Introduction
Silicosis is a serious occupational disease that is
characterized by the formation of early inflammatory cell nodular
lesions and late silicotic nodules, ultimately leading to
progressive silicosis (1). Autophagy
may be present in silicosis and can affect the incidence of
silicosis. Autophagy is a biological process conducted in the
majority of eucaryotic cells, which is also known as ‘cell
cannibalism’. During autophagy, cellular structures are degraded by
lysosomal activity and the cytoplasm is recycled in order to
maintain precisely calibrated environments to ensure the function
of cellular mechanisms. Thus, autophagy functions as a
‘double-edged sword’ in the fate of controlling the cells (2,3).
Autography is a normal process in cell growth, development and
homeostasis, and aids the maintenance of a balance between the
synthesis, degradation and subsequent recycling of cellular
products (3,4). Optimum rates of autophagy protect cells
against apoptosis and necrosis; however, excessive activation of
autophagy can result in type II programmed cell death (5).
A previous study indicated that bone marrow-derived
mesenchymal stem cells (BMSCs) may be effective in the treatment of
silicosis (6). BMSCs are a type of
multipotent stem cell, derived from the bone marrow, which possess
multidirectional differentiation potential (7). Furthermore, BMSCs exhibit a marked
immune function during treatment, which can prevent the immune
response-mediated rejection of allografts (8). In addition, BMSCs generate a variety of
paracrine cytokines that are effective for the promotion of
angiogenesis and wound repair (9).
In the present study, a rat model of silicosis was
established via the administration of silicon dioxide (SiO2) to the
rat bronchus using bronchoalveolar lavage (BAL). Alveolar
macrophages (AMs) were extracted using the BAL, and the activation
of autophagy in the AMs of the silicosis model rats was evaluated
using various morphological and biochemical analytical methods.
Thus, the aim of the present study was to determine the expression
levels of the autophagy-related proteins, microtubule-associated
protein light chain 3 (LC-3) and autophagy-related gene Beclin-1
(Beclin-1), in the AMs of the silicosis model rats using
morphological and biochemical detection methods, and to thereby
provide an experimental basis for the further study of the
pathogenesis of silicosis.
Materials and methods
Ethical approval
All procedures were performed in accordance with the
Institutional Guidelines for the Care and Use of Laboratory Animals
(Hebei United University, TangShan, Hebei, China), and conformed to
the National Institutes of Health (NIH) Guide for the Care and Use
of Laboratory Animals (NIH Publication no. 80–23, revised
1996).
BMSC culture. BMSCs were extracted from 5 male
Sprague-Dawley (SD) rats. Fresh bone marrow cells were collected by
flushing the medullary cavity of the rat femurs with Dulbeccos
modified Eagles medium (DMEM; Gibco Life Technologies, Grand
Island, NY, USA). After filtering, the cells were centrifuged at
167.7 × g for 5 min. The purified cells were dispersed in cell
culture flasks (Corning, Inc., Corning, NY, USA), grown in DMEM
media supplemented with 10% fetal calf serum (Gibco Life
Technologies), 100 U/ml penicillin and 100 µg/ml streptomycin
(Sigma-Aldrich, St. Louis, MO, USA), and subsequently cultured at
37°C with 5% CO2. After 48 h, the non-adherent cells
were removed and fresh media was added, with the media subsequently
exchanged every three days. Adhered cells were allowed to grow to
~90% confluency, after which the cells were trypsinized and
reseeded. Third-generation (P3) BMSCs were used for all the
subsequent experiments.
Flow cytometry. P3 BMSCs were harvested via
trypsinization (Gibco Life Technologies), and fixed in neutralized
2% paraformaldehyde solution for 30 min. Fixed cells were washed
three times with phosphate-buffered saline (PBS) and incubated for
25–30 min with antibodies against the following cell surface
antigens: CD29, anti-mouse monoclonal (cat. no. 102205); CD45,
rabbit polyclonal (cat. no. 202205); CD54, rabbit polyclonal (cat.
no. 202405); and CD106, anti-mouse monoclonal (cat. no. 200403; all
1:200; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The
primary antibodies were directly conjugated with fluorescein
isothiocyanate (CD29 and CD45) or phycoerythrin (CD54 and CD106).
Flow cytometry was performed using fluorescence-activated cell
sorting (FACS Calibur™; BD Biosciences, Franklin Lakes, NJ,
USA).
Animals and silicosis model. BMSCs were extracted
from five male SD rats (age, 6–8 weeks; weight, 200–220 g). The
recipient population of the BMSCs consisted of 60 female SD rats
(age, 3–5 weeks; weight, 100–120 g). All animals were provided by
Beijing Vital River Laboratory Animal Technology Co., Ltd.
(Beijing, China), and all procedures were performed in accordance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals (NIH Publication no. 80-23, revised 1996).
The rat model of silicosis was induced using a single 1.0-ml
infusion of SiO2 suspension (5 g/l; Sigma-Aldrich),
which was administered by non-exposed tracheal intubation. All the
rats were maintained in a room with a reversed 12-h light-dark
cycle at 21°C and 55% humidity.
Grouping and BMSC administration. The 60 female
recipient rats were allocated at random into three groups, namely
the control, model and BMSC treatment groups (n=20 per group).
Control group rats received a 1.0-ml intratracheal instillation of
sterile NaCl solution, while the model group rats received a 1.0-ml
intratracheal instillation of sterile NaCl solution and SiO2 dust
suspension (5 mg/ml). The BMSC treatment group received the same
1.0-ml SiO2 treatment (5 g/l) as the model group,
followed by a 1.0-ml suspension of BMSCs (1×106/ml) in DMEM at 12 h
after the administration of the SiO2. The rats in each
group received 10% chloral hydrate (3 ml/100 g) prior to being
sacrificed by decapitation. Five rats were sacrificed on each of
days 1, 7, 14 and 28 following treatment, after which AMs were
extracted using BAL.
AM extraction. AMs were acquired using BAL,
following which centrifugation was conducted at 377.325 × g for 10
min at 4°C. The supernatant was discarded, and the precipitate
containing the AMs and other cell types was collected. The AMs were
enriched and purified using the cell adhesion method, in which the
precipitate of the BAL fluid was washed 2–3 times with saline and
cultivated in a high glucose medium. The cells were subsequently
resuspended in DMEM with 10% fetal bovine serum, and diluted in a
suspension at 1×106 cells/ml.
Hematoxylin and eosin (HE) staining. The AMs were
placed on a glass slide, fixed with paraformaldehyde and
subsequently washed with running water. The specimens were
subjected to gradient alcohol dehydration and cleared with xylene,
after which the cytoplasm was subsequently stained with HE.
Finally, the samples were mounted with neutral gum and observed
under a microscope (AE31 inverted biological microscope; Motic
Incorporation Ltd., Causeway Bay, Hong Kong, China).
Immunocytochemical staining. The specimen slides
were purified and enriched, soaked in water to remove formaldehyde
and washed with PBS 2–3 times. The AMs were subjected to Triton
lysis for 15–20 min, incubated with 3% H2O2 for 10–15 min, antigen
retrieval was performed with trypsin at room temperature for 20
min, and sealed in 5% bovine serum albumin (Wuhan Boster Biological
Engineering, Wuhan, China) for 20 min. Next, the specimens were
incubated with rabbit anti-rat antibodies targeting LC-3 (PM036;
1:50; MBL International Corporation, Woburn, MA, USA) and Beclin-1
(ab195792; 1:100; Abcam, Cambridge, UK) at 4°C overnight. After the
samples were adjusted to room temperature, a goat anti-rabbit
secondary antibody (074–1506; 1:5,000; KPL, Inc., Gaithersburg, MA,
USA) was added and incubated at 37°C for 30 min, followed by a
streptavidin-biotin-peroxidase complex, which was incubated at 37°C
for 20 min. Specimens were visualized using 3,3-diaminobenzidine
chromogenic solution, dehydrated using an alcohol gradient,
clarified with dimethylbenzene and xylene and mounted with neutral
gum. A negative control was constructed using an identical quantity
of PBS instead of primary antibody, with all other protocol steps
unchanged.
Western blot analysis. Following removal of the cell
culture flask, the drained culture medium was washed with PBS three
times. Radioimmunoprecipitation assay lysis buffer was added to
cover the cell surface, and the solution was allowed to stand on
ice for 10–15 min. The cells were removed from the surface using a
clean and dry brush, and stored in an Eppendorf tube. Following
centrifugation at 2,414.8 × g for 15 min at 4°C, the supernatant,
which contained the cellular proteins, was extracted. The total
proteins were extracted and the protein concentration was
determined using a bicinchoninic acid assay (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China). Samples were
subjected to sodium dodecyl sulfate polyacrylamide gel
electrophoresis, and the separated proteins were transferred onto
polyvinylidene difluoride membranes (Roche Diagnostics GmbH,
Mannheim, Germany). The immunoblots were blocked with 5% fat-free
dry milk for 1 h at room temperature. Following blocking, the
membranes were incubated overnight at 4°C with polyclonal rabbit
anti-LC3 (1:500) and anti-Beclin-1 (1:200) antibodies and a
monoclonal mouse (074–1806; 1:5,000; KPL, Inc.) anti-β-actin
primary antibody (cat. no. M1210-2; 1:500; Epigentek Group Inc.,
Farmingdale, NY, USA). Next, the immunoblots were subsequently
incubated with horseradish peroxidase-conjugated anti-rabbit IgG
and anti-mouse IgG (1:5,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA) for 2 h at room temperature. The immunoblot on
the membrane was visualized following development using an enhanced
chemiluminescence detection system (ChemiDoc XRS; Bio-Rad
Laboratories, Inc., Hercules, CA, USA), and the densitometric
signals were quantified using an imaging program (Digital Medical
Image Analysis System 6.0; Motic). Immunoreactive bands for all the
proteins examined were normalized against β-actin. Western blot
results were analyzed using the National Institutes of Health Image
software, version 1.41 (Bethesda, MD, USA).
Statistical analysis. All experiments were repeated
three times and similar results were obtained. Statistical analysis
was performed using SPSS software, version 16.0 (SPSS, Inc.,
Chicago, IL, USA). Data are expressed as the mean ± standard error
of the mean. The statistical significance of the experimental
results was determined using one-way analysis of variance, where
P<0.05 was considered to indicate a statistically significant
difference.
Results
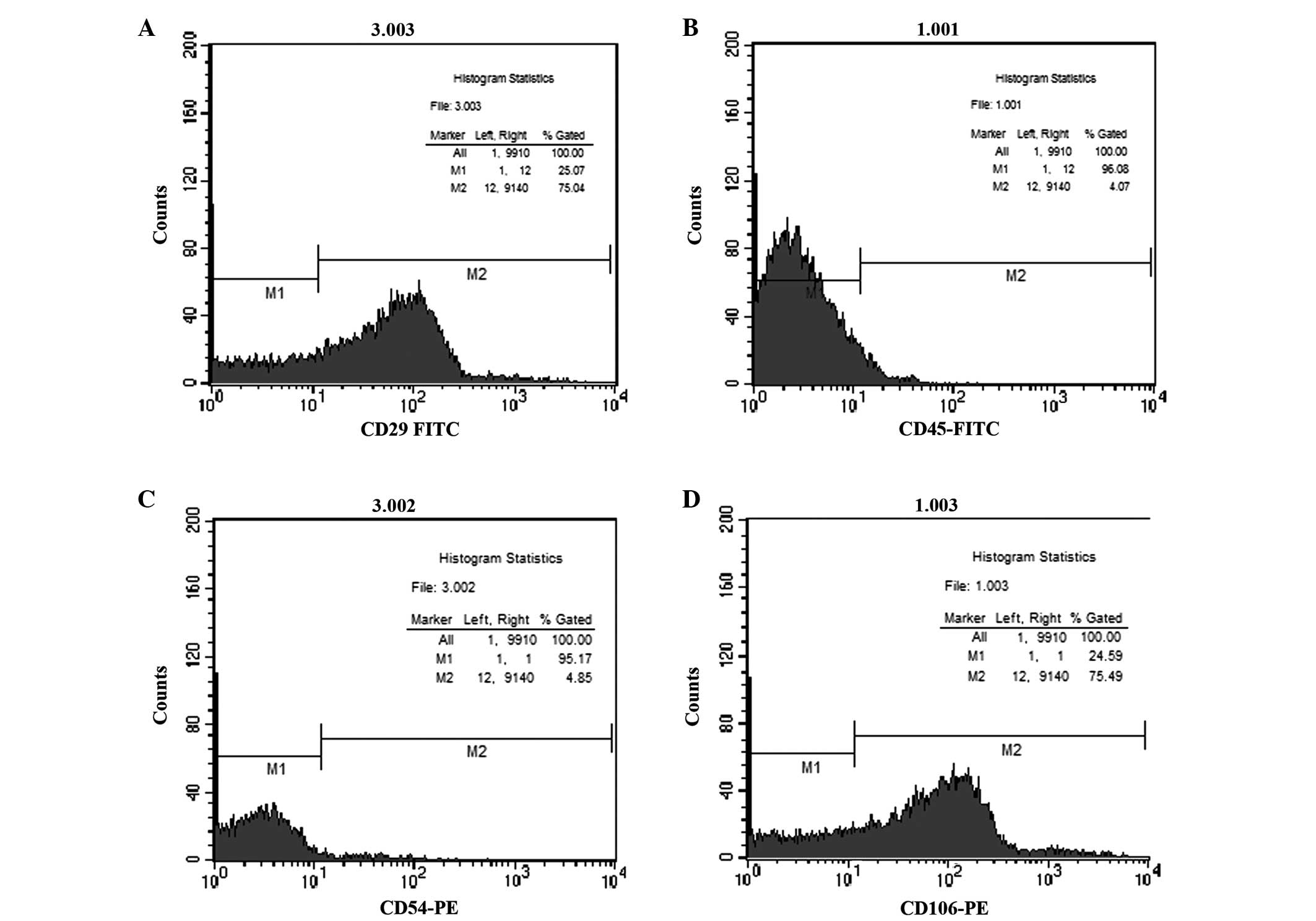
BMSC growth rate and cell surface marker detection.
P3 BMSCs were identified using flow cytometry to detect the
expression of a number of cell surface antigens. Tshe results of
these experiments revealed that the cell positivity rates for CD29
and CD106 antibodies were 75.04 and 75.49%, respectively. However,
the cell positivity rates for the expression of CD45 and CD54
antibodies were 4.07 and 4.85%, respectively (Fig. 1), indicating that the BMSCs were
positive for the expression of CD29 and CD106, but negative for the
expression of CD45 and CD54. Thus, the cells were confirmed as
BMSCs, and the cells used in the subsequent experiments were P3
BMSCs.
Morphology of the AMs. Results from the HE staining
revealed that the control group AMs were almost all round or oval,
and the nuclei were located in the center or inclined to one side
of the cells. In addition, the cytoplasm was unchanged, with no
evident engulfed SiO2 dust particles. When compared with
the control group, the cell volume of the AMs in the silicosis
model group increased (Fig. 2).
Furthermore, the majority of the cells presented with irregular,
basophilic nuclei, and were colored in the center or side of the
cell cytoplasm.
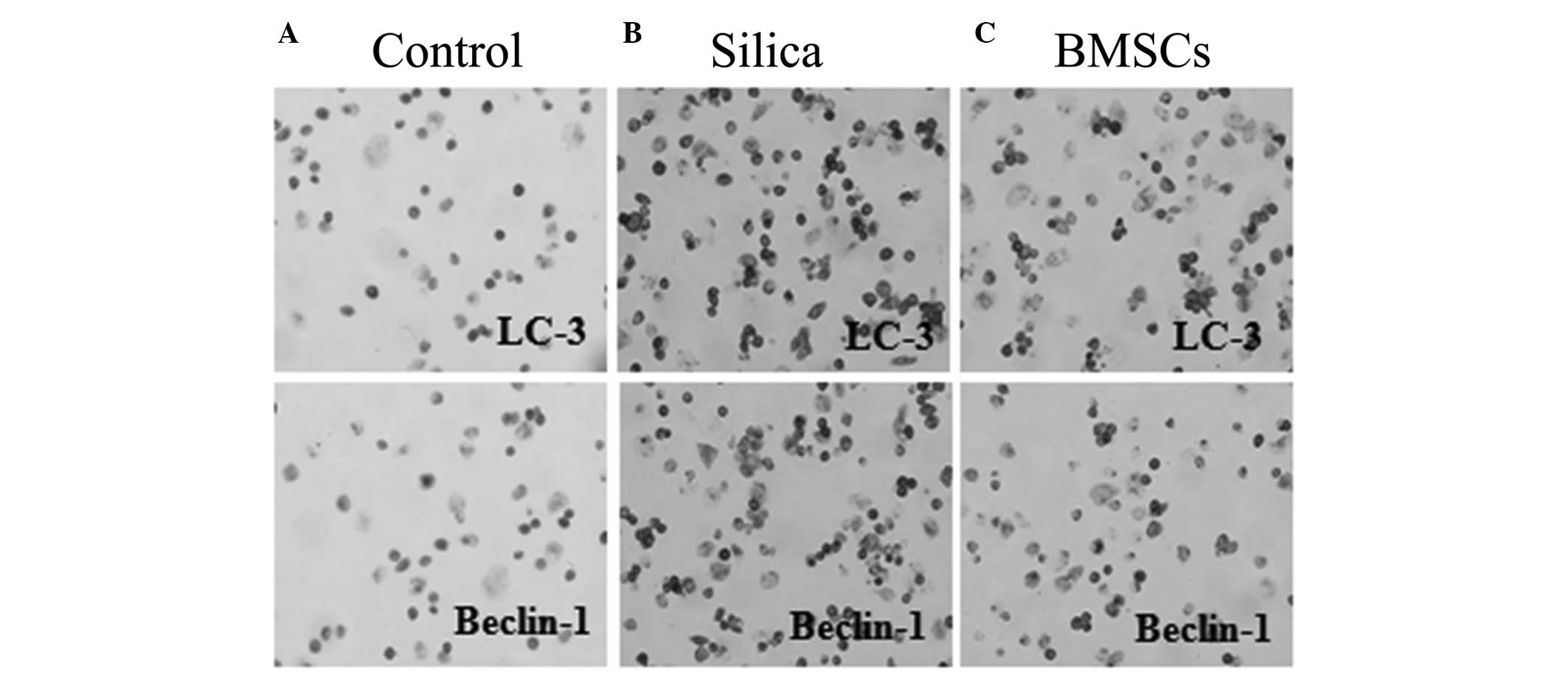
Location of LC-3 and Beclin-1 protein. Cytoplasmic
expression of the autophagy-associated proteins, LC-3 and Beclin-1,
appeared as brown particles in the AMs. The results indicated that
the control group cells were highly homogeneous, with a clear
structure and limited expression of LC-3 and Beclin-1 (Fig. 3). When compared with the control
group, the AMs in the model group exhibited a significantly
increased cell size, the shape and size was not uneven, and the
cell profile was not clear, with brown or reddish-brown coloration,
indicating a marked immune response. The BMSC treatment group
presented with significantly reduced numbers of LC-3- and
Beclin-1-positive cells when compared with the model group,
indicating attenuated immune reactivity.
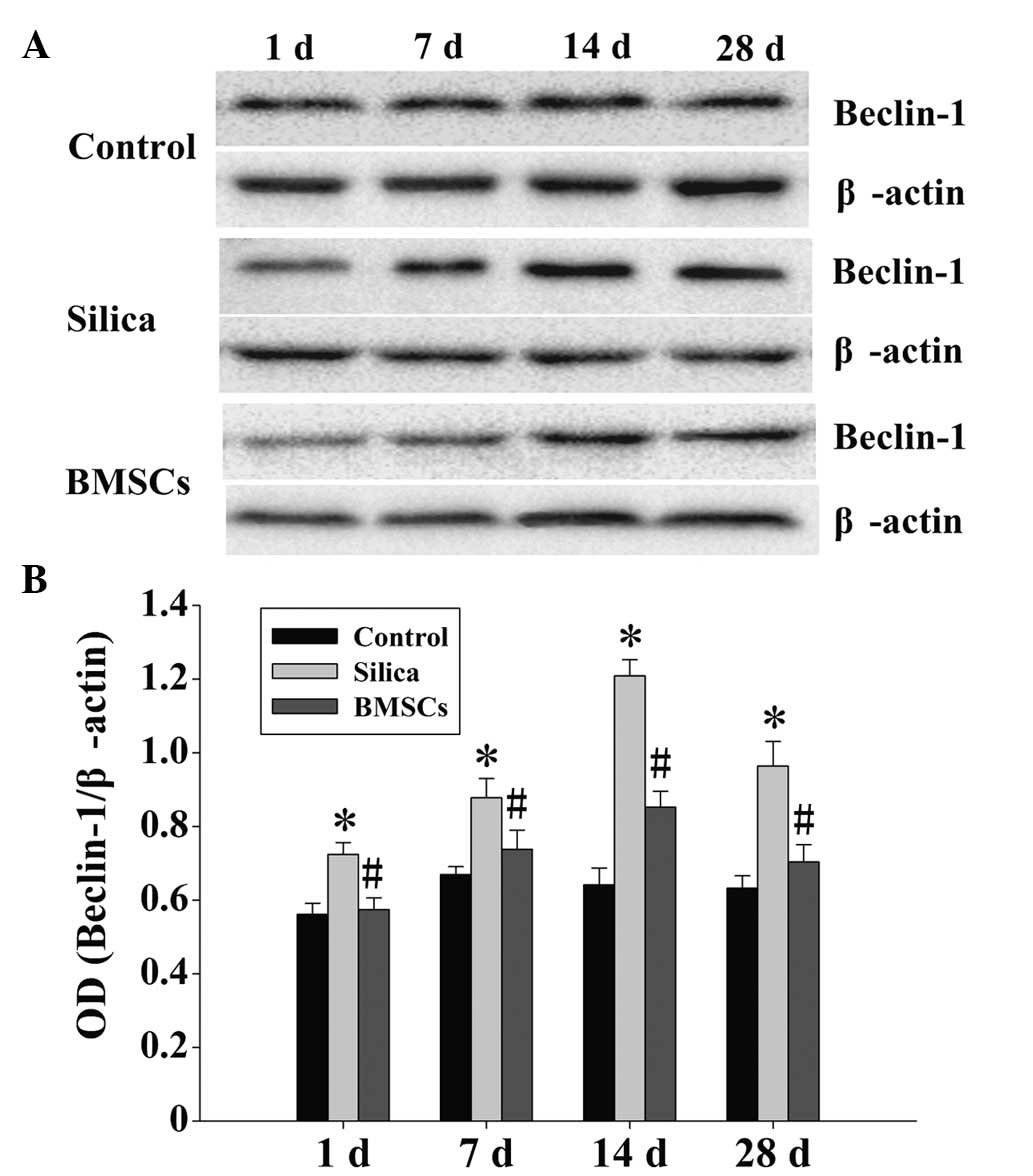
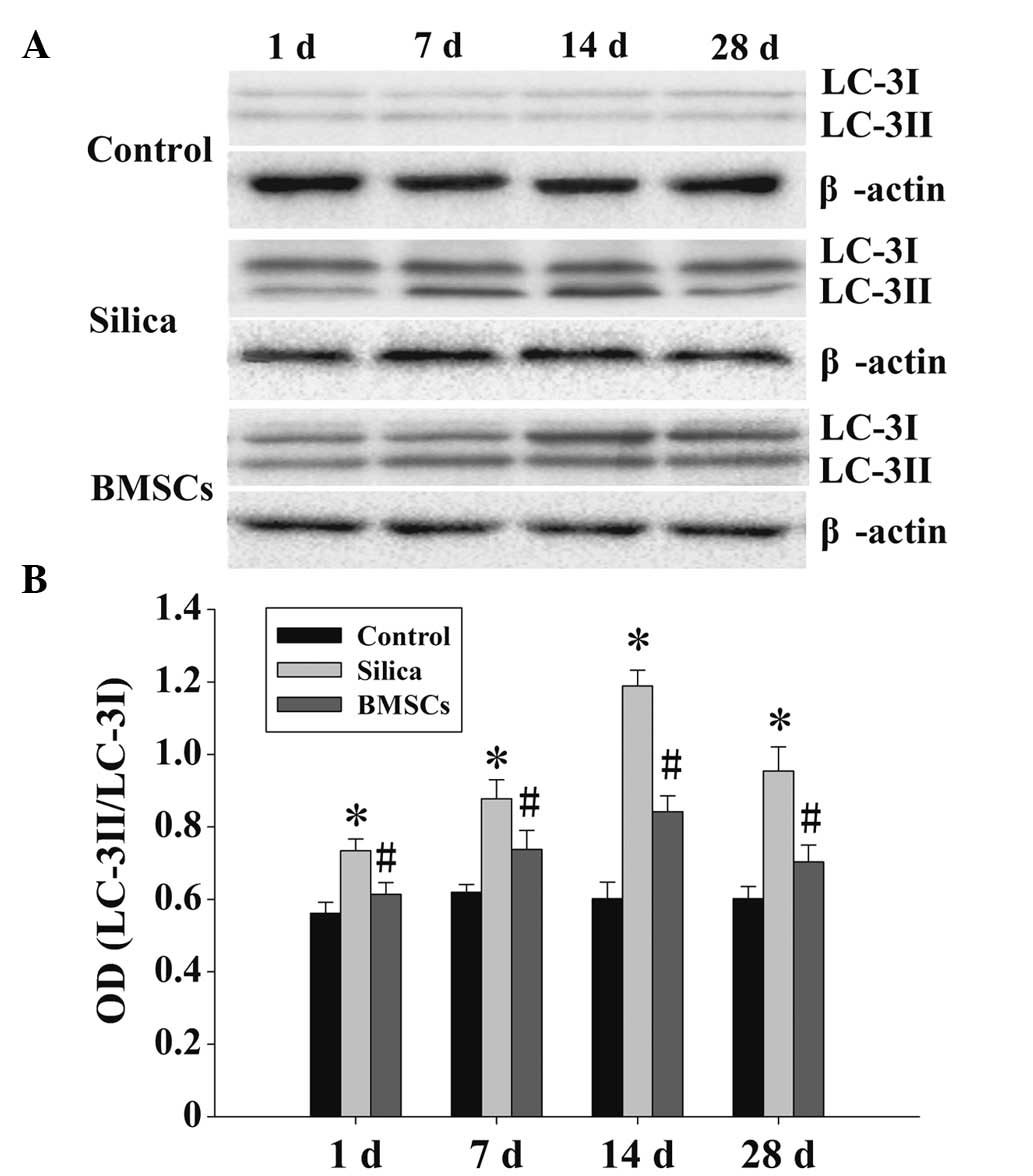
BMSC treatment inhibits the expression of LC-3 and
Beclin-1 in silicosis model AMs. In the control group, limited
basal expression levels of LC-3 and Beclin-1 protein were detected,
with negligible variation over time. However, the expression levels
of LC-3 and Beclin-1 protein in the model group AMs were
significantly increased at each time point when compared with the
control group AMs (Figs. 4 and
5; P<0.05). The expression levels
of LC-3 and Beclin-1 were shown to be dynamic, increasing at day 1
and peaking at day 14, while decreasing after day 28. However, the
expression levels of LC-3 and Beclin-1 after day 28 in the
silicosis model AMs remained elevated compared with those in the
control group AMs at the corresponding period. When compared with
the AMs in the model group, LC-3 and Beclin-1 protein expression
levels were significantly decreased in the BMSC treatment group AMs
at each time point (Figs. 4 and
5; P<0.05); however, the
expression trend was similar, with peak expression observed on day
14. The expression levels of LC-3 were presented as two bands in
different shades, representing LC-3I and LC-3II (Fig. 5).
Discussion
Silicosis is a occupational disease with a complex
pathogenesis. AM injury is a crucial factor in the development of
silicosis (10). A previous study
(11) extracted AMs using a BAL in
situ technique, and then performed in vitro transfection of the
cells with SiO2 in order to establish a model of SiO2 damage for
subsequent analysis (12). The
disadvantage of this approach is that the cells have been removed
from the native environment of the organism, in which coexisting
cells exert a variety of effects and interactions. A previous study
by the present research group indicated that autophagy is activated
in the lung tissue of rats with silicosis. The current study was
conducted on the basis of the aforementioned study using an in vivo
rat model of silicosis, induced by the SiO2 exposure method.
However, in the present study, the AMs remained in the living
environment in the organism following exposure to SiO2. Using BAL
in situ techniques, the AMs were removed from the organism and
follow-up experiments were conducted in vitro. This approach
can effectively avoid the in vitro transfection of dust and
interference caused by external factors, such as dust particles in
the air.
Autophagy is a key process in cellular energy
metabolism and a mechanism of self-renewal. The cellular process
aids the maintenance of body homeostasis via a
degradation/recycling system, which supports biosynthesis,
metabolism, growth and aging (13).
Autophagy was initially considered to be a type of programmed cell
death; however, subsequent studies (14) demonstrated that autophagy and
apoptosis are different processes. Apoptosis results in cell death
without exception, while autophagy may aid cell survival, since the
autophagic degradation and recycling of the internal cellular
structure may help cells adapt to external stimuli. Therefore,
autophagy serves a dual function in the control of cell fate
(15). However, the in vivo
experiments of the present study confirmed that autophagy is able
to promote the pathogenesis of silicosis. These results indicate
the control of autophagy as a novel treatment approach, and signify
autophagy as a target for candidate drugs for the treatment of
silicosis.
Recently, BMSCs have been increasingly recognized as
applicable to the treatment of lung injury. A number of studies
have indicated that the exogenous administration of BMSCs may
protect against a variety of pulmonary diseases, including acute
lung injury (16,17) and chronic lung disease (18). BMSCs exhibit a marked capacity for
self-renewal, multidirectional differentiation (19), perception of the position of damage
signals, repair and regeneration (20), immune function and the ability to
prevent the immune rejection of allografts (21). Furthermore, paracrine function,
angiogenesis promotion and the ability to repair associated damage
are key factors in the treatment of silicosis and necessary
mechanisms (11). In addition, BMSC
transplantation has been demonstrated to inhibit the expression of
autophagy-associated proteins in hippocampal cells in a rat model
of traumatic brain injury (22). For
these experiments provide the basic theory to ensure the
feasibility of the experiment.
The present study observed autophagic activation on
day 1 in the AMs following the successful modeling of silicosis in
rats. Furthermore, the expression levels of LC-3 and Beclin-1
continued to increase, peaking at day 14 and decreasing after day
28. These results confirm that AM autophagic activity occurs during
the formation and development of silicosis. Pathological changes
were significantly alleviated in the BMSC treatment group when
compared with the model group. However, the expression trend of
LC-3 and Beclin-1 remained unchanged, while the overall degree of
expression declined. These results indicate that the
transplantation of BMSCs may be used to reduce autophagic activity,
thereby effectively inhibiting the progress of silicosis caused by
AM damage and promoting fibrotic lung recovery. Therefore, BMSC
transplantation may be clinically applicable as a therapeutic
intervention for the treatment of silicosis.
In summary, a model of silicosis was established via
the administration of free SiO2 dust in situ in order to maintain a
response to body interactions. The results indicate that AMs may be
crucially involved in the initiation and progression of the lung
tissue inflammation and fibrosis associated with silicosis. The
present study employed a silicosis model manufactured in vivo,
while the experiments were conducted in vitro. The occurrence and
participation of autophagic activity in the development and
progression of silicosis was confirmed in the AMs of the rats.
Furthermore, BMSC transplantation therapy was demonstrated to
effectively reduce the expression of autophagy-associated proteins,
and promote the recovery of AMs. Thus, the present study provides a
novel theoretical and experimental basis for the treatment of
silicosis.
Acknowledgements
This study was supported by grants from the Science
and Technology Support Key Funding Project of Hebei Province (no.
09276191D) and Hebei Province Occupational Disease Prevention and
Control Research (no. 13277709D).
Glossary
Abbreviations
Abbreviations:
|
BMSCs
|
bone marrow-derived mesenchymal stem
cells
|
|
AM
|
alveolar macrophage
|
|
LC-3
|
microtubule-associated protein light
chain 3
|
|
Beclin-1
|
autophagy-related gene Beclin-1
|
|
SD
|
Sprague-Dawley
|
|
BAL
|
bronchoalveolar lavage
|
|
PBS
|
phosphate-buffered saline
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
HE
|
hematoxylin and eosin
|
References
|
1
|
Lee E, Lee EJ, Kim H, Jang A, Koh E, Uh
ST, Kim Y, Park SW and Park CS: Overexpression of apolipoprotein A1
in the lung abrogates fibrosis in experimental silicosis. PLoS One.
8:e558272013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Debnath J, Baehrecke EH and Kroemer G:
Does autophagy contribute to cell death? Autophagy. 1:66–74. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shintani T and Klionsky DJ: Autophagy in
health and disease: A double-edged sword. Science. 306:990–995.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mizushima N: Autophagy: Process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hale AN, Ledbetter DJ, Gawriluk TR and
Rucker EB III: Autophagy: Regulation and role in development.
Autophagy. 9:951–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Derubeis AR and Cancedda R: Bone marrow
stromal cells (BMSCs) in bone engineering: Limitations and recent
advances. Ann Biomed Eng. 32:160–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tohma Y, Dohi Y, Ohgushi H, Tadokoro M,
Akahane M and Tanaka Y: Osteogenic activity of bone marrow-derived
mesenchymal stem cells (BMSCs) seeded on irradiated allogenic bone.
J Tissue Eng Regen Med. 6:96–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang YG, Guo X, Xu P, Kang LL and Li J:
Bone mesenchymal stem cells transplanted into rabbit intervertebral
discs can increase proteoglycans. Clin Orthop Relat Res. 219–226.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng JF and Liang LJ: Intra-portal
transplantation of bone marrow stromal cells ameliorates liver
fibrosis in mice. Hepatobiliary Pancreat Dis Int. 7:264–270.
2008.PubMed/NCBI
|
|
10
|
Yao SQ, He QC, Yuan JX, Chen J, Chen G, Lu
Y, Bai YP, Zhang CM, Yuan Y and Xu YJ: Role of Fas/FasL
pathway-mediated alveolar macrophages releasing inflammatory
cytokines in human silicosis. Biomed Environ Sci. 26:930–933.
2013.PubMed/NCBI
|
|
11
|
Zhao MM, Cui JZ, Cui Y, Li R, Tian YX,
Song SX, Zhang J and Gao JL: Therapeutic effect of exogenous bone
marrow-derived mesenchymal stem cell transplantation on silicosis
via paracrine mechanisms in rats. Mol Med Rep. 8:741–746.
2013.PubMed/NCBI
|
|
12
|
Ferreira TPT, de Arantes ACS, do
Nascimento CVMF, Olsen PC, Trentin PG, Rocco PR, Hogaboam CM, Puri
RK, Martins MA and Silva PM: IL-13 immunotoxin accelerates
resolution of lung pathological changes triggered by silica
particles in mice. J Immunol. 191:5220–5229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Johansen T and Lamark T: Selective
autophagy mediated by autophagic adapter proteins. Autophagy.
7:279–296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chaabane W, User SD, El-Gazzah M, Jaksik
R, Sajjadi E, Rzeszowska-Wolny J and Los MJ: Autophagy, apoptosis,
mitoptosis and necrosis: Interdependence between those pathways and
effects on cancer. Arch Immunol Ther Exp (Warsz). 61:43–58. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen S, Kepp O, Michaud M, Martins I,
Minoux H, Métivier D, Maiuri MC, Kroemer RT and Kroemer G:
Association and dissociation of autophagy, apoptosis and necrosis
by systematic chemical study. Oncogene. 30:4544–4556. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Islam MN, Das SR, Emin MT, Wei M, Sun L,
Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S and
Bhattacharya J: Mitochondrial transfer from bone-marrow-derived
stromal cells to pulmonary alveoli protects against acute lung
injury. Nat Med. 18:759–765. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luan Y, Zhang X, Kong F, Cheng GH, Qi TG
and Zhang ZH: Mesenchymal stem cell prevention of vascular
remodeling in high flow-induced pulmonary hypertension through a
paracrine mechanism. Int Immunopharmacol. 14:432–437. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aslam M, Baveja R, Liang OD,
Fernandez-Gonzalez A, Lee C, Mitsialis SA and Kourembanas S: Bone
marrow stromal cells attenuate lung injury in a murine model of
neonatal chronic lung disease. Am J Respir Crit Care Med.
180:1122–1130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soleimani M, Abbasnia E, Fathi M, Sahraei
H, Fathi Y and Kaka G: The effects of low-level laser irradiation
on differentiation and proliferation of human bone marrow
mesenchymal stem cells into neurons and osteoblasts - an in vitro
study. Lasers Med Sci. 27:423–430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Le Blanc K and Ringdén O: Immunomodulation
by mesenchymal stem cells and clinical experience. J Intern Med.
262:509–525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grove JE, Lutzko C, Priller J, Henegariu
O, Theise ND, Kohn DB and Krause DS: Marrow-derived cells as
vehicles for delivery of gene therapy to pulmonary epithelium. Am J
Respir Cell Mol Biol. 27:645–651. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun L, Gao J, Zhao M, Jing X, Cui Y, Xu X,
Wang K, Zhang W and Cui J: The effects of BMSCs transplantation on
autophagy by CX43 in the hippocampus following traumatic brain
injury in rats. Neurol Sci. 35:677–682. 2014. View Article : Google Scholar : PubMed/NCBI
|