Introduction
Nasopharyngeal carcinoma (NPC) is a type of cancer
that affects the epithelial cells of the nasopharynx (1). Although NPC has a low prevalence among
Caucasian populations, the disease has an exceptionally high
incidence rate in the Eastern Malaysian state of Sarawak,
particularly in the Bidayuh ethnic community (2). NPC is also prevalent among populations
in Southeast Asia and Southern China, as well as Inuit populations
in Alaska and certain ethnic groups in North Africa (1,3).
Concurrent chemoradiation is the current standard therapy for NPC,
although this method appears to be more effective in patients with
early stage NPC, as compared with patients with advanced stage NPC
and distant tumour metastasis (3,4). One of
the treatment strategies being investigated for NPC involves the
addition of another therapeutic agent to the combination of
cisplatin and 5-fluorouracil, the standard chemotherapeutic drugs
for the treatment of NPC (4). This
approach requires a novel target agent that functions
synergistically with the standard chemotherapy drugs to treat
NPC.
The use of plant components as therapeutic agents
has attracted much attention. Higher plants, specifically plants
used in traditional medicine or as dietary supplements, are the
source of a considerable number of natural product-derived drugs
(5). Aglaia is a genus of
plant belonging to the family Meliaceae, and can be found primarily
in the forests in tropical Asia (6).
Several species within the genus are known to be sources of
cyclopenta[b]benzofuran flavaglines, a novel class of compound with
a unique structure that has been shown to be antineoplastic
(5). One member of this class of
compounds, silvestrol and its 5′-epimer episilvestrol, are isolated
from the twig, fruit, and bark of Aglaia stellatopilosa, a
species endemic to Borneo (7). The
mechanism underlying the anti-proliferative effects of the
cyclopenta[b]-benzofurans occurs via inhibition of protein
synthesis (8). Kinghorn et al
(5) described novel plant bioactive
agents with potential cancer chemotherapeutic properties, including
silvestrol. Investigations into the phytochemical effects,
synthetic methods, biological evaluation and mechanism of action of
cyclopenta[b]-benzofurans are described in Pan et al
(9). Rocaglates, silvestrol and
episilvestrol are translation initiation inhibitors (10). However, to the best of our knowledge,
the role of silvestrol and episilvestrol in the treatment of NPC
has yet to be evaluated.
The aim of the present study was to evaluate the
capacity of silvestrol and episilvestrol to inhibit proliferation,
induce apoptosis and perturb the cell cycle in NPC cells. The
results demonstrated that both silvestrol and episilvestrol are
effective at inhibiting the proliferation of NPC cells in
vitro by blocking the G2/M transition in the cell
cycle. In addition, in combination with cisplatin, the two
compounds exhibited a synergistic effect against NPC cells. These
results suggested that silvestrol and episilvestrol may serve as
NPC-targeting compounds in combination with existing chemoradiation
treatment regimens.
Materials and methods
Chemicals
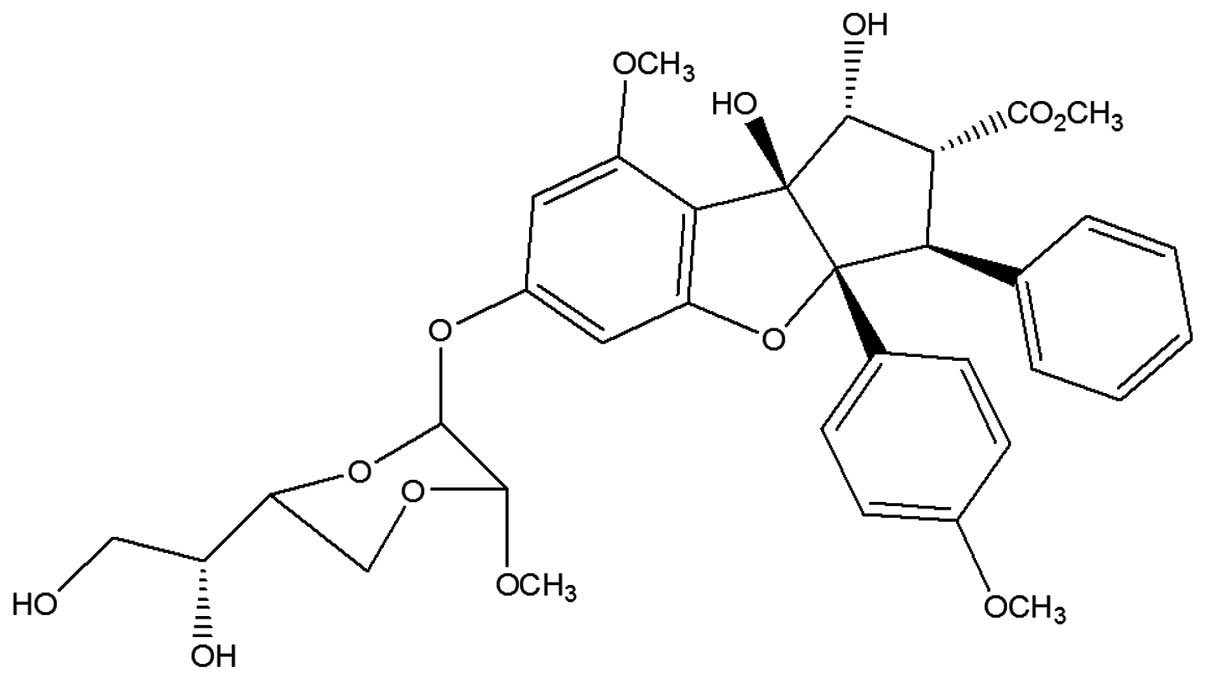
Silvestrol (Fig. 1)
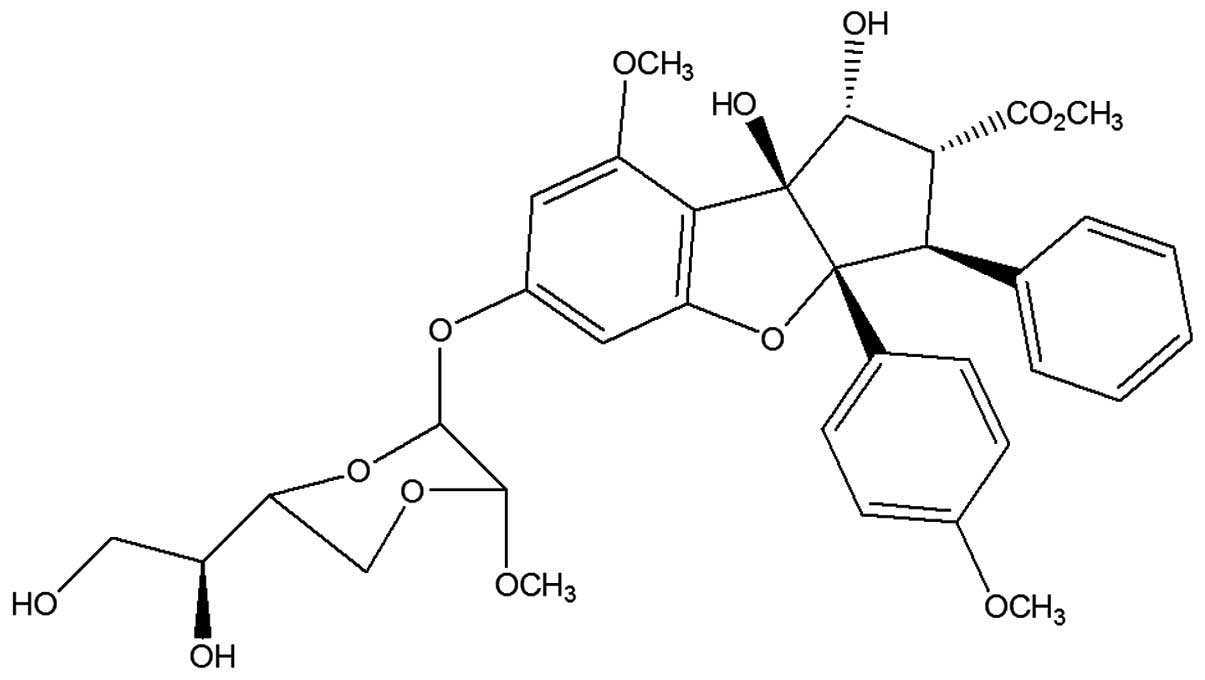
and episilvestrol (Fig. 2) were
purchased from Cerylid Biosciences. Ltd. (Richmond, Australia).
Cell lines and culture
HK1, an Epstein-Barr virus (EBV)-negative NPC cell
line (11), was provided by
Professor George Tsao (Department of Anatomy, Faculty of Medicine,
University of Hong Kong, Hong Kong, China). C666-1, an EBV-positive
NPC cell line (12), was donated by
Professor Kwok-Wai Lo (Department of Anatomical and Cellular
Pathology, Faculty of Medicine, Chinese University of Hong Kong,
Hong Kong, China). HK1 was maintained in the exponential growth
phase in RPMI-1640 medium supplemented with 10% heat-inactivated
foetal calf serum (FCS), 10 U/ml penicillin and 10 µg/ml
streptomycin (all from Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C in a humidified atmosphere containing 5%
CO2. C666.1 was maintained under similar conditions,
although the FCS concentration was increased to 15%. Passage levels
of the NPC cells were in the range of 10–30. The identity of HK1
and C666.1 cells were confirmed by DNA fingerprinting using an
AmpFISTR Identifiler® Polymerase Chain Reaction (PCR)
Amplification kit (part no. 4322288; Applied Biosystems; Thermo
Fisher Scientific, Inc.). The short tandem repeat profiles were
consistent with published data (13). Detection of mycoplasma using an
e-Myco™ Mycoplasma PCR Detection kit (cat. no. 25235; Intron
Biotechnology, Inc., Seongnam, Korea) were conducted routinely and
contamination-free cells were used throughout this study.
Mycoplasma-free stocks were frozen in 10% v/v dimethyl sulfoxide
(DMSO; Sigma-Aldrich, St. Louis, MO, USA), 40% v/v FCS and 50% v/v
RPMI-1640, then stored in liquid nitrogen for subsequent
re-culturing.
Sulforhodamine B (SRB) bioassay
SRB assays were conducted in order to ascertain the
stability of silvestrol and episilvestrol activity against the
NCI-H460 non-small cell lung cancer and MCF-7 breast cancer cell
lines over a period of time. Both cell lines were obtained from
American Type Culture Collection (Manassas, VA, USA), and were
maintained in RPMI-1640 medium supplemented with 10%
heat-inactivated FCS at 37°C in 5% CO2. For the SRB
assay, 0.1% (w/v) gentamycin (Amresco, LLC, Solon, OH, USA) was
added to the culture medium, after which 100 µl cells were plated
in 96-well flat bottomed microtiter plates (Nalge Nunc
International, Penfield, NY, USA) at 7,500 cells/well and 10,000
cells/well for NCI-H460 and MCF-7, respectively. The cells were
incubated for 24 hours for recovery, after which 100 µl culture
medium or culture medium containing silvestrol or episilvestrol
(3819, 381.9, 38.19, 3.819 or 0.3819 nM) was added to the wells for
72 h. Subsequently, the cells were fixed with 10% (w/v)
trichloroacetic acid (Scharlab, S.L., Barcelona, Spain) at 4°C for
1 h. After five washings with reversed osmosis water, the cells
were stained with 0.4% (w/v) SRB dye (MP Biomedicals, Santa Ana,
CA, USA) in 1% (v/v) acetic acid (Merck Millipore, Darmstadt,
Germany) for 10 min at room temperature. Unbound stain was removed
by washing three times with 1% acetic acid. The plates were then
air-dried and the bound protein stain was solubilized with 100 µl
of 10 mM Tris base (Avantor Performance Materials, Center Valley,
PA, USA). The optical density was read at 515 nm using the Sunrise
Basic Microplate Reader from Tecan Group Ltd. (Männedorf,
Switzerland).
xCELLigence cell proliferation
assay
HK1 cells were seeded at a density of
1×104 cells/well into an E-Plate 16 (ACEA Biosciences,
Inc., San Diego, CA, USA). For C666.1 cells, 3×104
cells/well were seeded. At 24 h following seeding, the culture
medium was aspirated and replaced with fresh medium containing 6.25
or 50 nM silvestrol or episilvestrol. The compounds were dissolved
in DMSO with a final concentration of DMSO in the cell culture
≤1.0%. Vehicle control cultures received DMSO alone. Cells treated
with 33.3 µM cisplatin served as the positive control. Cells were
monitored dynamically for ~70 h using the impedance-based
xCELLigence real-time cell analyzer (ACEA Biosciences, Inc.). The
cell index, automatically calculated from the change in electrical
impedance as the living cells interacted with electrodes in the
E-plate wells, correlated with the number of cells, viability
and/or cytotoxicity over time.
MTS cell viability assay
A total of 1×104 HK1 cells/well or
3×104 C666.1 cells/well were seeded into 96-multiwell
microtiter plates using a Hamilton Microlab®STARlet
robotic liquid handling workstation (Hamilton Robotics, Inc., Reno,
NV, USA). At 24 h following seeding, the medium was aspirated and
replaced with fresh medium containing various concentrations of
silvestrol or episilvestrol. Vehicle control cultures received DMSO
alone. The cells were then incubated for 24 h at 37°C in an
atmosphere containing 5% CO2. The number of viable cells
at the end of the incubation period was measured using a CellTiter
96®AQueous One Solution Cell Proliferation
(MTS) assay (Promega Corporation, Madison, WI, USA). Absorbance at
490 nm was read using an EnVision multilabel plate reader
(PerkinElmer, Inc., Waltham, MA, USA) and subtracted with
non-specific absorbance measured at 630 nm. Wells containing medium
without cells served as blanks. Cell viability was calculated as a
percentage compared to the control cells, which were arbitrarily
assigned 100% viability. The half maximal inhibitory concentration
(IC50) values, defined as the concentration that
inhibited 50% cell growth relative to control cells, were
graphically obtained from the dose-response curves.
Apoptosis assay
HK1 and C666.1 cells were seeded at a density of
1×106 cells/ml in culture dishes containing RPMI-1640
medium supplemented with 10 (HK1) or 15% (C666.1) heat-inactivated
FCS, and allowed to adhere and reach ~80% confluence overnight at
37°C. Subsequently, the medium was aspirated and the cells were
treated with 50 nM silvestrol or episilvestrol. Vehicle control
cultures received DMSO alone, whereas cells treated with 100–175 µM
cisplatin served as the control. Apoptosis was evaluated at 24 h
following treatment using a FACSCalibur flow cytometry system
(model no. 342975; BD Biosciences, San Jose, CA, USA) using an
Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit
(cat. no. 556547; BD Pharmingen, San Diego, CA, USA). Data
acquisition and analysis were performed using BD CellQuest Pro
software, version 6.0 (BD Biosciences). A total of 1×104
events were collected for each sample. The lower right and upper
right quadrants represented cells undergoing apoptosis. Annexin
V-FITC-propidium iodide-stained cells were imaged using an IN Cell
Analyzer 2000 (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA)
with a 20X objective. Hoechst 33342 (Cell Signaling Technology,
Inc., Danvers, MA, USA) was used for nuclear staining. Briefly, 10
µl Hoechst 33342 was added to the cell suspension to a final
concentration of 1 µg/ml, and then incubated in the dark for 15
min. The following filter combinations were used: Green (490/20
ex., 525/20 em.) for detection of Annexin V-FITC; red (579/34 ex.,
624/40 em.) for detection of propidium iodide; and blue (350/50
ex., 455/50 em.) for detection of Hoechst 33342.
Cell cycle analysis assay
HK1 and C666.1 cells were seeded and grown in
culture dishes, as described for the apoptosis assay. Cultured
cells were pulsed with 10 µM bromodeoxyuridine (BrdU) daily. Cell
cycle progression was determined 24 and 48 h following treatment on
a FACSCalibur flow cytometry system using an FITC BrdU Flow kit
(cat. no. 559619; BD Pharmingen). ModFit LT™ software, version
3.3.11 from Verity Software House, Inc. (Topsham, ME, USA) was used
to analyze the DNA patterns in the flow cytometry histograms.
Combined drug analysis
Cell seeding was performed as described above for
the MTS cell viability assay using the Hamilton
Microlab®STARlet robotic liquid handling workstation. To
maintain similar experimental conditions, 96-multiwell microtiter
plates were assigned simultaneously for single-drug and two-drug
treatment (14). At 24 h following
seeding, non-fixed ratio combinations of silvestrol-cisplatin or
episilvestrol-cisplatin were evaluated (Table I). Following drug treatment, the
microtiter plates were incubated for a further 24 h following which
an MTS assay was conducted to determine cell viability. Drug
interaction was determined using the previously described
combination index (CI) method (14).
CalcuSyn version 2.0 software (Premier Biosoft International, Palo
Alto, CA, USA) was used to generate the dose-response curves,
dose-effect analysis, and CI-effect plot. A CI <1 implied
synergism, CI=1 was additive, and CI >1 implied antagonism. In
addition, CI<0.1 implied very strong synergism, CI=0.1–0.3
implied strong synergism, CI=0.3–0.7 implied synergism, CI=0.7–0.85
was moderate synergism, CI=0.85–0.9 implied slight synergism
(14).
 | Table I.Description of CI values for each
fraction of the cells and the corresponding DRI. |
Table I.
Description of CI values for each
fraction of the cells and the corresponding DRI.
| A, HK1 cell
line |
|---|
|
|---|
| Compound (nM) | Fraction
affected | CI | Synergism | DRIa | Cisplatin (µM) | Cisplatin DRI |
|---|
| Silvestrol |
|
|
|
|
|
|
| 5 | 0.211 | 0.04 | Very strong | 98.879 | 12.5 |
33.057 |
| 10 | 0.208 |
0.087 | Very strong | 47.213 | 25 |
15.128 |
| 50 | 0.347 |
0.016 | Very strong | 83.851 | 50 | 236.646 |
|
500 | 0.391 |
0.070 | Very strong | 15.067 | 100 | 298.111 |
|
1,000 | 0.336 |
0.294 | Strong |
3.603 | 150 |
62.114 |
|
5,000 | 0.394 |
0.645 | Synergism |
1.567 | 200 | 158.505 |
| Episilvestrol |
|
|
|
|
|
|
| 10 | 0.172 |
0.209 | Strong | 778.01 | 25 |
4.811 |
| 50 | 0.270 |
0.025 | Very strong |
7.67×106 | 50 |
40.295 |
|
500 | 0.324 |
0.014 | Very strong |
9.83×107 | 100 |
71.468 |
|
1,000 | 0.261 |
0.093 | Very strong |
1.61×105 | 150 |
10.720 |
|
5,000 | 0.440 |
0.003 | Very strong |
1.03×1011 | 200 | 399.788 |
|
| B, C666.1 cell
line |
|
| Compound (nM) | Fraction
affected | CI | Synergism | DRIa | Cisplatin (µM) | Cisplatin DRI |
|
| Silvestrol |
|
|
|
|
|
|
| 5 | 0.350 | 0.098 | Very strong |
83.104 |
33.3 | 11.638 |
| 10 | 0.467 | 0.048 | Very strong | 230.427 |
66.7 | 23.053 |
| 50 | 0.378 | 0.570 | Synergism |
12.633 | 266.7 |
2.037 |
|
500 | 0.445 | 0.644 | Synergism |
3.297 | 400 |
2.936 |
|
1,000 | 0.561 | 0.214 | Strong |
8.276 | 400 | 10.735 |
| Episilvestrol |
|
|
|
|
|
|
| 5 | 0.461 | 0.033 | Very strong | 114.652 |
33.3 | 41.370 |
| 10 | 0.451 | 0.071 | Very strong |
52.574 |
66.7 | 19.103 |
| 50 | 0.647 | 0.038 | Very strong |
58.864 | 133.3 | 46.547 |
|
500 | 0.564 | 0.444 | Synergism |
2.786 | 266.7 | 11.700 |
|
1,000 | 0.670 | 0.325 | Synergism |
3.666 | 400 | 18.987 |
Statistical analysis
Calculations were performed using IBM®
SPSS® version 22.0 statistical software (IBM SPSS,
Armonk, NY, USA). Differences between mean values were evaluated
with a one-way analysis of variance and Tukey's post-hoc analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Compound profiles
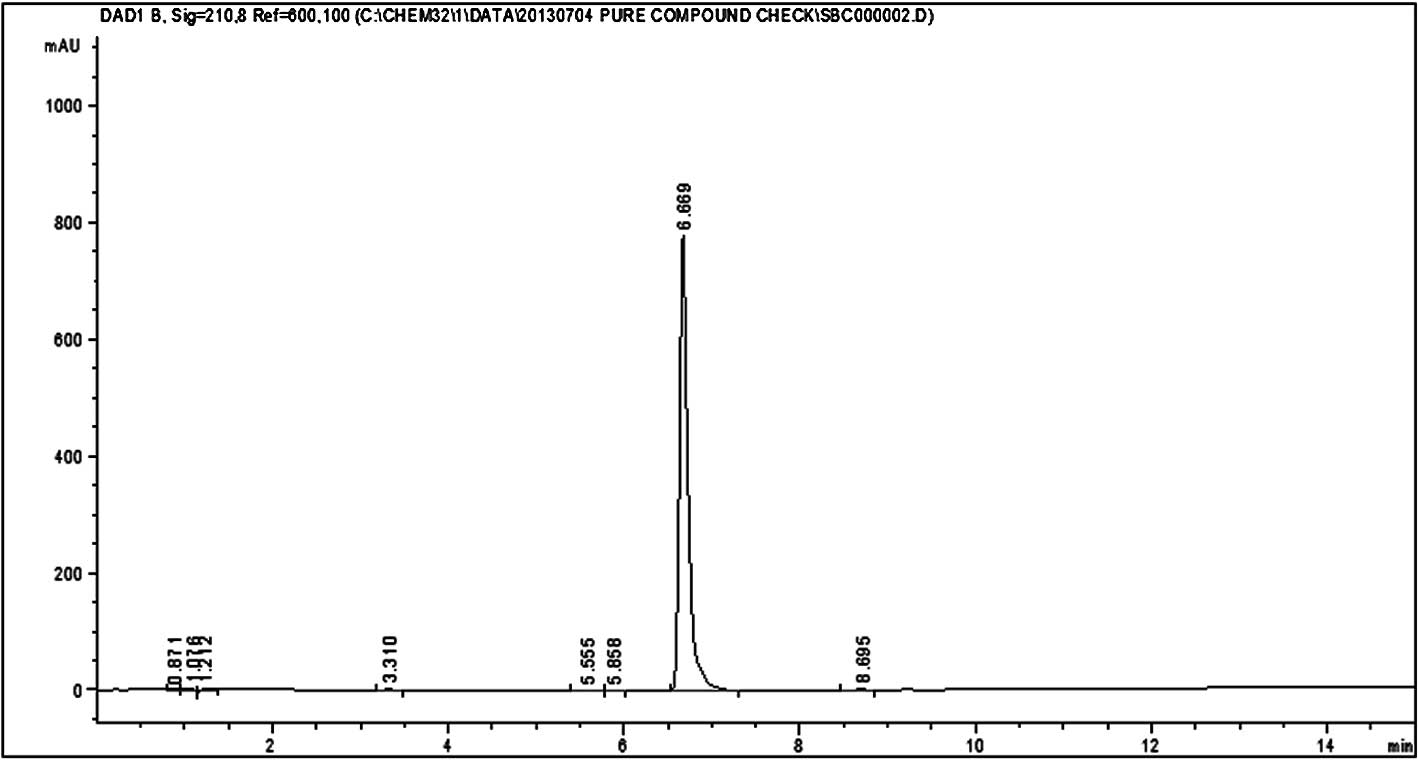
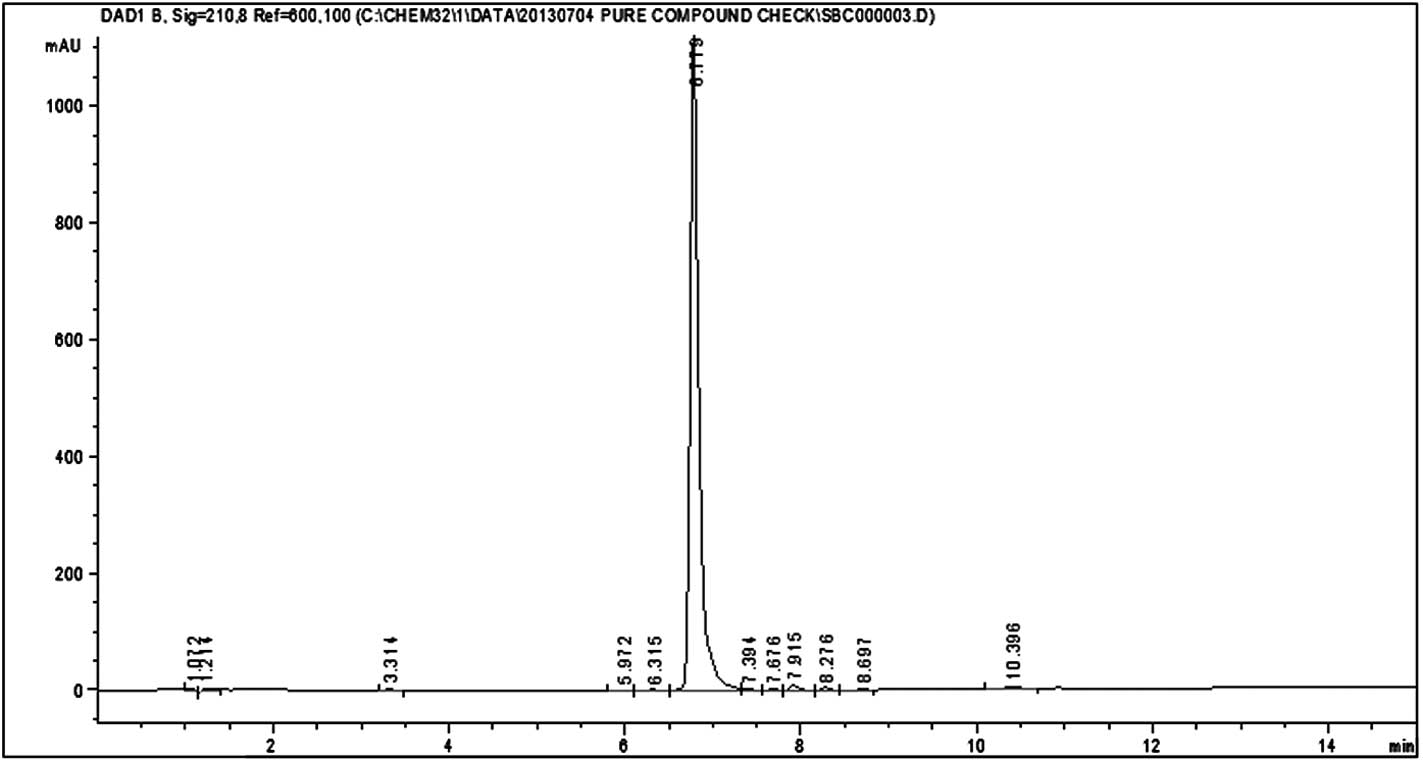
HPLC analysis of silvestrol and episilvestrol
demonstrated that the compounds were pure (Figs. 3 and 4).
Stability of silvestrol and
episilvestrol
Preliminary data (unpublished) from on-going
experiments demonstrated that the silvestrol and episilvestrol
compounds were stable over time. Silvestrol and episilvestrol
reconstituted with 100% DMSO and stored at −20°C produced
consistent GI50 results as determined by the SRB
bioassay (72 h treatment period) on NCI-H460 non-small cell lung
cancer and MCF-7 breast adenocarcinoma cell lines (Tables II and III).
 | Table II.GI50 for silvestrol. |
Table II.
GI50 for silvestrol.
|
| GI50
value (nM) |
|---|
|
|
|
|---|
| SRB bioassay
performed | NCI-H460 | MCF-7 |
|---|
| First test | 16.90 | 19.71 |
| 3 months | 17.81 | 18.50 |
| 6 months | 19.70 | 19.02 |
| 9 months | 18.10 | 16.90 |
| 12 months | 18.60 | 17.60 |
 | Table III.GI50 for
episilvestrol. |
Table III.
GI50 for
episilvestrol.
|
| GI50
value (nM) |
|---|
|
|
|
|---|
| SRB bioassay
performed | NCI-H460 | MCF-7 |
|---|
| First test | 17.96 | 19.09 |
| 2 months | 15.60 | 18.70 |
Dynamic monitoring of cell
proliferation
Based on screening experiments on NCI-H460 and MCF-7
cell lines (Tables II and III), the effective inhibition
concentrations of silvestrol and episilvestrol were in the
nano-molar range. Therefore, one low and one moderate dose were
selected for use in the xCELLigence system; a real-time cell
proliferation, viability and cytotoxicity analyzer. The cell index
generated represents growth over time.
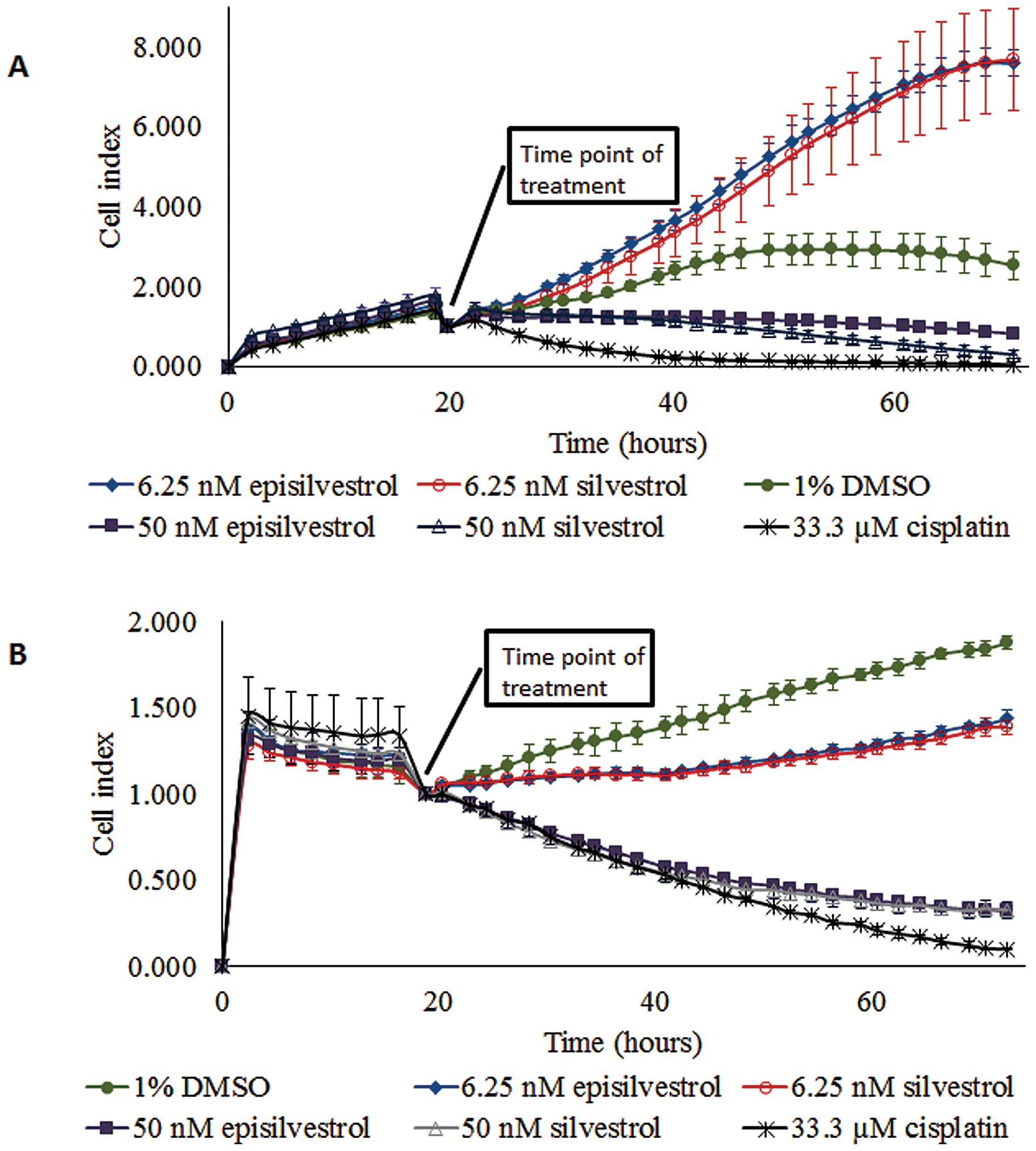
Proliferation of HK1 cells cultured in 6.25 nM
silvestrol was not inhibited (Fig.
5A). However, 50 nM silvestrol exerted an immediate inhibitory
effect and caused near-static cell index compared with the control
cells. This observation suggests that a lower concentration of
silvestrol (6.25 nM) enhanced proliferation more than the vehicle
control-treated cells, whereas a higher concentration of silvestrol
(50 nM) could inhibit cell proliferation. Similar observations were
obtained with episilvestrol. A total of 50 nM silvestrol or
episilvestrol were as effective as 33.33 µM cisplatin in reducing
C666.1 cell index (Fig. 5B).
Following treatment, there was a rapid decline in cell index.
However, C666.1 cell proliferation was not entirely inhibited by
6.25 nM silvestrol or episilvestrol, although the cell index
generated was markedly lower compared with that of the control,
indicating that the hyper-proliferation effect observed in HK1
cells was cell-specific. The pattern of growth inhibition by 50 nM
silvestrol and episilvestrol were comparable to that of cisplatin,
a standard NPC chemotherapy drug. Since 50 nM silvestrol and
episilvestrol were sufficient for inhibiting cell proliferation,
all further experiments were conducted at this minimum
concentration only.
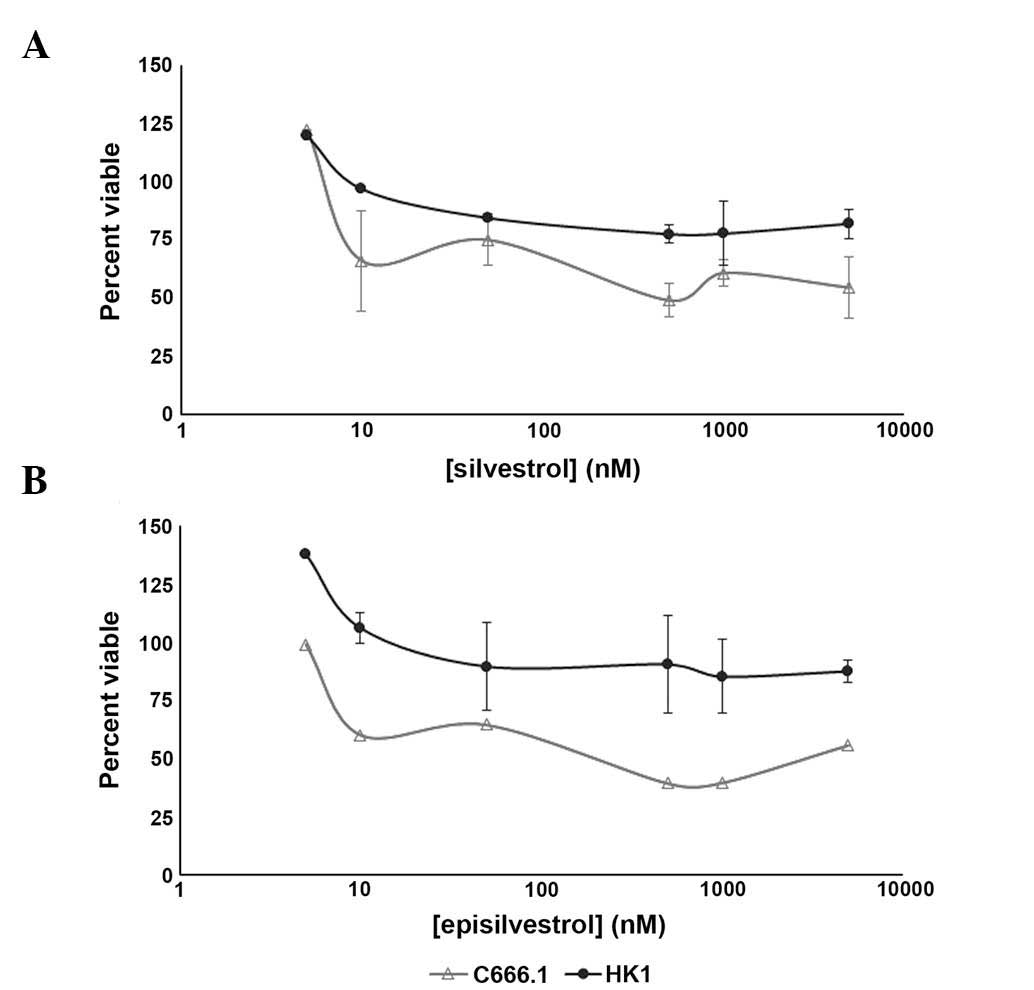
Determination of viable cells
To further examine the anti-proliferative effects
exhibited by the xCELLigence system, HK1 and C666.1 cells treated
with various concentrations of silvestrol (Fig. 6A) and episilvestrol (Fig. 6B) were assessed for viability using
an MTS assay. In HK1 cells, increasing the treatment concentrations
from 50 nM to 10 and 100-fold higher did not elicit further
response, as evidenced by the near-plateau in cell viability ≥50 nM
silvestrol and ≥50 nM episilvestrol. The trend in effect of
silvestrol and episilvestrol against C666.1 cell proliferation was
not as smooth, as compared with HK1. However, both compounds were
potent against EBV-positive C666.1 NPC cells, with the effective
concentrations required to inhibit IC50 values
attainable within 24 h compared with EBV-negative HK1 NPC cells
(Table IV). These results are concordant with those obtained from
xCELLigence dynamic monitoring of cell proliferation, whereby
following treatment with 50 nM silvestrol or episilvestrol, the
cell index of C666.1 cells continued to decline over time, whereas
that of the HK1 cells remained static. The IC50 value of
episilvestrol in C666.1 cells was markedly lower compared with that
of silvestrol, indicating increased efficacy and suggesting that
stereoisomerism may be involved. DMSO showed no influence on cell
viability (data not shown).
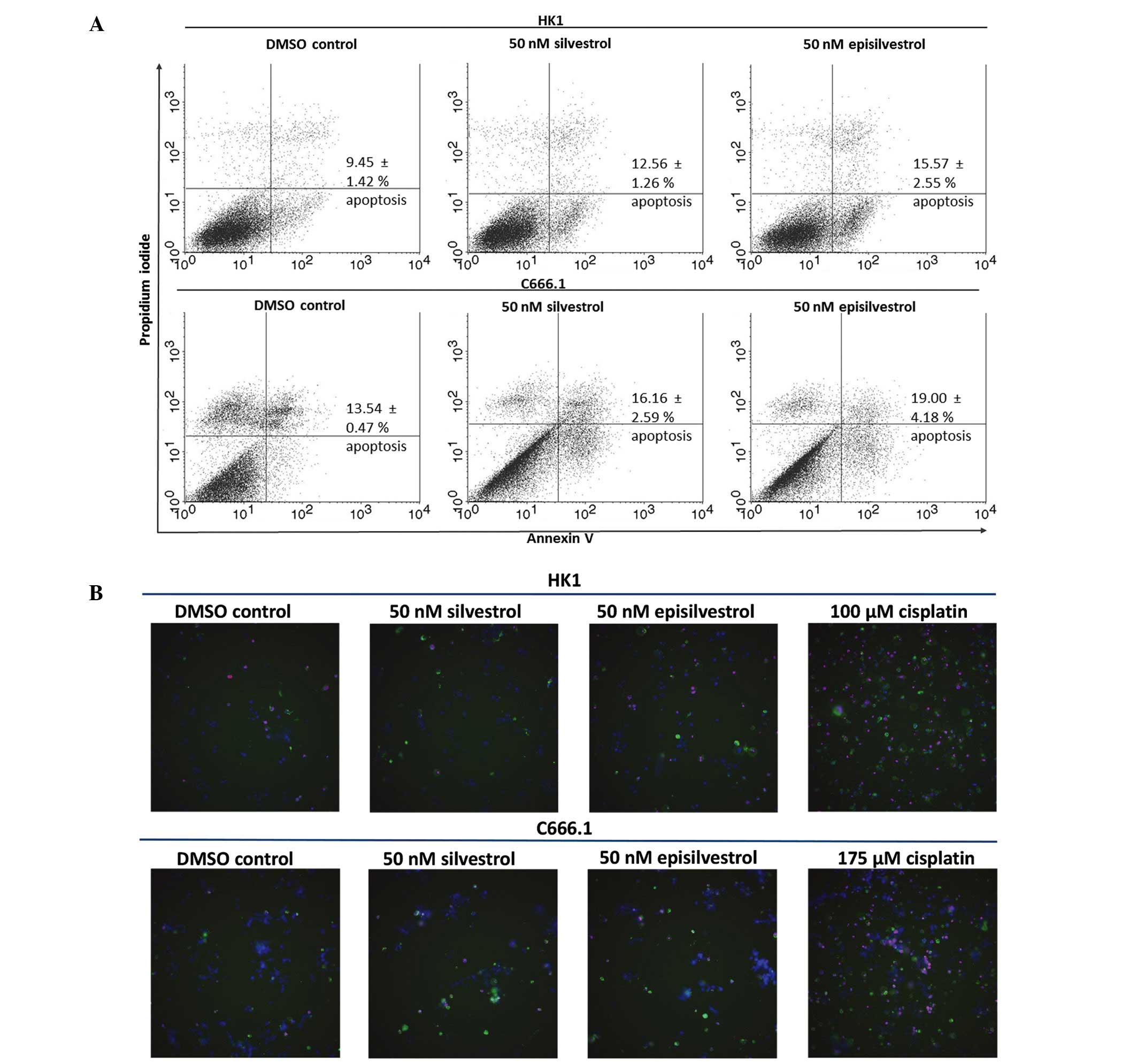
Silvestrol and episilvestrol do not
induce apoptosis
To determine if inhibited cell proliferation was
associated with apoptosis induction, HK1 and C666.1 cells cultured
in silvestrol or episilvestrol were subjected to Annexin V-FITC and
propidium iodide assay, and apoptotic cells were identified by flow
cytometry. Following 24 h exposure to 50 nM silvestrol or
episilvestrol, there was no significant difference in the
percentage of apoptotic cells between the treated cells and the
control (Fig. 7A). Therefore,
apoptosis did not account for the differences in cell proliferation
observed in the present study. Cisplatin, an NPC chemotherapy drug,
was used for instrument set-up and comparison of apoptosis
induction (data not shown). Consistent with the flow cytometry
results, no differences were detected in silvestrol or
episilvestrol-treated cells compared with the control, as
determined by the IN Cell 2000 high content cell analyzer. However,
apoptosis was detected in cisplatin-treated HK1 and C666.1 cells,
as shown in Fig. 7B and demonstrated
by the greater quantity of green (Annexin V-FITC) and red
(propidium iodide) stained cells. Cisplatin is used in cancer
therapy due to its apoptosis-inducing activity. The amount of green
and red fluorescence in silvestrol or episilvestrol-treated cells
and vehicle control-treated cells were similar and were markedly
lower compared with cisplatin-treated cells. These results were
concordant with the flow cytometry results which demonstrated that
silvestrol and episilvestrol did not induce apoptosis with the
experimental dose and time.
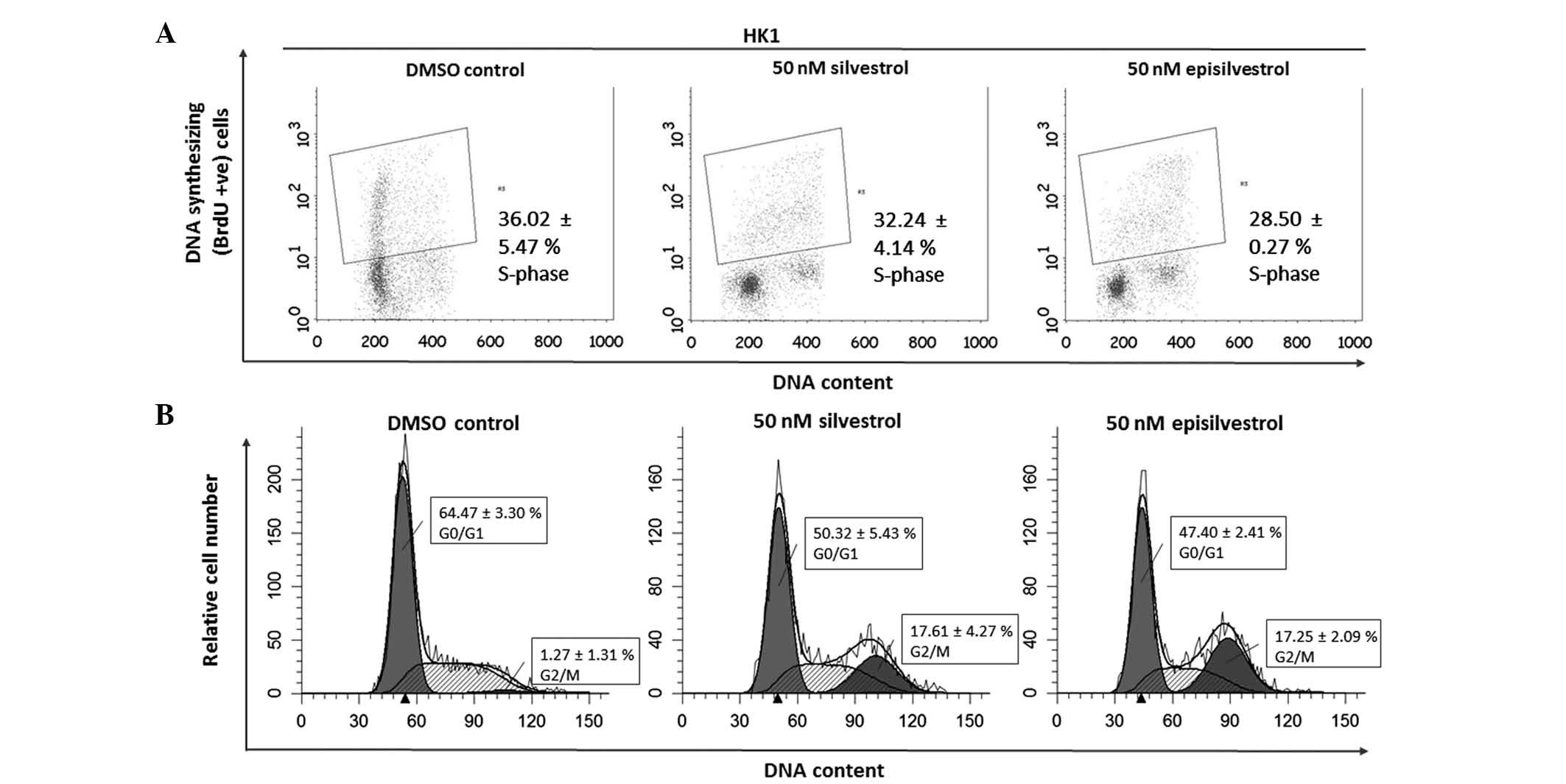
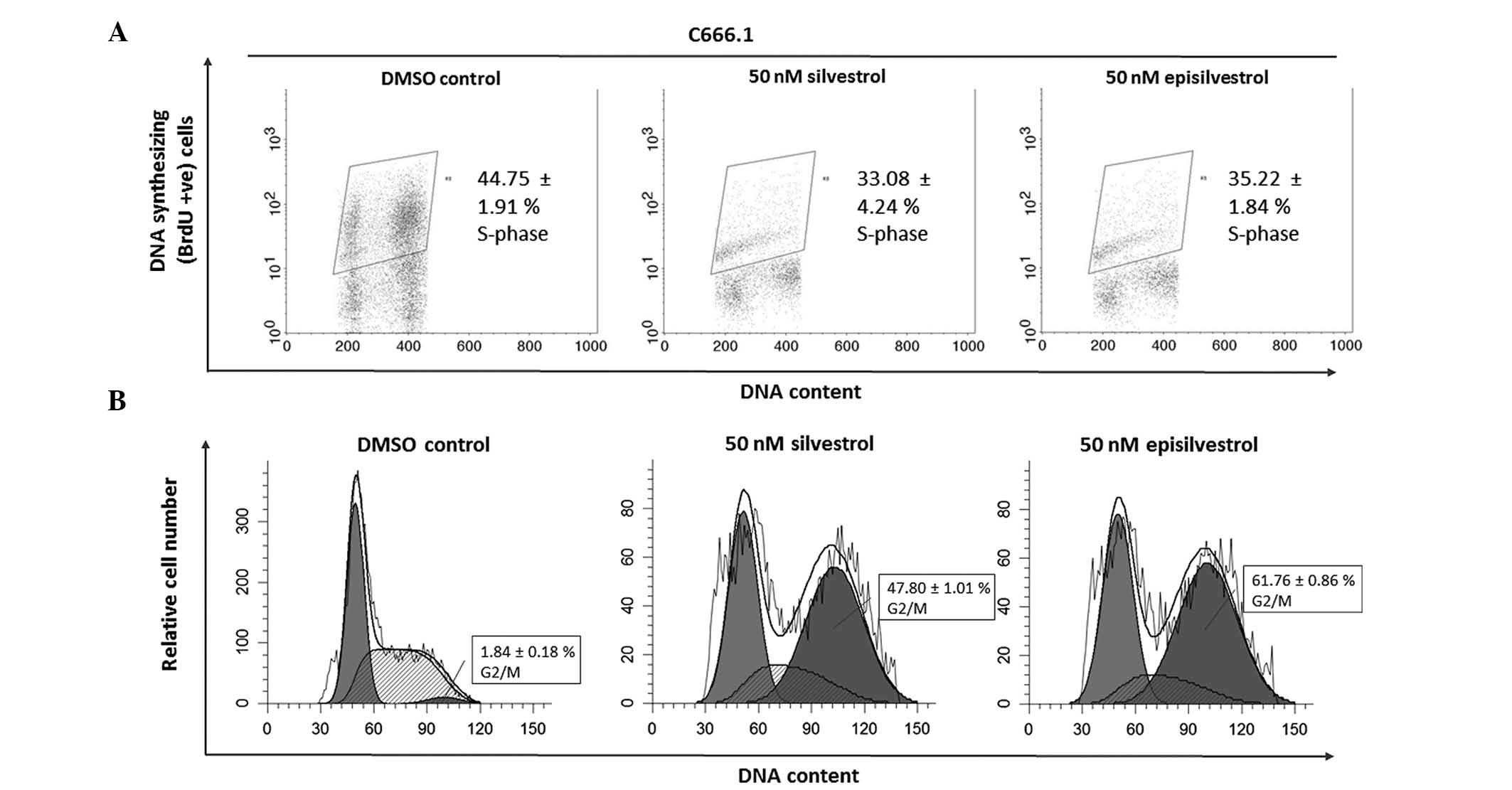
Cell cycle analysis assay
DNA synthesis was monitored by BrdU-labelling and
cell cycle progression by flow cytometry. When BrdU is added to
cell cultures it is incorporated into newly synthesized DNA of
cells entering and progressing through the S-phase (DNA synthesis
phase) of the cell cycle. In HK1 cells, 24 h after cell culture in
50 nM silvestrol or episilvestrol, there was no observable
difference in BrdU-positive cells compared with the control
(Fig. 8A). Silvestrol or
episilvestrol did not affect cell distribution in the S-phase.
However, there was a significant reduction of cells in the
G0/G1 phase (silvestrol, P=0.011;
episilvestrol, P=0.002) and increase in the G2/M phase
(silvestrol, P=0.006; episilvestrol, P=0.016) in treated cells
compared with the control (Fig. 8B).
This cell cycle inhibition observed in HK1 cells corresponds to the
plateaued cell index observed following xCELLigence assay. The
cells were neither proliferating nor dying. There was no
significant observable effect on C666.1 cells within 24 h. However,
after 48 h there was a significant reduction in BrdU-positive
C666.1 cells (silvestrol, P=0.019; episilvestrol, P=0.042) compared
with the control (Fig. 9A). This was
accompanied by a significant increase in G2/M phase
cells (silvestrol, P=0.000; episilvestrol, P=0.000) compared with
the control (Fig. 9B). The reduction
of DNA synthesis in C666.1 cells may have caused the cell index
(determined by the xCELLigence assay) to rapidly decrease. These
differences in cellular effects and the time point at which the
compounds exert their effects support the hypothesis that the
activity of silvestrol and episilvestrol is cell-specific in HK1
and C666.1 cells.
Silvestrol and episilvestrol display
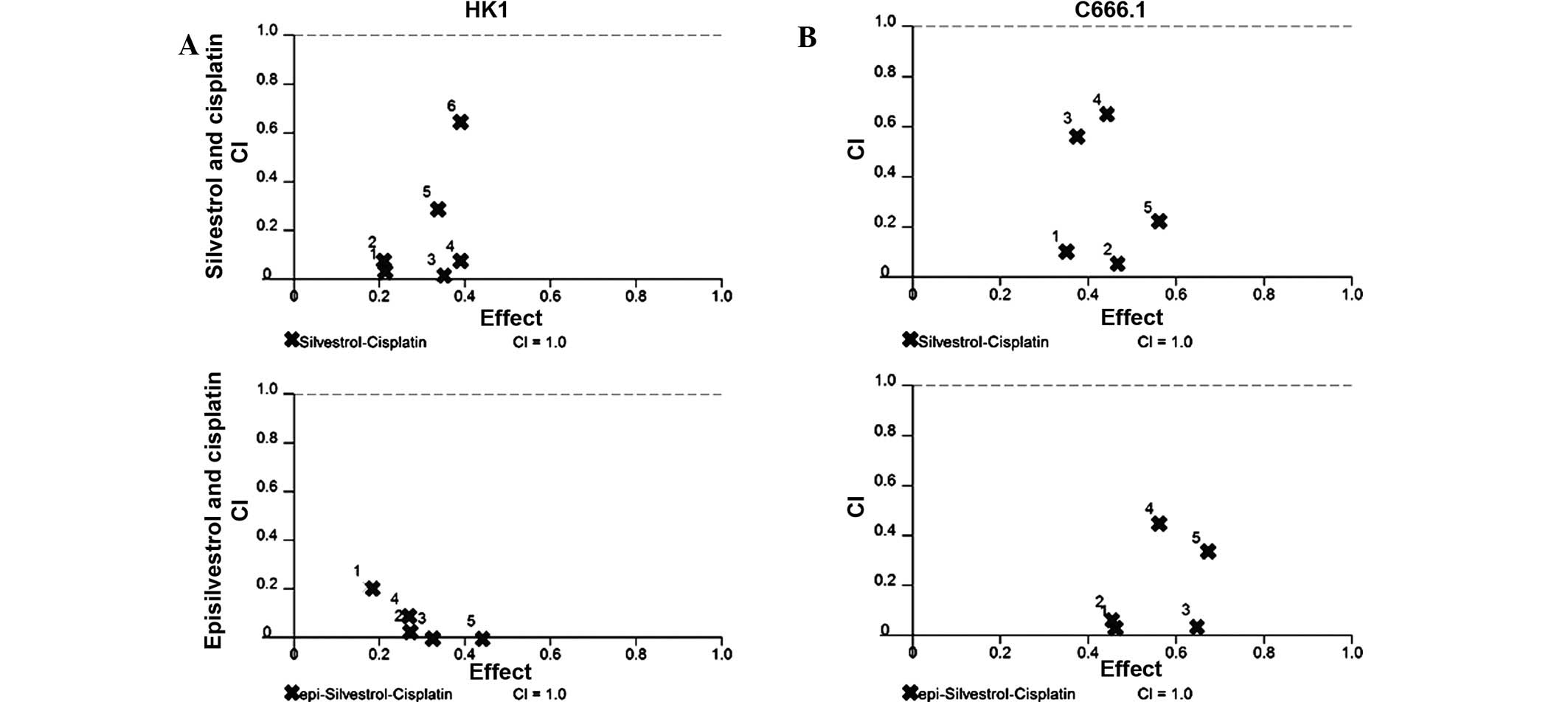
synergistic effects in combination with cisplatin
As the results of the xCELLigence system and the MTS
cell viability assay revealed that silvestrol or episilvestrol
alone did not exert significant effects on HK1, both compounds were
further investigated to determine whether they were able to
sensitize NPC cells to the treatment and effect of cisplatin. Using
CalcuSyn software, the CI was determined to ascertain the
combinatorial effect of silvestrol or episilvestrol with cisplatin.
The CI values are presented in Table
I. The CI method (14), revealed
a remarkable synergistic activity in HK1 cells treated
simultaneously with silvestrol or episilvestrol and cisplatin
(Fig. 10). Similarly, in C666.1
cells, silvestrol or episilvestrol synergized with cisplatin.
Discussion
Plants have been an important source of novel drugs
of natural origin over the past decade (5). Our previous study demonstrated that
quercetin, a polyphenolic flavonoid found in vegetables and fruits,
and trans-cinnamaldehyde obtained from the stem bark of
Cinnamomum burmannii, exhibited synergism with cisplatin in
inhibiting the growth of NPC cells (15,16).
Notably, the skeletal structures of the rocaglamide derivatives
include a flavonoid unit and a cinnamic acid amide moiety (9). The synthesis of racemic rocaglamide
from the benzofuran intermediate with trans-cinnamaldehyde
was described by Pan et al (9).
The results of the present study showed that
silvestrol and episilvestrol were able to inhibit the proliferation
of EBV-positive C666.1 and EBV-negative HK1 NPC cells. NPC is often
associated with the EBV. EBV-infected NPC cells exhibit type II
latency and may express among others, latent membrane proteins 1
and 2 (LMP12A and B), of which LMP1 is oncogenic (3). According to Patton et al
(17), silvestrol modulates direct
anti-tumor activity against EBV-associated lymphomas while sparing
innate and antigen-specific adaptive immunity. There is an urgent
requirement for the development of anti-cancer agents that are
effective against EBV-positive diseases. The study on
EBV-transformed lymphoblastoid cell lines (EBV-LCLs) demonstrated
that silvestrol (2–50 nM) was highly potent against
anti-proliferation, with minimal cell death (18). These results were concordant with
those of the present study that demonstrated that the inhibition of
NPC cell proliferation was not accompanied by apoptosis induction
at 50 nM. Notably, the study demonstrated that the
anti-proliferative activity of silvestrol was associated with the
loss of LMP1 expression (18). In
addition, it has previously been reported that silvestrol treatment
had indirect anti-proliferative effects on EBV-transformed
lymphoblastoid cell lines by enhancing the innate immune function
(17).
As a single agent, silvestrol was markedly
effective, with IC50 values of 4–10 nM. Silvestrol
exhibits nanomolar potency against multiple solid tumour cell lines
(10,19,20).
Similarly, the data of the present study obtained from the
preliminary screening experiments conducted on NCI-H460 and MCF-7
showed a similar potency. The NPC results were in concordance with
these findings. However, contrary to a previous study (10) which stated that silvestrol was
approximately three times more potent than episilvestrol, the
results herein suggested that episilvestrol had higher efficacy
against NPC cells.
The present flow cytometry data indicated that
silvestrol and episilvestrol inhibit the G2/M transition
in the NPC cell cycle. A previous study evaluating the cytotoxicity
of silvestrol in LNCaP human prostate carcinoma cells indicated a
similar mode of action (21). In
addition, another study demonstrated that rocaglaol, which is a
cyclopenta[b]benzofuran flavagline, inhibited G2/M cell
cycle progression (22). Tumours
often have increased proliferation rates. Evaluation of the
percentage of cells in S phase may be useful, as it may convey
prognostic value. To evaluate the number of cells in S phase, a
pulse-chase experiment was performed herein. In C666.1 cells,
silvestrol and episilvestrol were able to reduce DNA synthesis.
However, this was not observed in HK1 cells under the present
experimental conditions. Conversely, Kim et al (19) reported that while certain members of
the plant-derived cyclopenta[b]benzofuran class were cytostatic
against various human cancer cell lines, silvestrol exhibited a
cytotoxic rather than cytostatic effect, as determined by a colony
formation assay with LNCaP cells. Furthermore, silvestrol induced
an apoptotic response. A previous study demonstrated that
silvestrol induced cell growth arrest and apoptosis in AML cell
lines and primary blasts (23).
Another study reported the ability of silvestrol to inhibit
eIF4F-dependent translation correlated with the ability to inhibit
cell proliferation (24).
Concurrent chemotherapy and radiotherapy or
chemoradiation is the standard treatment regimen for NPC. The
method of treatment uses the chemotherapy drug cisplatin,
5-fluorouracil, or a combination of both in addition to
radiotherapy to achieve local control of the disease (4). However, treatment of metastatic NPC
remains a clinical challenge. One of the strategies currently under
investigation involves the use of additional therapeutic agents in
combination to the standard chemoradiation regimen to treat
patients with metastatic NPC. The results of the present study
demonstrated that silvestrol and episilvestrol, natural products
from the A. stellatopilosa tree, exhibit in vitro
synergism with cisplatin for the inhibition of NPC cell growth.
Previous studies have shown that tumour cells can be sensitized by
silvestrol to standard chemotherapeutic agents such as doxorubicin
(8,25) or daunorubicin, etoposide or
cytarabinose-C (26), thereby
producing improved therapeutic effects. One recent study
demonstrated that the growth of hepatocellular cancer cell lines
was inhibited by silvestrol, and that there was a synergistic
effect of the compound with chemotherapy drugs sorafenib and
rapamycin (27). The data obtained
from the present investigation demonstrated that concomitant
treatment of silvestrol or episilvestrol with cisplatin at various
ratios showed marked synergistic growth inhibitory effects on NPC
cells. The results also showed that the dose reduction index, which
indicates how many fold the dose of each drug may be reduced in a
synergistic combination compared with the dose of that drug alone,
were >1. This is advantageous as a reduced dose leads to
decreased toxicity in the host, while maintaining the same
therapeutic efficacy (14). When
administered as a single agent to nude mice, in vivo
anti-tumour effects of silvestrol were observed with no obvious
toxicity (8). This allowed the use
of silvestrol with established agents as a novel therapeutic
strategy (9,26).
Silvestrol has been shown to exhibit in vivo
anti-tumor activity in B-cell acute lymphoblastic leukemia and
chronic lymphocytic leukemia (20).
In a previous in vivo study, silvestrol administration
suppressed the growth of MDA-MB-231 breast cancer and PC-3 human
prostate cancer xenografts (8).
Notably, vehicle and silvestrol-treated animals displayed similar
blood cell profiles and silvestrol appeared to be well-tolerated in
animals. However, the use of silvestrol in studies in vivo
is hindered by poor absorption, distribution, metabolism and
secretion and sensitivity to P-glycoprotein-mediated multidrug
resistance (22). Gupta et al
(28) described silvestrol as a
substrate of phosphoglycolate phosphatase that was likely to result
in the poor oral absorption of silvestrol observed in mice.
Phosphoglycolate phosphatase efflux is a crucial aspect to overcome
for the successful drug development of oral formulations (28). It was reported that intraperitoneal
systemic availability was 100%; however, the oral bioavailability
of silvestrol was markedly lower (29). A study conducting pharmacokinetics
analysis of silvestrol via the development and validation of a
sensitive LC-MS/MS method for accurate quantification of silvestrol
in mouse plasma has previously been described (29). The data suggested an overall
favorable pharmacokinetic profile of silvestrol in mice (29).
In conclusion, silvestrol and episilvestrol may
function as adjunct therapeutic agents in the standard NPC
treatment regimen of cisplatin and 5-fluorouracil. The synergism of
these compounds with standard therapeutic agents may help in
reducing drug toxicity in patients with NPC. Further investigation
is required in order to understand the exact mechanism underlying
the synergism between silvestrol or episilvestrol and
cisplatin.
Acknowledgements
The authors thank the Director General of Health
(Malaysia) Datuk Dr Noor Hisham Bin Abdullah for permission to
publish this article and the Director of the Institute for Medical
Research, Dr Jasbir Singh Dhaliwal for support. The present study
was financially supported by the Ministry of Health of Malaysia
(grant no. NMRR-14-815-22074).
References
|
1
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Devi BC, Pisani P, Tang TS and Parkin DM:
High incidence of nasopharyngeal carcinoma in native people of
Sarawak, Borneo island. Cancer Epidemiol Biomarkers Prev.
13:482–486. 2004.PubMed/NCBI
|
|
3
|
Tao Q and Chang AT: Nasopharyngeal
carcinoma: Molecular pathogenesis and therapeutic developments.
Expert Rev Mol Med. 9:1–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wee J: Nasopharyngeal cancer: A promising
future. Lancet Oncolo. 13:116–118. 2012. View Article : Google Scholar
|
|
5
|
Kinghorn AD, Pan L, Fletcher JN and Chai
H: The relevance of higher plants in lead compound discovery
programs. J Nat Prod. 74:1539–1555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pannel CM: Aglaia (Meliaceae). Tree flora
of Sabah and Sarawak. 6:Soepadmo E, Saw LG, Chung RCK and Kiew R:
(Kuala Lumpur). Forest Research Institute Malaysia. 27–107.
2007.
|
|
7
|
Ng BL, Omarzuki M, Lau GS, Pannel CM and
Yeo TC: A nucleotide signature for indentification of Aglaia
stellatopilosa pannel. Mol Biotechnol. 56:671–679. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cencic R, Carrier M, Galicia-Vázques G,
Bordeleau ME, Sukarieh R, Bourdeau A, Brem B, Teodoro JG, Greger H,
Tremblay ML, et al: Antitumor activity and mechanism of action of
the cyclopenta[b]benzofuran, silvestrol. PLoS One. 4:e52232009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan L, Woodard JL, Lucas DM, Fuchs JR and
Kinghorn AD: Rocaglamide, silvestrol and structurally related
bioactive compounds from aglaia species. Nat Prod Rep. 31:924–939.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hwang BY, Su BN, Chai H, Mi Q, Kardono LB,
Afriastini JJ, Riswan S, Santarsiero BD, Mesecar AD, Wild R, et al:
Silvestrol and episilvestrol, potential anticancer rocaglate
derivatives from Aglaia silvestris. J Org Chem.
69:3350–3358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang DP, Ho JH, Poon YF, Chew EC, Saw D,
Lui M, Li CL, Mak LS, Lai SH and Lau WH: Establishment of a cell
line (NPC/HK1) from a differentiated squamous carcinoma of the
nasopharynx. Int J Cancer. 26:127–132. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheung ST, Huang DP, Hui AB, Lo KW, Ko CW,
Tsang YS, Wong N, Whitney BM and Lee JC: Nasopharyngeal carcinoma
cell line (C666-1) consistently harbouring Epstein-Barr virus. Int
J Cancer. 83:121–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan SY, Choy KW, Tsao SW, Tao Q, Tang T,
Chung GT and Lo KW: Authentication of nasopharyngeal carcinoma
tumor lines. Int J Cancer. 122:2169–2171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Daker M, Ahmad M and Khoo AS:
Quercetin-induced inhibition and synergistic activity with
cisplatin - a chemotherapeutic strategy for nasopharyngeal
carcinoma cells. Cancer Cell Int. 12:342012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Daker M, Voon YL, Akowuah GA, Yam MF and
Mariam A: Inhibitory effects of Cinnamomum burmannii Blume
stem bark extract and trans-cinnamaldehyde on nasopharyngeal
carcinoma cells; synergism with cisplatin. Exp Ther Med.
5:1701–1709. 2013.PubMed/NCBI
|
|
17
|
Patton JT, Lustberg ME, Lozanski G, Garman
SL, Towns WH, Drohan CM, Lehman A, Zhang X, Bolon B, Pan L, et al:
The translation inhibitor silvestrol exhibits direct anti-tumor
activity while preserving innate and adaptive immunity against
EBV-driven lymphoproliferative disease. Oncotarget. 6:2693–2708.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Patton JT, Lustberg ME, Garman SL,
Kinghorn AD, Pan L, Lucas DM, Grever MR and Baiocchi RA: Silvestrol
Modulates Direct Anti-Tumor Activity Against Epstein-Barr Virus
(EBV)-Associated Lymphomas While Sparing Innate and Antigen
Specific Adaptive Immunity. Presented at 53rd ASH Annual Meeting
and Exposition. (abstract 104). 2011.
|
|
19
|
Kim S, Hwang BY, Su BN, Chai H, Mi Q,
Kinghorn AD, Wild R and Swanson SM: Silvestrol, a potential
anticancer rocaglate derivative from Aglaia foveolata,
induces apoptosis in LNCaP cells through the
mitochondrial/apoptosome pathway without activation of executioner
caspase-3 or −7. Anticancer Res. 27:2175–2183. 2007.PubMed/NCBI
|
|
20
|
Lucas DM, Edwards RB, Lozanski G, West DA,
Shin JD, Vargo MA, Davis ME, Rozewski DM, Johnson AJ, Su BN, et al:
The novel plant-derived agent silvestrol has B-cell selective
activity in chronic lymphocytic leukemia and acute lymphoblastic
leukemia in vitro and in vivo. Blood. 113:4656–4666. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mi Q, Kim S, Hwang BY, Su BN, Chai H,
Arbieva ZH, Kinghorn AD and Swanson SM: Silvestrol regulates G2/M
checkpoint genes independent of p53 activity. Anticancer Res.
26:3349–3356. 2006.PubMed/NCBI
|
|
22
|
Mi Q, Su BN, Chai H, Cordell GA,
Farnsworth NR, Kinghorn AD and Swanson SM: Rocaglaol induces
apoptosis and cell cycle arrest in LNCaP cells. Anticancer Res.
26:947–952. 2006.PubMed/NCBI
|
|
23
|
Alachkar H, Santhanam R, Harb JG, Lucas
DM, Oaks JJ, Hickey CJ, Pan L, Kinghorn AD, Caligiuri MA, Perrotti
D, et al: Silvestrol exhibits significant in vivo and in vitro
antileukemic activities and inhibits FLT3 and miR-155 expressions
in acute myeloid leukemia. J Hematol Oncol. 6:212013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boussemart L, Malka-Mahieu H, Girault I,
Allard D, Hemmingsson O, Tomasic G, Thomas M, Basmadjian C, Ribeiro
N, Thuaud F, et al: eIF4F is a nexus of resistance to anti-BRAF and
anti-MEK cancer therapies. Nature. 513:105–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bordeleau ME, Robert F, Gerard B,
Lindqvist L, Chen SM, Wendel HG, Brem B, Greger H, Lowe SW, Porco
JA Jr and Pelletier J: Therapeutic suppression of translation
initiation modulates chemosensitivity in a mouse lymphoma model. J
Clin Invest. 118:2651–2660. 2008.PubMed/NCBI
|
|
26
|
Cencic R, Carrier M, Trnkus A, Porco JA
Jr, Minden M and Pelletier J: Synergistic effect of inhibiting
translation initiation in combination with cytotoxic agents in
acute myelogenous leukemia cells. Leuk Res. 34:535–541. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kogure T, Kinghorn AD, Yan I, Bolon B,
Lucas DM, Grever MR and Patel T: Therapeutic potential of the
translation inhibitor silvestrol in hepatocellular cancer. PLoS
One. 8:e761362013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gupta SV, Sass EJ, Davis ME, Edwards RB,
Lozanski G, Heerema NA, Lehman A, Zhang X, Jarjoura D, Byrd JC, et
al: Resistance to the translation initiation inhibitor silvestrol
is mediated by ABCB1/P-glycoprotein overexpression in acute
lymphoblastic leukemia cells. AAPS J. 13:357–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saradhi UV, Gupta SV, Chiu M, Wang J, Ling
Y, Liu Z, Newman DJ, Covey JM, Kinghorn AD, Marcucci G, et al:
Characterization of silvestrol pharmacokinetics in mice using
liquid chromatography-tandem mass spectrometry. AAPS J. 13:347–356.
2011. View Article : Google Scholar : PubMed/NCBI
|