Introduction
Stress is a common factor in everyday life and is
known to induce circulatory diseases and ulceration of the
digestive tract (1). A number of
early clinical reports have suggested that stress serves a major
role in the initiation, course and outcome of liver diseases.
Hirose et al (2) demonstrated
that emotional stress significantly decreases hepatic blood flow.
In addition, Tissari et al (3) reported that electric foot-shock stress
exacerbates liver injury in mice treated with carbon tetrachloride
(CCl4). Furthermore, it has been observed that stress
can aggravate α-galactosylceramide-induced hepatitis (4). Therefore, growing evidence continues to
demonstrate that stress can influence the progression of liver
disease.
The term ‘restraint stress’ refers to a specific
type of stress resulting from the restriction of movement. Two
primary experimental restraint stress animal models have been
established: The first model involves confinement when the animal's
movement is limited within a restricted space (5). The second model utilizes immobilization
of the limbs and body of the animal using tape or plaster (6). Panuganti et al (7) demonstrated that restraint for periods
of 0.5 or 1.5 h did not significantly enhance liver injury in
healthy animals, whereas restraint for 2.5 h caused a significant
increase in liver injury. However, whether restraint for 0.5 or 1.5
h has an effect on CCl4-induced liver injury in animals
remains unknown.
In the present study, mice were restrained in 50 ml
centrifuge tubes in order to investigate the effects of chronic
restraint stress on CCl4-induced liver fibrosis. A
previous study reported that stimulation of 5-hydroxytryptamine 2B
(5-HT2B) receptor on hepatic stellate cells (HSCs) promotes HSC
activation, and that antagonism of 5-HT2B receptor attenuates
fibrogenesis (8). Therefore, in the
present study, the effects of chronic restraint stress on 5-HT2B
expression and HSC activation were investigated.
Materials and methods
Chemicals
CCl4, hematoxylin and eosin (HE) solution
and a Masson's trichrome staining kit were obtained from
Sigma-Aldrich (Shanghai, China). Peanut oil was obtained from
Shandong Luhua Group Co., Ltd. (Beijing, China).
Animals and chronic restraint stress
model
A total of 30 male BALB/c mice (Beijing Merial Vital
Laboratory Animal Technology Co., Ltd., Beijing, China), weighing
18–20 g and aged 6–8 weeks, were acclimatized for 7–10 days in the
Capital Medical University (Beijing, China) animal care facility.
Throughout the experiments, animals were housed under a 12 h
light/dark cycle with controlled temperature (24°C) and humidity
(50%). Animals were allowed access to food and water ad
libitum, except when restrained. All procedures were approved
by the Institutional Animal Care and Use Committee of Capital
Medical University of China. On day 1, 30 male BALB/c mice were
randomly divided into three groups (n=10 each): Oil-treated control
group; CCl4-treated group; and CCl4 +
restraint-treated group, which mice were treated with
CCl4 and exposed to chronic restraint stress. Mice in
the oil- and CCl4-treated groups were injected
intraperitoneally with 10 µl/g peanut oil or 0.5% CCl4
solution once every 3 days over a 42-day experimental period.
Following injection of 0.5% CCl4 solution, animals in
the restraint group were placed in 50 ml centrifuge tubes (Axygen;
Corning Incorporated, Corning, NY, USA) for 0.5 h every 3 days over
the 42-day experimental period. The restraint cage was well
ventilated and prevented animals from turning or ambulating, but
did not squeeze them. Following exposure to restraint, the mice
were transferred to their home cages.
Immediately after restraint on day 42 of the
experimental period, all mice were sacrificed via CO2
narcosis. Blood samples were collected from the vena cava. The
livers were removed and fixed in 2.5%
glutaraldehyde-polyoxymethylene solution (Leagene Biotech Co.,
Ltd., Beijing, China) for 72 h for analysis. Liver tissues were
then processed into paraffin and 5-µm sections were prepared for
HE, Masson's trichrome, 5-HT2B (Abgent Biotech Co., Ltd., Suzhou,
China) and α-smooth muscle actin (α-SMA; Abcam, Shanghai, China)
immunohistochemical staining.
Liver injury assessment
A total of 0.1 ml serum was isolated from blood
samples by centrifugation at 2,500 × g for 15 min. The alanine
aminotransferase (ALT) and aspartate aminotransferase (AST)
expression levels in serum samples were measured using a
commercially available colorimetric assay kits (Kinghawk
Pharmaceutical Co., Ltd., Beijing, China). A highly colored end
product from colorimetric assay was detected at 490–520 nm by a
spectrophotometer (736-10; Hitachi, Ltd., Beijing, China), as the
absorbance of each end product is proportional to the activity of
the enzyme.
HE staining
Liver sections were stained with HE, and the degree
of fibrosis in each section was classified according to grades 0–4,
as previously described (9), where
grade 0, 0% fibrosis; grade 1, <10% fibrosis; grade 2, <30%
fibrosis; grade 3, <50% fibrosis; and grade 4, ≥50% fibrosis.
Each tissue section was examined using an Olympus BH-2 microscope
(Olympus Optical Co. Ltd., Beijing, China). In addition,
histopathological changes were investigated using a Motic Images
2000 microscope (Motic China Group Co. Ltd, Guangzhou, China).
Masson's trichrome staining for
collagen level detection
A Masson's trichrome staining kit (Sigma-Aldrich)
was used to detect the collagen levels in the liver tissue. The
blue-stained areas in the tissue sections were assessed using an
Image-Pro Plus image analyzer (Media Cybernetics, Inc., Rockville,
MD, USA) for semi-quantitative analysis. Results were expressed as
the area density, which was defined the area of the positive cells
/ area of the entire field.
Measurement of 5-HT2B receptor and
α-SMA expression levels
Immunohistochemical staining for 5-HT2B receptor and
α-SMA was performed using Streptavidin Biotin Complex
immunohistochemistry kits (Wuhan Boster Biological Engineering Co.,
Ltd., Wuhan, China) according to the manufacturer's instructions.
The yellow-stained areas in the tissue sections were assessed and
area density was recorded using a similar method as for collagen
levels.
Statistical analysis
Experimental data were analyzed by one-way analysis
of variance using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
The results are expressed as the mean ± standard deviation.
Difference were considered as statistically significant at
P<0.05.
Results
Serum biochemistry
On day 42, no mortalities were reported in any of
the experimental groups. However, all CCl4-treated mice
displayed progressive jaundice, ascites and hepatomegaly, whereas
these conditions were observed to a lesser degree in the
oil-treated control and CCl4 + restraint-treated mice.
Liver injury was assessed by determining the serum levels of the
liver enzymes alanine aminotransferase (ALT) and aspartate
aminotransferase (AST), and the AST/ALT ratio. As presented in
Fig. 1, the serum levels of AST and
ALT, and the AST/ALT ratio were significantly higher in
CCl4-treated mice in comparison with those in the
oil-treated control mice (P<0.01). Treatment with restraint
significantly reduced the expression levels of AST and ALT compared
with the CCl4-treated mice (P<0.05; Fig. 1), but did not reduce the AST/ALT
ratio.
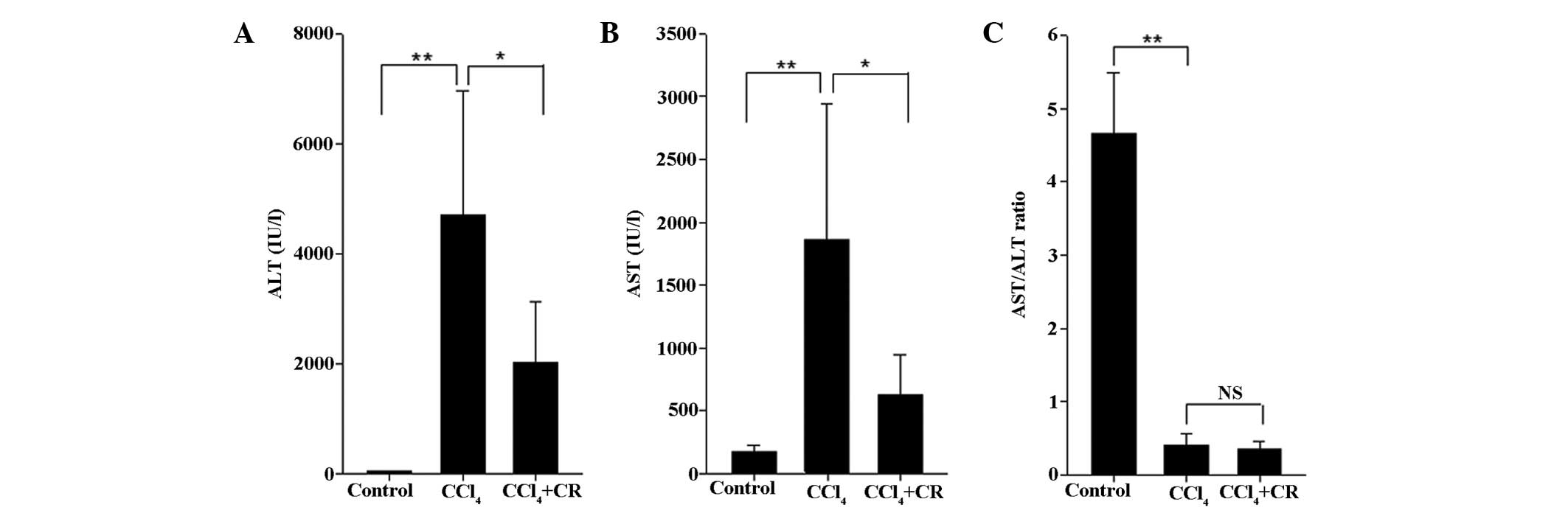 | Figure 1.Serum levels of biochemical
parameters. (A) ALT, (B) AST, and (C) AST/ALT ratio of the control,
CCl4, and CCl4 + CR groups. n=10; error bars
represent standard deviation; *P<0.05, **P<0.01. ALT, alanine
aminotransferase; AST, aspartate aminotransferase; CCl4,
carbon tetrachloride; CR, chronic restraint; NS, no significant
difference. |
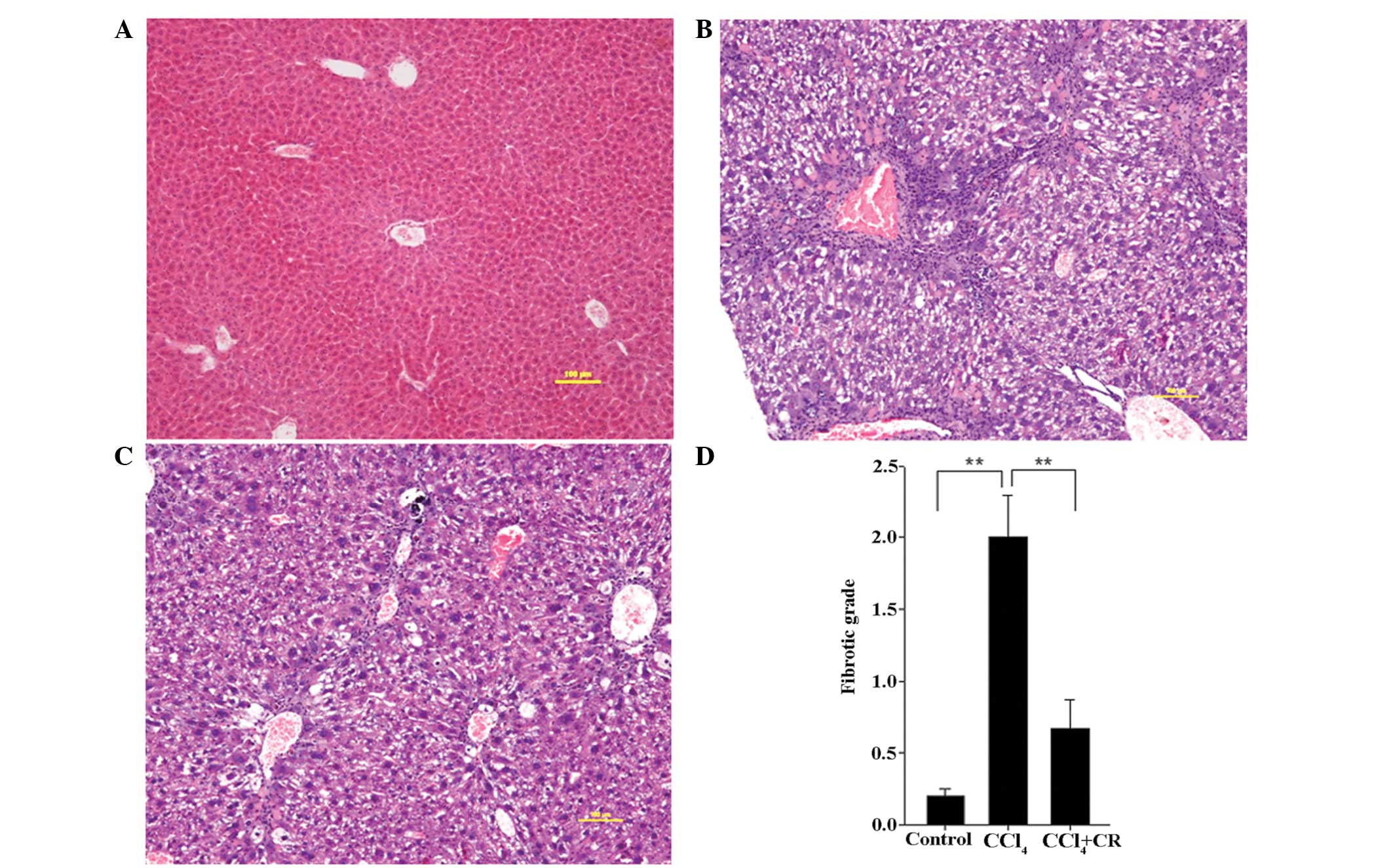
HE staining
As presented in Fig.
2, the fibrotic grade of the livers from
CCl4-treated mice was significantly higher on day 42 in
comparison with the grade in the control mice (P<0.01), while
restraint significantly reduced this fibrotic grade (P<0.01). HE
staining detected centrilobular necrosis and macrovesicular lipid
droplets in the CCl4-treated group (Fig. 2B), in which collagen had accumulated
around the blood vessels and pseudolobuli had formed by thin
fibrous septa. In the CCl4 + restraint group, the area
of centrilobular necrosis and the degree of macrovesicular lipid
droplets were decreased (Fig. 2B).
In addition, collagen accumulation surrounding the blood vessels
was reduced in the CCl4 + restraint group compared with
that in the CCl4-treated group, and no evident
pseudolobuli had formed (Fig.
2C).
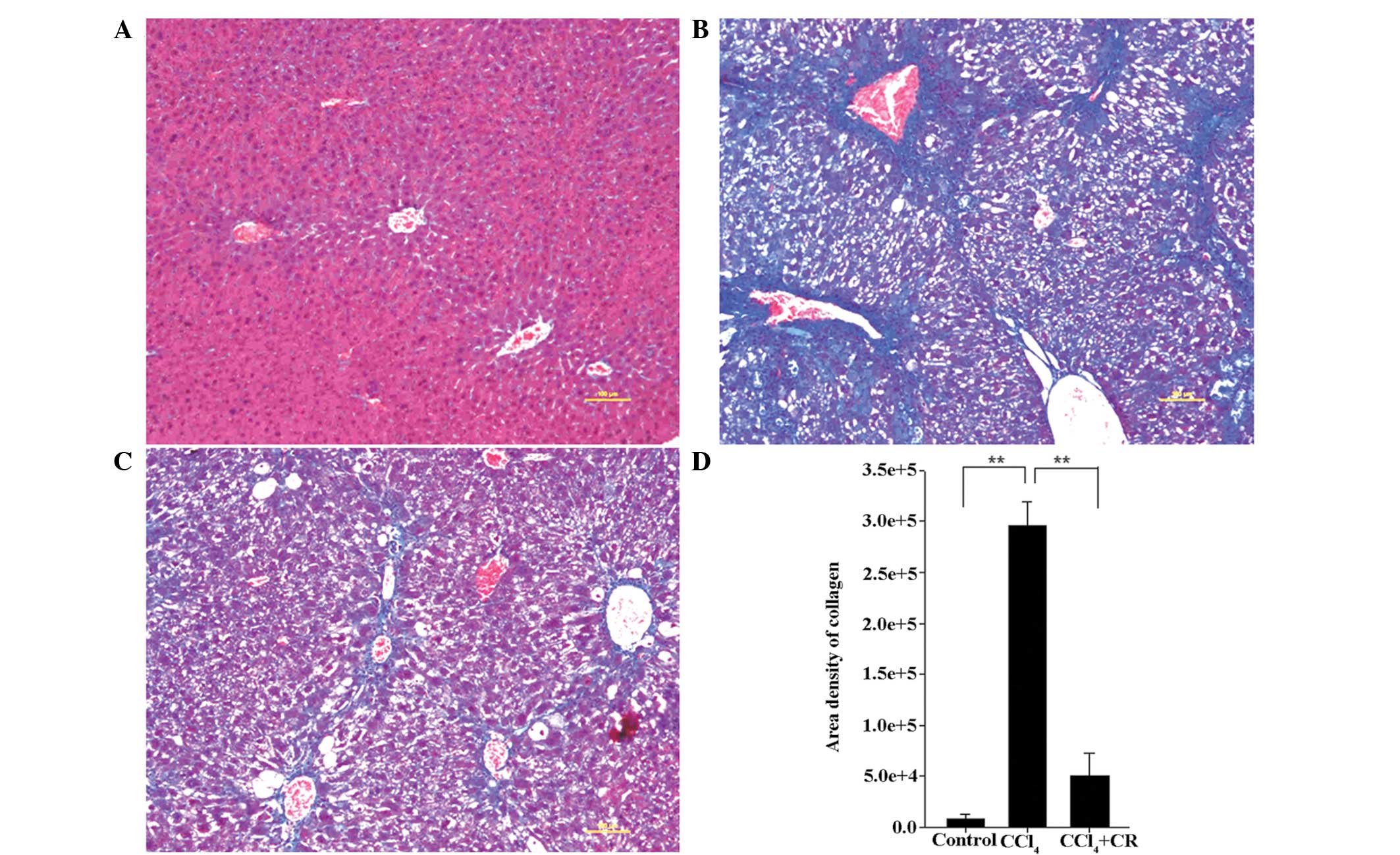
Masson's trichrome staining
As presented in Fig.
3, the area density of collagen in liver sections from
CCl4-treated mice was significantly higher on day 42
compared with that in sections from the oil-treated control mice
(P<0.01). In addition, the density of collagen in
CCl4 + restraint-treated mice was significantly lower
compared with that in CCl4-treated mice (P<0.01),
indicating that restraint significantly reduced the
CCl4-induced collagen production.
In CCl4-treated mice, collagen fibers
were more abundant in the centrilobular area and neighboring
central veins were bridged by fibrous septa (Fig. 3B). In addition, pseudolobuli actively
formed, macrovesicular lipid droplets were detected and the
collagenous septa were much thicker compared with the oil-treated
control group (Fig. 3A and B).
Following restraint stress treatment, the prevalence of collagen
fibers and macrovesicular lipid droplets was reduced and no
pseudolobuli were identified (Fig.
3C).
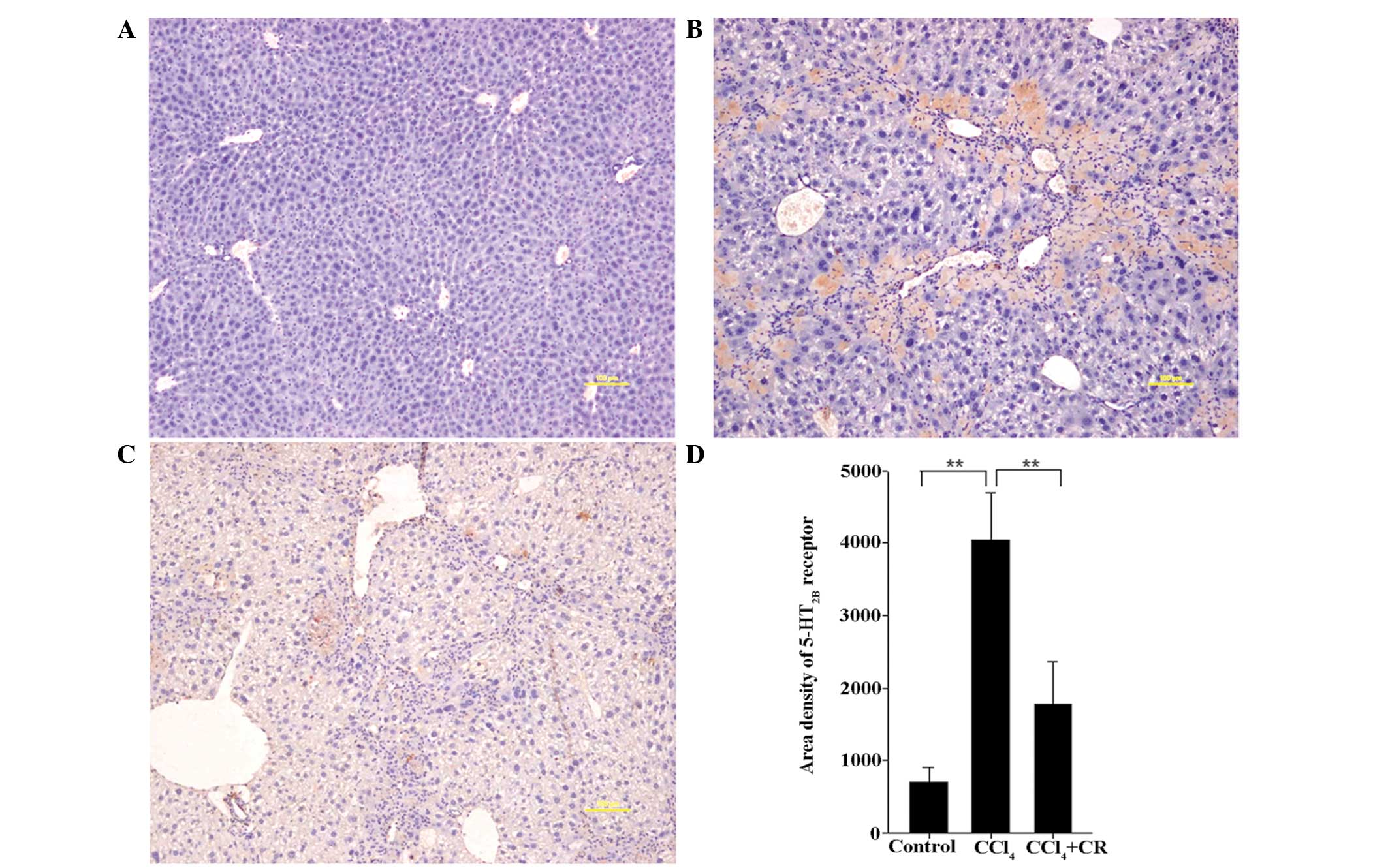
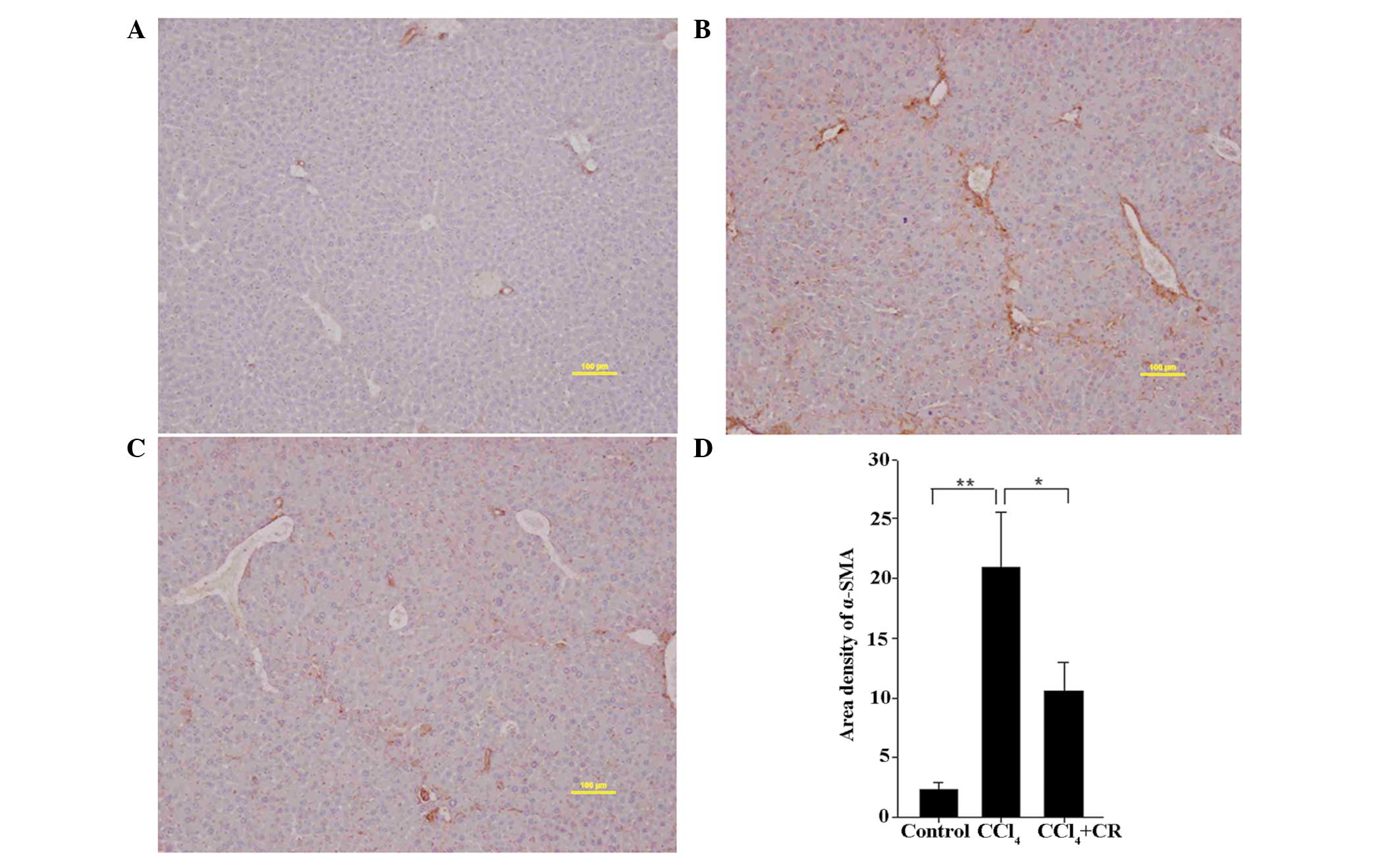
Expression levels of 5-HT2B receptor
and α-SMA
As presented in Figs.
4 and 5, the area densities of
5-HT2B receptor and α-SMA in liver sections from
CCl4-treated mice were significantly higher on day 42 in
comparison with oil-treated control mice (P<0.01). In addition,
the densities in CCl4-treated mice were significantly
higher compared with CCl4 + restraint-treated mice
(P<0.05), indicating that restraint can significantly reduce the
expression levels of 5-HT2B receptor and α-SMA that were induced by
CCl4 treatment. The expression of 5-HT2B receptor and
α-SMA in the CCl4-treated group was highly localized
within the portal area, confirming that the increased expression
levels were induced by CCl4 (Figs. 4B and 5B). In comparison with the
CCl4-treated group, 5-HT2B receptor and α-SMA staining
in the CCl4 + restraint group was significantly reduced
and covered a smaller area (Figs. 4C
and 5C), indicating that the
expression levels of 5-HT2B receptor and α-SMA are associated with
the effects of restraint.
Discussion
In the present study, animals were restrained in 50
ml centrifuge tubes for 0.5 h following the injection of
CCl4 once every 3 days for 42 consecutive days, in order
to evaluate the effect of chronic restraint stress on
CCl4-induced liver injury. Notably, chronic restraint
stress was found to reduce CCl4-induced increases in the
ALT and AST expression levels. Furthermore, liver fibrosis was
alleviated by chronic restraint stress treatment, which may prove
to be a novel therapy for chronic liver disease.
Stress has been associated with a number of
definitions in scientific literature. Certain life-changing or
threatening events are considered to be ‘stressors’ that can be
acute or chronic factors, depending on their duration (10). In addition, stressors have been
associated with a number of immune system dysfunctions, independent
of whether an individual is affected by a chronic or acute disease
(11).
Stress has been suggested to result in liver damage,
since it affects hepatic blood flow by inducing vasospasm and
centrilobular hypoxia (12). As the
understanding of stress mediators increases, research focuses on
the effect of stress on the onset and development of liver damage
in acute and chronic liver diseases (13,14).
Several animal models have demonstrated that there is a close
association between stress and liver disease. For instance,
electric foot-shock stress was found to exacerbate liver injury in
mice treated with CCl4, and to aggravate
α-galactosylceramide-induced hepatitis (4) that is associated with malaria and
Salmonella infection-induced liver injury, and viral hepatitis B
and C (15–18). In addition, restraint and electric
foot-shock stress were demonstrated to induce mild liver injury in
healthy rodents, which was confirmed by slightly elevated ALT
expression levels (19,20). Furthermore, social isolation stress
has been demonstrated to increase the spontaneous hepatocellular
carcinoma incidence in transforming growth factor-α transgenic mice
(21) and to accelerate the
development of liver metastasis in the colon of mice injected with
carcinoma cells (22–24).
In the present study, it was observed that 0.5 h of
restraint was able to reduce CCl4-induced liver
fibrosis. This may be associated with the fact that the restraint
model in the present study limited the animals' movement by
restricting the amount of available space, however the animals'
limbs and body were not completely immobilized. Furthermore, the
time and frequency of restraint were lower than those applied in a
previous study (6).
The activation of the hypothalamic-pituitary-adrenal
(HPA) axis that is induced by stress results in an inhibitory
effect on the immune and inflammatory responses, as all the immune
response components can be inhibited by glucocorticoids (25). Glucocorticoids are the final HPA axis
effector molecules released from the adrenal cortex that
participate in the regulation of homeostasis in each organ
(26). Thus, the ability of chronic
restraint stress to reduce liver fibrosis may result from changes
in glucocorticoid levels. Further research is, therefore, required
to evaluate the role of glucocorticoids in the reduction of
CCl4-induced liver fibrosis following the application of
restraint.
A previous study reported that stimulation of 5-HT2B
receptor on HSCs promotes HSC activation. The present study
demonstrated that 5-HT2B receptor and α-SMA expression levels in
the CCl4 + restraint-treated mice were significantly
reduced compared with those in CCl4-treated mice. These
results indicate that chronic restraint stress may inhibit HSC
activation via the 5-HT2B receptor.
In conclusion, restraint has been identified as an
important factor in the progression and outcome of liver
pathologies. The results of the present study demonstrated that
proper restraint stress may be a potential therapeutic strategy for
the treatment of chronic liver disease. However, the effects of
chronic strain stress may vary depending on the time, type, or
equipment of restraint. An increased understanding of how restraint
alters hepatic inflammation will provide important information for
the development of novel therapies for managing liver diseases.
Acknowledgements
The present study was supported by the Capital
Medical University Basic-Clinical Research Project (grant no.
JL1271), National Natural Science Foundation of China (grant no.
31540094) and Scientific Research Common Program of Beijing
Municipal Commission of Education (grant no. KM201510025003).
References
|
1
|
Chiba S, Numakawa T, Ninomiya M, Richards
MC, Wakabayashi C and Kunugi H: Chronic restraint stress causes
anxiety- and depression-like behaviors, downregulates
glucocorticoid receptor expression, and attenuates glutamate
release induced by brain-derived neurotrophic factor in the
prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry.
39:112–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirose S, Hirayama C and Ikemi Y: The
influence of emotional stress on the liver blood flow. Kyushu J Med
Sci. 12:319–323. 1961.PubMed/NCBI
|
|
3
|
Tissari AH, Argiolas A, Fadda F, Serra G
and Gessa GL: Foot-shock stress accelerates non-striatal dopamine
synthesis without activating tyrosine hydroxylase. Naunyn
Schmiedebergs Arch Pharmacol. 308:155–157. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chida Y, Sudo N, Sonoda J, Sogawa H and
Kubo C: Electric foot shock stress-induced exacerbation of
α-galactosylceramide-triggered apoptosis in mouse liver.
Hepatology. 39:1131–1140. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim JG, Jung HS, Kim KJ, Min SS and Yoon
BJ: Basal blood corticosterone level is correlated with
susceptibility to chronic restraint stress in mice. Neurosci Lett.
555:137–142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Golub MS, Campbell MA, Kaufman FL, Iyer P,
Li LH, Donald JM and Morgan JE: Effects of restraint stress in
gestation: Implications for rodent developmental toxicology
studies. Birth Defects Res B Dev Reprod Toxicol. 71:26–36. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Panuganti SD, Khan FD and Svensson CK:
Enhanced xenobiotic-induced hepatotoxicity and Kupffer cell
activation by restraint-induced stress. J Pharmacol Exp Ther.
318:26–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ebrahimkhani MR, Oakley F, Murphy LB, Mann
J, Moles A, Perugorria MJ, Ellis E, Lakey AF, Burt AD, Douglass A,
et al: Stimulating healthy tissue regeneration by targeting the
5-HT2B receptor in chronic liver disease. Nat Med.
17:1668–1673. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujiwara K, Ogata I, Ohta Y, Hayashi S,
Mishiro S, Takatsuki K, Sato Y, Yamada S, Hirata K, Oka H, et al:
Decreased collagen accumulation by a prolyl hydroxylase inhibitor
in pig serum-induced fibrotic rat liver. Hepatology. 8:804–807.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feliu J, Mel JR, Camps C, Escudero P,
Aparicio J, Menéndez D, García Girón C, Rodriguez MR, Sánchez JJ
and González Barón M: Oncopaz Cooperative Group Associated
Hospitals: Raltitrexed in the treatment of elderly patients with
advanced colorectal cancer: an active and low toxicity regimen. Eur
J Cancer. 38:1204–1211. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Segerstrom SC and Miller GE: Psychological
stress and the human immune system: A meta-analytic study of 30
years of inquiry. Psychol Bull. 130:601–630. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Steingrub JS: Pregnancy-associated severe
liver dysfunction. Critical Care Clinics. 20:763–776. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chida Y, Sudo N and Kubo C: Does stress
exacerbate liver diseases? J Gastroenterol Hepatol. 21:202–208.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Swain MG: Stress and hepatic inflammation.
Am J Physiol Gastrointest Liver Physiol. 279:G1135–G1138.
2000.PubMed/NCBI
|
|
15
|
Gonzalez-Aseguinolaza G, de Oliveira C,
Tomaska M, Hong S, Bruna-Romero O, Nakayama T, Taniguchi M,
Bendelac A, Van Kaer L, Koezuka Y and Tsuji M:
α-Galactosylceramide-activated Vα14 natural killer T cells mediate
protection against murine malaria. Proc Natl Acad Sci USA.
97:8461–8466. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kakimi K, Guidotti LG, Koezuka Y and
Chisari FV: Natural killer T cell activation inhibits hepatitis B
virus replication in vivo. J Exp Med. 192:921–930. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishigami M, Nishimura H, Naiki Y, Yoshioka
K, Kawano T, Tanaka Y, Taniguchi M, Kakumu S and Yoshikai Y: The
roles of intrahepatic Valpha14(+) NK1.1(+) T
cells for liver injury induced by Salmonella infection in mice.
Hepatology. 29:1799–1808. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nuti S, Rosa D, Valiante NM, Saletti G,
Caratozzolo M, Dellabona P, Barnaba V and Abrignani S: Dynamics of
intra-hepatic lymphocytes in chronic hepatitis C: Enrichment for
Valpha24+ T cells and rapid elimination of effector
cells by apoptosis. Eur J Immunol. 28:3448–3455. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chida Y, Sudo N, Motomura Y and Kubo C:
Electric foot-shock stress drives TNF-alpha production in the liver
of IL-6-deficient mice. Neuroimmunomodulation. 11:419–424. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fernández G, Mena MP, Arnau A, Sánchez O,
Soley M and Ramirez I: Immobilization stress induces c-Fos
accumulation in liver. Cell Stress Chaperones. 5:306–312. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hilakivi-Clarke L and Dickson RB: Stress
influence on development of hepatocellular tumors in transgenic
mice overexpressing TGF alpha. Acta Oncol. 34:907–912. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu W, Yamaura T, Murakami K, Murata J,
Matsumoto K, Watanabe H and Saiki I: Social isolation stress
enhanced liver metastasis of murine colon 26-L5 carcinoma cells by
suppressing immune responses in mice. Life Sci. 66:1827–1838. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu W, Murata J, Murakami K, Yamaura T,
Hayashi K and Saiki I: Social isolation stress augments
angiogenesis induced by colon 26-L5 carcinoma cells in mice. Clin
Exp Metastasis. 18:1–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu W, Yamaura T, Murakami K, Ogasawara M,
Hayashi K, Murata J and Saiki I: Involvement of TNF-alpha in
enhancement of invasion and metastasis of colon 26-L5 carcinoma
cells in mice by social isolation stress. Oncol Res. 11:461–469.
1999.PubMed/NCBI
|
|
25
|
Charmandari E, Achermann JC, Carel JC,
Soder O and Chrousos GP: Stress response and child health. Sci
Signal. 5:mr12012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McEwen BS, Biron CA, Brunson KW, Bulloch
K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH,
Spencer RL and Weiss JM: The role of adrenocorticoids as modulators
of immune function in health and disease: Neural, endocrine and
immune interactions. Brain Res Brain Res Rev. 23:79–133. 1997.
View Article : Google Scholar : PubMed/NCBI
|