Introduction
Mycobacterium tuberculosis (M.
tuberculosis) is a small, rod-shaped, aerobic,
non-spore-forming bacillus and the causative agent of the majority
of cases of tuberculosis (1). Humans
are the only known reservoirs of M. tuberculosis (1). The most frequently used diagnostic
methods for tuberculosis include the tuberculin skin test,
acid-fast stain and chest radiographs (1). M. tuberculosis infections may
result in myocarditis (2),
pericarditis (3) and myopericarditis
(4). An anatomical predilection for
the right-side mediastinal lymph nodes has been described in
cardiac tuberculosis, such that the right side of the heart is the
most vulnerable area of the myocardium owing to the potential for
contiguous spread (5).
Myopericarditis is primarily a ‘pericarditic
syndrome’ in which acute pericarditis is often accompanied by a
certain degree of myocarditis (6).
The incidence of myopericarditis was 14.6% in a clinical series of
274 consecutive cases of acute pericarditis (7). Its typical symptoms include fever,
fatigue, pleuritic chest pain, a decreased exercise capacity, and
palpitations secondary to cardiac arrhythmias (6). In addition, patients with acute
myopericarditis may present with chest pain, focal ST-segment
elevation and significant elevation of cardiac enzyme levels, thus
mimicking acute myocardial infarction (AMI) (6,8).
Therefore, the differentiation of acute myopericarditis from AMI is
of great importance due to differences in the complications,
treatment strategies and prognoses between the two conditions. A
large number of studies have investigated the differentiation
between acute myopericarditis and AMI since 2008 (6,8–18). However, to the best of our knowledge,
acute myopericarditis resulting from M. tuberculosis
infection and simulating AMI has not previously been reported. The
present study reports the case of a 21-year-old male patient with
acute tuberculous myopericarditis in whom focal ST-segment
elevation and elevated cardiac enzymes were detected and mimicked
AMI.
Case report
A 21-year-old male patient presented at the
Emergency Department of Shandong Provincial Chest Hospital (Jinan,
China) in April 2014 with a sharp and nonradiating substernal chest
pain lasting for 30 min. The pain increased with inspiration and
upon lying in supine position, while it decreased significantly by
leaning forward. No rash, palpitation, dyspnea, nausea, vomiting
and diarrhea were reported. The patient smoked 10 cigarettes daily
for the last 4 years. In addition, the patient had experienced
fever, coughing, expectoration and night sweats 2 weeks prior to
admission, and had been treated with antibiotics on the basis of a
presumptive diagnosis of upper respiratory tract infection at a
local clinic. However, the symptoms did not subside significantly.
No history of hyperlipidemia, hypertension, diabetes and cocaine
abuse, or family history of premature coronary artery diseases was
reported.
Upon presentation, the patient's vital signs were
normal: Body temperature, 37.2°C; heart rate, 72 beats/min;
respiratory rate, 17 breaths/min; blood pressure, 115/70 mm Hg; and
oxygen saturation in room air, 99%. However, auscultation revealed
crackles in the left upper lung fields. The patient's heart rate
was regular and heart sounds were normal, with no murmurs, clicks,
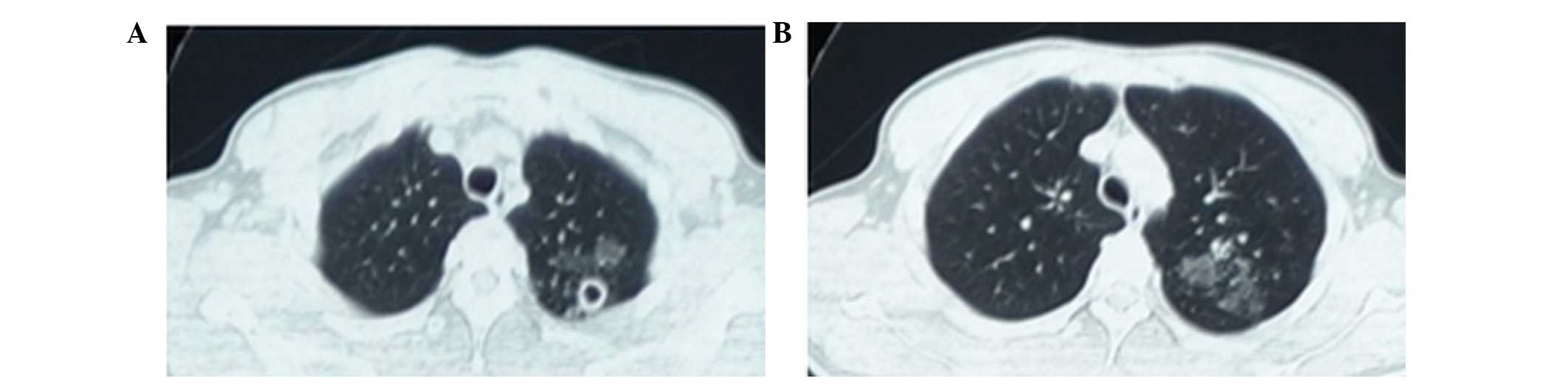
gallops or rubs. Thoracic computed tomography (CT) was performed
using a GE LightSpeed VCT 64 slice CT scanner (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA) and detected small round
thick-walled cavities in the apex of the left lung and multiple
patchy ground-glass opacities in the left upper lobe of lung
(Fig. 1).
A preliminary diagnosis of inferolateral myocardial
infarction was presumed on the basis of an abnormal
electrocardiogram (ECG; FX-8322; Beijing Fukuda Denshi Medical
Instruments Co., Ltd., Beijing, China) performed at the Emergency
Department. Subsequent to sublingual treatment with 0.5 mg
nitroglycerin (Shandong Xinyi Pharmaceutical Co., Ltd., Dezhou,
China), the patient was transferred to the Coronary Care Unit (CCU)
of the Shandong Provincial Chest Hospital with relief of chest pain
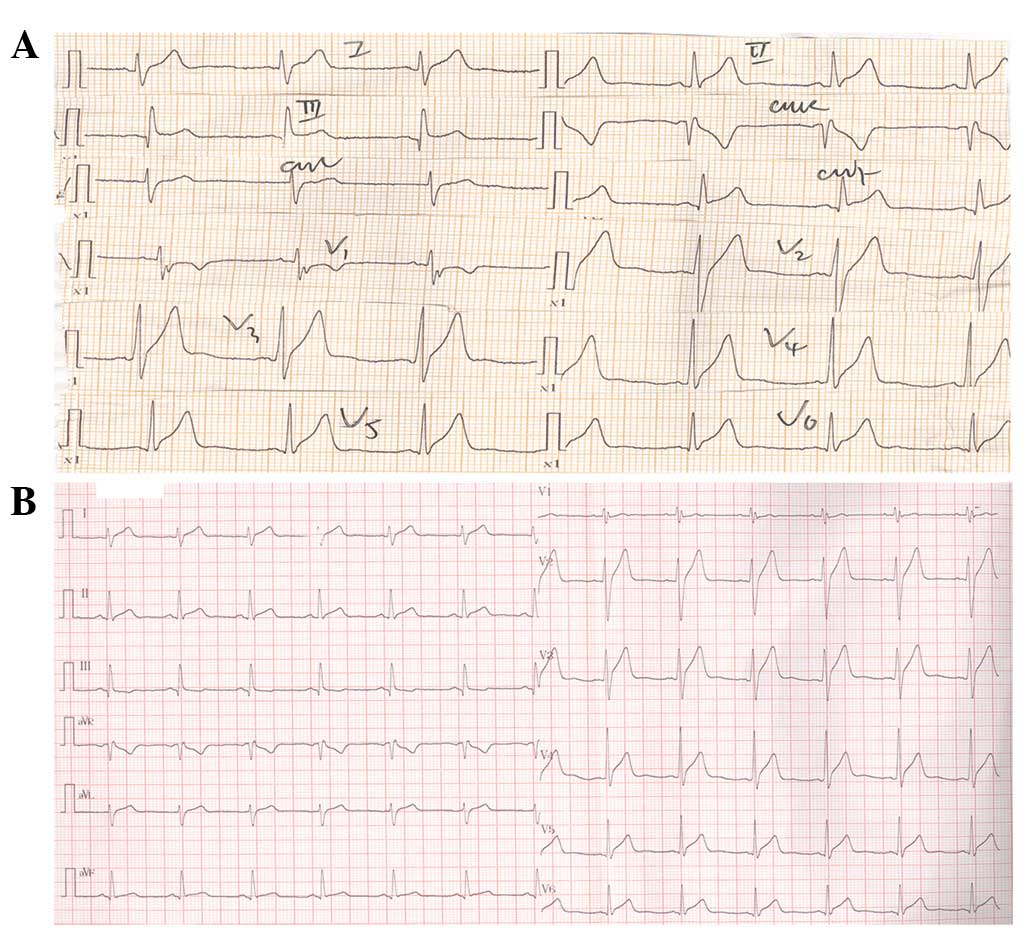
within 20 min following admission. The initial ECG revealed a
1–4-mm ST-segment elevation with coved upward pattern in the
inferior leads (II, III and AVF) and lateral precordial leads
(V5 and V6), as well as a 0.5-mm PR segment
depression in lead II (Fig. 2A). The
second ECG performed in the CCU when the patient was not presenting
chest pain showed tall positive T waves in leads
V2-V6 (Fig.
2B). The patient was found to have a history of exposure to
M. tuberculosis upon more detailed investigation.
Laboratory evaluation revealed that the erythrocyte
sedimentation rate (ESR) was 50 mm/h (normal range, 0–15 mm/h), the
levels of C-reactive protein (CRP) were 37.2 mg/l (normal range,
0–8.0 mg/l), and the cardiac enzyme levels were significantly
elevated, with cardiac troponin I (cTnI) at 1.32 µg/l (normal
level, <0.01 µg/l) and creatine kinase-MB (CKMB) fraction at
26.3 ng/ml (normal level, <5.1 ng/ml). Other laboratory values
were found to be normal. A transthoracic echocardiogram showed
slight global hypokinesis with left ventricular ejection fraction
of 55% (normal range, 50–70%).
All the aforementioned findings favored the
diagnosis of acute myopericarditis and pulmonary tuberculosis.
Therefore, the patient was administered indomethacin (50 mg, thrice
daily for 10 days; Shanxi Yunpeng Pharmaceutical Co., Ltd., Linfen,
China), imidapril (2.5 mg, once daily for 1 month; Tianjin Tianbian
Pharmaceutical Co., Ltd., Tianjin, China) and metoprolol (6.25 mg,
twice daily for 1 month; AstraZeneca, Wuxi, China), along with
antituberculosis therapy, including isoniazid (300 mg, once daily),
rifampicin (450 mg, once daily), pyrazinamide (750 mg, twice daily)
and ethambutol (750 mg, once daily; all Shanghai Sine
Pharmaceutical Co., Ltd., Shanghai, China) for 2 months, followed
by isoniazid (300 mg, once daily) and rifampicin (450 mg, once
daily) for a further 10 months.
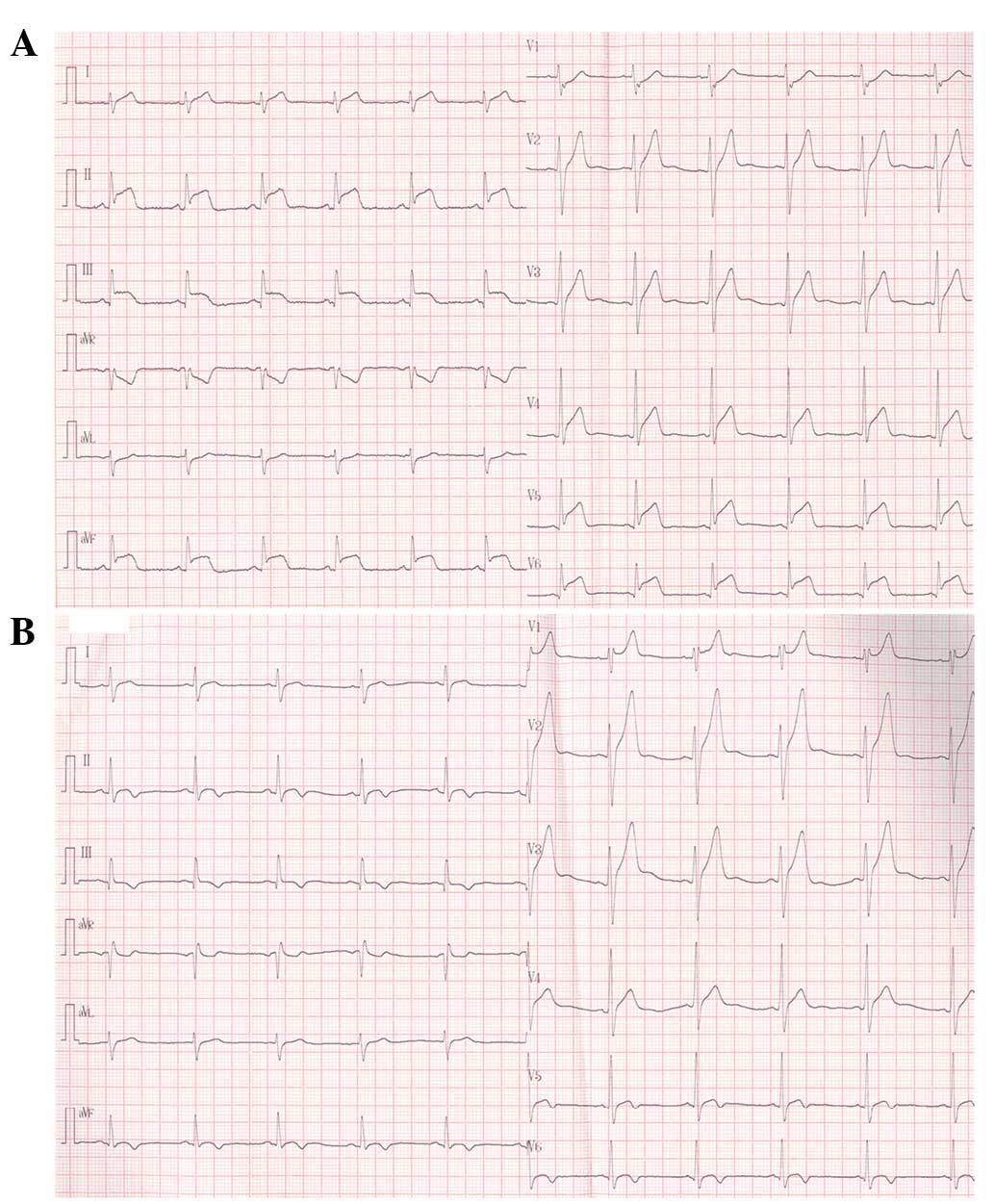
Approximately 14 h after admission, the patient
experienced a sudden relapse of squeezing chest pain. A repeat ECG
revealed 3–6-mm ST-segment elevation with coved upward pattern in
leads II, III, AVF and V4-V6 (Fig. 3A). The patient received 50 mg
indomethacin orally and 3 mg morphine (Shenyang First
Pharmaceutical Factory, Shenyang, China) intravenously. The chest
pain subsided within 5 min and did not recur. On the following day,
a coronary angiography (CAG; GE Innova 2000; GE Healthcare
Bio-sciences) revealed normal coronary anatomy and no ischemia or
occlusions. Additionally, sputum acid-fast staining was highly
positive.
Serum cTnI and CKMB levels rose to a maximum of 2.95
µg/l and 65.19 ng/ml, respectively, within 26 h after admission,
and returned simultaneously to the normal levels at day 11. The
tuberculin skin test (Chengdu Institute of Biological Products Co.,
Ltd., Chengdu, China) with a 22-mm diameter induration was strongly
positive at day 4, and the patient's temperature fluctuated between
35.8°C and 38.4°C for 6 days and normalized after day 7 of
admission. Sputum culture yielded M. tuberculosis after 20
days of incubation and was sensitive to all antituberculous agents,
while no pathogens were isolated in blood cultures.
During the subsequent 3-week hospitalization period,
the symptoms of the patient subsided gradually. The elevated
ST-segment was gradually resolved, and the T waves began to invert
in the inferolateral leads with tall and prominent positive T waves
in leads V1-V3 in May 2014 (Fig. 3B). The CRP level and ESR returned to
normal, and the echocardiogram was normalized, with an ejection
fraction of 66%. A sputum smear was negative for acid-fast bacilli.
Furthermore, serum viral titers for Coxsackie virus, adenovirus,
Epstein-Barr virus, cytomegalovirus, respiratory syncytial virus,
hepatitis A, B and C virus, as well as human immunodeficiency virus
(HIV), were all negative. A variety of immunological tests
[Euroimmun (Hangzhou) Medical Laboratory Diagnostics Co., Ltd.,
Hangzhou, China], including assays for rheumatoid factor and
anti-nuclear antibodies, such as anti-dsDNA, anti-Sm,
anti-ribonucleoprotein, anti-Ro, anti-La, anti-Jo-1 and
anti-Scl-70, anti-neutrophil cytoplasmic antibodies and anti-cyclic
citrullinated peptide antibody, excluded autoimmune disease.
In May 2014, the patient was discharged
approximately 23 days after admission with instructions to take
imidapril (2.5 mg, once daily) and metoprolol (6.25 mg, twice
daily) for 1 week, as well as antituberculous drugs as mentioned
above. In addition, the patient was advised to limit his physical
activity for 4 weeks. During the 7-month follow-up period, the
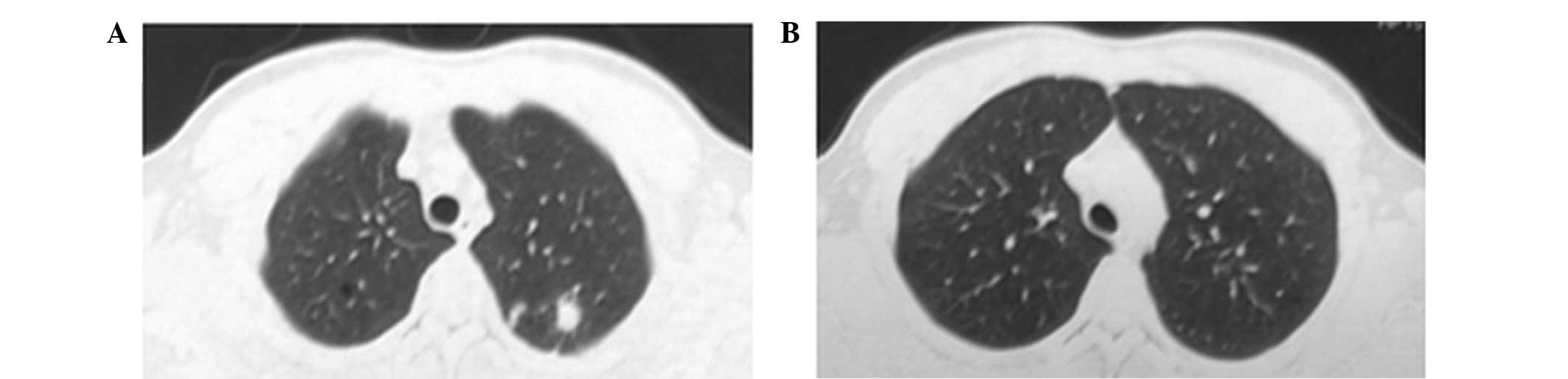
patient remained asymptomatic and the ECG was normal. Thoracic CT
scans revealed patchy shadows of high density in the left upper
lobe of lung (Fig. 4), suggesting a
significant improvement over the initial CT prior to
antituberculosis therapy. Following the withdrawal of isoniazid and
rifampicin in June 2015, the patient recovered well and had normal
CT lung images. Written informed consent was obtained from the
patient for the publication of this study.
Discussion
In clinical practice, pericarditis and myocarditis
coexist since they share common etiologic agents. However, these
two conditions are rarely of equivalent intensity, resulting in
clinical syndromes that are mainly ‘pericarditic’ or ‘myocarditic’.
Myopericarditis indicates a primarily pericarditic syndrome with
minor degrees of myocardial involvement, whereas the term
perimyocarditis suggests a mainly myocarditic syndrome (6).
The most common cause of myopericarditis is viral
infection, followed by bacterial infection. Among bacterial causes,
tuberculosis is possibly the most important cause worldwide due to
the high number of cases in developing countries, particularly in
the setting of HIV coinfection (19,20).
Tuberculous pericarditis caused by M. tuberculosis is found
in ~1% of all autopsied cases of tuberculosis and in 1–2% of cases
with pulmonary tuberculosis (21).
In the present case, tubercle bacilli detected in a sputum smear
and culture demonstrated indirectly the diagnosis of tuberculous
myopericarditis based on the exclusion of viral infection, other
bacterial infections and autoimmune disease. Although a previous
case of tuberculous myopericarditis has recently been published
(4), to the best of our knowledge,
the present study reports the first case of acute tuberculous
myopericarditis mimicking AMI.
The present study performed a literature search to
identify previous reports of acute myopericarditis or
perimyocarditis mimicking AMI. The following search terms in
‘Titles and Abstracts’ were used to retrieve relevant articles from
Scopus (http://www.scopus.com), MEDLINE
(http://www.ncbi.nlm.nih.gov/pubmed)
and ScienceDirect (www.sciencedirect.com) databases from inception to
December 2014: ‘myopericarditis or perimyocarditis’ and ‘mimicking
or simulating or masquerading’ and ‘myocardial infarction or acute
coronary syndrome’ and ‘English’. The wildcard term ‘*’ was used to
increase the sensitivity of the search strategy. Only the
literature denoting myopericarditis or perimyocarditis mimicking
AMI were included. A total of 11 cases (9–18) of
acute myopericarditis or perimyocarditis mimicking AMI (Table I) were identified by this search, of
which there was 1 case with Streptococcus infection, 1 case
with Campylobacter jejuni, 1 case with herpes simplex virus
II and 1 case with varicella, whereas unclear causes were reported
in the remaining cases. All patients in previous cases were males
aged 12–26 years old, with the exception of a 58-year-old man. One
or more risk factors for coronary artery disease (CAD), included
smoking, obesity and dyslipidemia, were found in 3 patients.
 | Table I.Reported cases of acute
myopericarditis/perimyocarditis mimicking acute myocardial
infarction. |
Table I.
Reported cases of acute
myopericarditis/perimyocarditis mimicking acute myocardial
infarction.
| Case | Year | Age/gender | Risk factors for
CAD | Organism | ST-segment elevation
leads | Symptoms other than
chest pain | Therapy | Refs. |
|---|
| 1 | 2008 | 25/M | Smoking |
Streptococcus | I, AVL | Vomiting,
dyspnea | Penicillin | 9 |
| 2 | 2009 | 26/M | No | HSV-2 | All but AVR | Fever | Valacyclovir | 10 |
| 3 | 2009 | 19/M | Smoking, obesity | C. jejuni | All but AVR | Diarrhoea | Erythromycin,
carvedilol, ramipril | 11 |
| 4 | 2009 | 18/M | No | Unclear | II, III, AVF,
V5, V6 | Fever | Indomethacin | 12 |
| 5 | 2009 | 20/M | No | Unclear | II, III, AVF,
V3-V6 | Syncope, dyspnea | NA | 13 |
| 6 | 2010 | 15/M | No | Unclear | II, III, AVF,
V5, V6 | Fever | NA | 14 |
| 7 | 2011 | 17/M | No | Varicella | II, III, AVF,
V4-V6 | Fever | Indomethacin,
acyclovir, esomeprazole | 15 |
| 8 | 2013 | 58/M | Dyslipidemia | Unclear | II, III, AVF,
V5, V6 | No | NA | 16 |
| 9 | 2014 | 16/M | No | Unclear | II, III, AVF | No | NSAID, carvedilol,
enalapril | 17 |
| 10 | 2014 | 15/M | No | Unclear | III, AVF,
V3-V6 | Palpitation | NA | 17 |
| 11 | 2014 | 12/M | No | Unclear | II, III, AVF,
V4-V6 | Nausea, vomiting,
chills | NSAID,
morphine | 18 |
| 12 | 2014 | 21/M | Smoking | M.
tuberculosis | II, III, AVF,
V5, V6 | Fever, cough,
expectoration, night sweats | Indomethacin,
morphine, imidapril, metoprolol, isoniazid, rifampicin
pyrazinamide, ethambutol | Present |
Myopericarditis can be clinically defined as a
definite diagnosis of acute pericarditis and elevation of cardiac
markers of injury (cTnI or troponin T, and CKMB fraction), without
further focal or diffuse depressed left ventricular function
observed on a echocardiogram or cardiac magnetic resonance (CMR)
scan (8). In the present study, the
patient's symptoms of sharp, positional chest pain and minimal
pericardial effusion were suggestive of acute pericarditis. The
levels of cTnI and CKMB were markedly elevated, while the left
ventricular function was normal. These observations are in
accordance with the clinical diagnosis of myopericarditis. However,
the focal ST segment elevation in inferolateral leads resulted in
difficulties in distinguishing acute myopericarditis from AMI. A
typical pattern of ECG evolution includes diffuse ST elevation and
PR depression, followed by normalization of the ST and PR segments,
and subsequent diffuse T-wave inversions (22). Imazio et al (7) demonstrated that certain findings
occurred significantly more frequently in patients with
myopericarditis compared with those in acute pericarditis patients,
and these findings included: Atypical ECG changes and T-wave
inversion prior to ST-segment normalization; and cardiac
arrhythmias, including supraventricular or ventricular ectopic
beats, as well as nonsustained ventricular tachycardia.
Although a triad of acute chest pain, elevated
cardiac enzymes and localized ST-segment elevation in inferolateral
leads mimicked AMI, the present patient had a concave upwards
morphology of the ST-segment in leads II, III, AVF and
V5-V6 and a PR-segment deflection opposite to
P-wave polarity in lead II, suggesting a diagnosis of
myopericarditis. ST-segment elevations were almost localized in
inferolateral leads of the 11 previously-reported cases (Table I). Electrocardiographic abnormalities
associated with acute myopericarditis have been previously
attributed to changes in repolarization involving the ventricle and
atrium, which are due to epicardial inflammation. These
repolarization changes consequently affect the morphology of the PR
segment, ST segment, and T wave (22).
Differential diagnosis of acute myopericarditis
based on the ECG findings included benign early repolarization
(BER) and AMI (22). A previous
study found that the morphology of ST segment elevation in BER
patients is similar to that of acute myopericarditis, presenting an
indistinct J point and an initial concave upsloping. Unlike in
acute myopericarditis, the ST segment elevation in BER is mainly
observed in the precordial leads, with most prominent appearance in
the right precordial leads and less commonly in lead V6
(where the ST segment is frequently isoelectric) (23). Furthermore, ST segment elevation is
often short term in acute myopericarditis, whereas its short-term
resolution is not observed in BER and it may transiently return to
the baseline upon exercise. However, differentiating between AMI
and acute myopericarditis on an ECG can be challenging. Regarding
the ST morphology, the elevation is initially convex in AMI,
whereas it is concave in acute myopericarditis. In addition, Q
waves may be observed in AMI, however, they are rarely reported in
acute myopericarditis ECGs. By contrast, PR segment depression
suggests a diagnosis of acute myopericarditis rather than AMI, as
indicated by positional chest pain (22).
In the present case, a number of evidence favored
the diagnosis of myopericarditis, including the young age of the
patient, the single risk factor for CAD (smoking 10 cigarettes
daily for 4 years), history of pulmonary tuberculous infection,
concave upward ST segment elevation, PR segment depression and the
absence of regional wall motion abnormalities on an echocardiogram.
However, the elevated cardiac enzyme levels and the ST segment
elevation in inferolateral leads mimicked a diagnosis of AMI, which
was subsequently excluded by CAG examination and
echocardiography.
Other imaging examinations for the differentiation
between acute myopericarditis and AMI include contrast-enhanced
CMR, 64-slice CT coronary angiography and endomyocardial biopsy. In
acute myocarditis, CMR shows an abnormal patchy myocardial signal
with delayed enhancement suggesting myonecrosis and edema, without
presenting in the subendocardial region, which exclude the
diagnosis of CAD (24). In acute
myopericarditis, similar findings upon the use of CMR further
confirm that the subepicardial myocardium is involved and the
subendocardial region is spared (13,16,17).
Although biopsy is the main technique used for the diagnosis of
myocarditis, it may be of limited clinical value in certain cases,
particularly in myopericarditis, where pericardial involvement is
prevalent. In the 11 identified cases of acute myopericarditis
mimicking AMI (9–18), endomyocardial biopsy of the right
ventricle was only performed in 1 case, and the results
demonstrated a number of the expected histological features of
myocarditis, including endomyocardial fibrosis, myocytolytic
changes and interstitial edema (14).
The management of acute myopericarditis is primarily
aimed at the pathogen, pericarditis and myocarditis, with the
exception of the recommendation of rest and avoiding of physical
activity for 4–6 weeks (6). Therapy
for the pathogen involves the use of antiviral drugs, antibiotics
or antituberculosis medications (6).
In the absence of significant myocardial failure, the management of
myopericarditis is similar with that in acute pericarditis, with
nonsteroidal anti-inflammatory drugs (NSAIDs) being the commonly
administered therapy (6). Ibuprofen
may be the preferred NSAID since side effects are rare, and it has
a favorable impact on coronary artery blood flow and a large dose
range (25). Other medications
lacking evidence-based data in myopericarditis include
corticosteroid, colchicine and intravenous immunoglobulin (6). In acute myopericarditis with decreased
left ventricular ejection fraction, treatment includes myocardial
protection and inhibition of cardiac remodeling. In the present
case, the prescribed therapeutic measures, including rest,
indomethacin, imidapril, metoprolol and antituberculosis agents,
were completely performed and the patient recovered well.
In conclusion, the current study reported for the
first time a case of acute tuberculous myopericarditis mimicking
AMI in a 21-year-old male patient. The case highlights the
importance of a detailed collection of medical history,
comprehensive explanations of serial ECGs, thoracic CT scan,
echocardiogram and CAG in the diagnosis and differentiation of
acute tuberculous myopericarditis mimicking AMI.
Acknowledgements
The present study was sponsored by grants from the
Natural Science Foundation of China (no. 81270238) and the
Scientific Research Foundation for the Doctoral Degree, State
Education Ministry of China (no. 20100131110059), and was supported
by the Scientific Development Plan of Shandong Province of China
(no. 2012G0021850).
References
|
1
|
Goldman L and Schafer AI: Tuberculosis.
Goldman-Cecil Medicine. 1:(25th). Elsevier Saunders. (Philadelphia,
PA). 2030–2037. 2014.
|
|
2
|
Michira BN, Alkizim FO and Matheka DM:
Patterns and clinical manifestations of tuberculous myocarditis: A
systematic review of cases. Pan Afr Med J. 21:1182015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lazaros G and Tousoulis D: Tuberculous
Pericarditis: A Complex Puzzle to Put Together. EBioMedicine.
2:1570–1571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Desai N, Desai S, Chaddha U and Gable B:
Tuberculous myopericarditis: A rare presentation in an
immunocompetent host. BMJ Case Rep 2013. 2013.
|
|
5
|
Maeder M, Ammann P, Rickli H and Schoch
OD: Fever and night sweats in a 22-year-old man with a mediastinal
mass involving the heart. Chest. 124:2006–2009. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Imazio M and Trinchero R: Myopericarditis:
Etiology, management and prognosis. Int J Cardiol. 127:17–26. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Imazio M, Cecchi E, Demichelis B,
Chinaglia A, Ierna S, Demarie D, Ghisio A, Pomari F, Belli R and
Trinchero R: Myopericarditis versus viral or idiopathic acute
pericarditis. Heart. 94:498–501. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Imazio M and Cooper LT: Management of
myopericarditis. Expert Rev Cardiovasc Ther. 11:193–201. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khavandi A, Whitaker J, Elkington A,
Probert J and Walker PR: Acute streptococcal myopericarditis
mimicking myocardial infarction. Am J Emerg Med. 26:638.e1–e2.
2008. View Article : Google Scholar
|
|
10
|
Tian W, Zhang Z, Bai X, Zeng D and Qi G:
Acute myopericarditis masquerading as acute myocardial infarction.
J Nanjing Med Uni. 22:130–133. 2008. View Article : Google Scholar
|
|
11
|
Lai T, Yadav R and Schrale R: Mimicking
myocardial infarction: Localized ST-segment elevation in
Campylobacter jejuni myopericarditis. Intern Med J. 39:422–423.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Omar HR, Fathy A, Rashad R and Elghonemy
M: Acute perimyocarditis mimicking transmural myocardial
infarction. Int Arch Med. 2:372009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Poh KK, Chan EH, Chia BL and Chai P: Acute
perimyocarditis masquerading as acute coronary syndrome with
spontaneous resolution of increased left ventricular wall
thickness. Ann Acad Med Singapore. 38:278–279. 2009.PubMed/NCBI
|
|
14
|
Nisbet BC and Breyer M: Acute
myopericarditis with focal ECG findings mimicking acute myocardial
infarction. J Emerg Med. 39:e153–e158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De A, Myridakis D, Kerrigan M and Kiblawi
F: Varicella myopericarditis mimicking myocardial infarction in a
17-year-old boy. Tex Heart Inst J. 38:288–290. 2011.PubMed/NCBI
|
|
16
|
Bolognesi M and Bolognesi D: Acute
coronary syndrome vs. myopericarditis-not always a straightforward
diagnosis. Am J Case Rep. 14:221–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gupta SK and Naheed Z: Chest pain in two
athletic male adolescents mimicking myocardial infarction. Pediatr
Emerg Care. 30:493–495. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharma J, Fernandes N, Alvarez D and
Khanna S: Acute myopericarditis in an adolescent mimicking acute
myocardial infarction. Pediatr Emerg Care. 31:427–430. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mayosi BM: Contemporary trends in the
epidemiology and management of cardiomyopathy and pericarditis in
sub-Saharan Africa. Heart. 93:1176–1183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krauss MR, Harris DR, Abreu T, Ferreira
FG, Ruz NP, Worrell C and Hazra R: NISDI Pediatric Study Group:
Tuberculosis in HIV-infected infants, children and adolescents in
Latin America. Braz J Infect Dis. 19:23–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fowler NO: Tuberculous pericarditis. JAMA.
266:99–103. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan TC, Brady WJ and Pollack M:
Electrocardiographic manifestations: Acute myopericarditis. J Emerg
Med. 17:865–872. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shabetai R: Acute pericarditis. Cardiol
Clin. 8:639–644. 1990.PubMed/NCBI
|
|
24
|
Kern J, Modi R, Atalay MK and Kochilas LK:
Clinical myocarditis masquerading as acute coronary syndrome. J
Pediatr. 154:612–615. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maisch B, Seferović PM, Ristić AD, Erbel
R, Rienmüller R, Adler Y, Tomkowski WZ, Thiene G and Yacoub MH:
Task Force on the Diagnosis and Management of Pricardial Diseases
of the European Society of Cardiology: Guidelines on the diagnosis
and management of pericardial diseases executive summary; The Task
force on the diagnosis and management of pericardial diseases of
the European Society of Cardiology. Eur Heart J. 25:587–610. 2004.
View Article : Google Scholar : PubMed/NCBI
|