Influence on cardiac form and
function. Physiol Rev. 87:1285–1342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takenaka H, Kihara Y, Iwanaga Y, Onozawa
Y, Toyokuni S and Kita T: Angiotensin II, oxidative stress and
extracellular matrix degradation during transition to LV failure in
rats with hypertension. J Mol Cell Cardiol. 41:989–997. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Castro MM, Rizzi E, Figueiredo-Lopes L,
Fernandes K, Bendhack LM, Pitol DL, Gerlach RF and Tanus-Santos JE:
Metalloproteinase inhibition ameliorates hypertension and prevents
vascular dysfunction and remodeling in renovascular hypertensive
rats. Atherosclerosis. 198:320–331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mujumdar VS, Smiley LM and Tyagi SC:
Activation of matrix metalloproteinase dilates and decreases
cardiac tensile strength. Int J Cardiol. 79:277–286. 2001.
View Article : Google Scholar : PubMed/NCBI
|