Introduction
During the past few decades, nondepleting monoclonal
antibody agents specifically targeting activated T cells have been
developed, which are intended to provide effective
immunosuppression without the adverse effects of profound and
aspecific T lymphocyte depletion that are associated with
polyclonal antibody agents (1).
Basiliximab (Simulect®) is a high affinity, chimeric
monoclonal antibody, with murine variable and human constant
regions, directed against the α-chain (also known as CD25 subunit)
of the interleukin (IL)-2 receptor on T lymphocytes (2). The expression of CD25 is specific to
activated T cells; thus, basiliximab selectively targets activated
lymphocytes and does not affect resting cells (3). The chimerization of the murine antibody
with human immunoglobulin G1 is associated with reduced
immunogenicity.
Basiliximab has been widely used as an induction
therapy in kidney transplantation, with a two-dose regimen of 20 mg
on days 0 and 4, respectively. However, pharmacokinetic studies
have shown that the half-life of basiliximab is 7.2±3.2 days in
adults, suggesting that a shorter regimen might be effective as an
induction therapy (4). Recently, a
single-dose regimen of basiliximab has been described in cadaveric
kidney transplantation, with good efficacy and safety (4).
The objective of the present study was to observe
the efficacy and safety of induction immunosuppression with
single-dose basiliximab in 33 patients with donation after cardiac
death (DCD) kidney transplantation in a single hospital.
Materials and methods
Patients
In this retrospective study, the clinical data of 33
DCD kidney surgeries from 25 donors performed between December 2010
and July 2013 at the Affiliated Urology and Nephrology Hospital of
Ningbo University (Ningbo, China) were analyzed.
The study protocol was approved by the hospital's
Committee for Medical and Health Research Ethics. It was conducted
in accordance with the ethical guidelines of the 1975 Declaration
of Helsinki. All patients included provided consent for the use of
their clinical data in research.
All donors were China Category III donors (DCD or
brain death patients). Donors' data were obtained from The Chinese
Red Cross (Beijing, China).
The donor selection criteria were as follows: <60
years of age; terminal urine output >30 ml/h; normal kidney size
and morphology; absence of pre-existing renal disease; no medical
history of cancer; absence of untreated systemic infection;
negative serologic tests for human immunodeficiency virus, syphilis
or hepatitis B or C infection.
The surgical technique of organ procurement is
briefly described as follows: The donor was transported to the
operating room so that no time was lost in transportation and the
warm ischemia time (WIT) was reduced. The patient was pretreated
with an intravenous injection of 10,000 units heparin to decrease
the risk of thrombosis. After withdrawal of life support and
declaration of cardiac death, rapid laparotomy was conducted with
direct cannulation of the aorta and in situ perfusion with
UW solution (Bristol-Myers Squibb, New York, NY, USA). In all
cases, abdominal organs were cooled using ice-slush. Then, multiple
organ procurement was performed using conventional organ recovery
techniques (5). Subsequently, the
organs were rapidly flushed, cooled, and preserved in UW
preservation solution.
Immunosuppressive regimen
Prior to this study, basiliximab (Novartis
Pharmaceuticals Corp., East Hanover, NJ, USA) was used in induction
therapy in conventional two doses (days 0 and 4
post-transplantation). However, following careful observation and
an accumulation of clinical experience, in 2006, the induction
protocol was changed and a single-dose basiliximab protocol was
introduced.
In this study, for all the DCD kidney recipients, a
bolus of 20 mg basiliximab was given 1 h prior to the surgery,
lasting until after the completion of the vascular anastomoses. In
addition, all recipients received 500 mg intravenous
methylprednisolone (Pfizer, Inc,, New York, NY, USA) during the
surgery and a 3-day bolus of intravenous methylprednisolone therapy
(10 mg/kg per day) post-transplantation. Subsequently, the patients
received oral prednisone (Xianju Pharmaceutical Co., Ltd.,
Hangzhou, China) at 80 mg per day, with further tapering to 20 mg
per day at 10 days, and 10 mg per day at 3 to 4 months.
The standard immunosuppressive protocol for all
kidney transplant recipients consisted of the following: A
calcineurin inhibitor, such as cyclosporine A (CsA;
Neoral®; Novartis, Basel, Switzerland) or tacrolimus
(Astellas Pharma, Deerfield, IL, USA); mycophenolate mofetil (MMF;
Roche Pharmaceuticals, Nutley, NJ, USA); and corticosteroids
(prednisone). Initial target CsA blood concentration at 2 h after
dose (C2) levels were 900–1,100 ng/ml, tapering to 800–1,000 ng/ml
by 2 months and thereafter CsA C2 levels were 600–800 ng/ml with
gradual reduction towards a CsA C2 of 500–600 ng/ml in the
long-term maintenance phase. Initial tacrolimus trough levels were
targeted at 8–10 ng/ml in the first 3 months, while subsequent
doses were adjusted to 6–8 ng/ml thereafter. For mycophenolate
mofetil, a dose of 1,000 mg twice daily was given at the time of
transplantation, lasting for 1 week; then the dose was adjusted to
750 mg twice daily for 1 week, and a further reduction to 500 mg
twice daily continued thereafter.
Diagnosis of acute rejection
In this study, the incidence and severity
(determined by histological grade) of acute rejection during the
post-transplant period were observed. Acute rejections were
diagnosed by persistent increase of serum creatinine (Cr; by ≥15%
from the baseline) not explained by CsA or tacrolimus levels above
the target levels or by kidney hypoperfusion, vascular thrombosis,
or urinary tract obstruction; clinical signs of rejection including
fever (>38.0°C), decreased urinary output, pain over an enlarged
kidney graft, hypertension, an increased kidney graft size
indicated by color Doppler and elevated vascular resistance index
(>0.8). Acute rejection was confirmed by biopsy. Biopsies
obtained were graded according to the updated Banff Classification
(6).
Prophylaxis of infection
All patients received perioperative ceftriaxone for
bacterial prophylaxis. Two weeks after the surgery, all recipients
received anti-Pneumocystis prophylaxis with
sulfamethoxazole/trimethoprim for 3 months. Antiviral prophylaxis
consisted of intravenous ganciclovir for ≥1 month, depending on the
donor and recipient cytomegalovirus (CMV) serologic status.
Patient monitoring and follow-up
Post-transplantation renal allograft function was
evaluated by measuring serum Cr levels as well as Cr clearance. In
all patients, routine clinical and laboratory parameters, including
serum Cr concentration, red blood cell, white blood cell (WBC) and
platelet counts, were monitored daily up to post-transplant day 14
while hospitalized, then followed up at regular intervals following
discharge. Furthermore, patient and graft survivals, and the
incidence of delayed graft function (DGF) were also observed. DGF
was defined as a requirement for dialysis treatment within 1 week
after transplantation.
After discharge from the hospital, the patients were
followed up at the out-patient clinic on a weekly basis during the
first 3 months, every 2 weeks until month 6, and then monthly until
the end of the first post-transplant year. On every visit, complete
blood counts, serum Cr concentration, and urine examination results
were monitored and recorded on a database. Color Doppler studies
were performed as per requirement, and additional evaluations were
performed whenever deemed clinically appropriate. The follow-up
period was 12 months.
Safety assessment
Safety was assessed by monitoring adverse events
during the course of the study, including the incidence and type of
possible side effects associated with basiliximab. In particular,
the incidence and severity of infections, malignancy and abnormal
results of laboratory tests were monitored.
Statistical analysis
All data are expressed as mean ± standard deviation.
The SPSS 16.0 statistical software package (SPSS, Inc., Chicago,
IL, USA) was used.
Results
Patient characteristics
Characteristics of DCD donors and recipients are
summarized in Tables I and II, respectively.
 | Table I.Demographic and clinical
characteristics of donors after cardiac death. |
Table I.
Demographic and clinical
characteristics of donors after cardiac death.
| Characteristic | Donors (n=25) |
|---|
| Age, years |
|
|
Meana | 29.3±14.5 |
|
Range | 2–53 |
| Gender |
|
| Male | 15 |
|
Female | 10 |
| sCr concentration on
the day of transplantation, µmol/la | 150.7±31.9 |
| Warm ischemia time,
mina | 13.9±6.2 |
| Cold ischemia time,
mina | 187.4±33.7 |
| Cause of death,
n |
|
| Traumatic
brain injury | 20 |
|
Drowning | 3 |
|
Others | 2 |
 | Table II.Demographic and clinical
characteristics of recipients. |
Table II.
Demographic and clinical
characteristics of recipients.
| Characteristic | Recipients
(n=33) |
|---|
| Age, years |
|
|
Meana | 41.1±15.7 |
|
Range | 12–63 |
| Gender |
|
| Male | 19 |
|
Female | 14 |
| Weight ratio
(graft-to-recipient), g/kga | 2.8±0.7 |
| Underlying kidney
disease, n |
|
| Chronic
glomerulonephritis | 18 |
|
Hypertension | 7 |
|
Diabetes | 6 |
|
Polycystic kidney | 2 |
| Mode of
pretransplantation dialysis, n |
|
|
Hemodialysis | 26 |
|
Peritoneal dialysis | 7 |
| Duration of
pretransplantation dialysis, mina | 10.6±4.5 |
| PRA, %a | 4.1±2.5 |
| sCr clearance,
ml/mina |
|
| 1
month | 37.9±12.5 |
| 6
month | 56.7±16.1 |
| 12
month | 70.4±20.6 |
The mean age of the DCD donors was 29.3 years
(range, 2–53 years; Table I). There
were 15 male and 10 female donors. The mean WIT was 13.9 min, with
a mean cold ischemia time of 187.4 min. The causes of mortality
included traumatic brain injury (20 cases, 80.0%), drowning (3
cases, 12.0%) and other reasons (1 case of ganglia glioma and 1
case of Guillian-Barre syndrome, 8.0%). The serum Cr (sCr) level
prior to organ procurement was 150.7±31.9 µmol/l. Among the donors,
the paired kidneys from one 2-year-old donor were en bloc engrafted
into one 14-year-old female recipient.
The 33 kidney recipients had a mean age of 41.1
years (range, 2–63 years; Table
II), and included 19 males and 14 females. Original diseases
leading to end stage renal disease (ESRD) were chronic
glomerulonephritis (n=18), hypertension (n=7), diabetic nephropathy
(n=6) and polycystic kidney (n=2). All the recipients had a panel
reactive antibody (PRA) value of <10%, with a mean of 4.1%. In
addition, the proportion for human leukocyte antigen (HLA) mismatch
at 1, 2, 3, 4, 5 and 6 loci was 6.1, 15.2, 48.4, 18.2, 9.1 and
3.0%, respectively. None of the recipients presented anti-HLA
antibodies.
Acute rejection episodes (AREs)
At 1 year post-transplantation, there were a total
of 3 AREs observed in 3 patients, with an overall incidence of
9.1%. Renal biopsy confirmed the diagnosis of rejection in these
patients. Among them, 2 patients had grade I rejection; and in 1
case, acute rejection was graded as II. All patients with acute
rejection were treated with a combination of intravenous
administration of methylprednisolone pulses (8 mg/kg per day) and
cyclophosphamide (CTX, 0.2 g per day) for 3 days followed by a
steroid taper. The majority of AREs responded to this therapy, and
only 1 patient was treated with anti-thyroglobulin (ATG) antibodies
(2 mg/kg per day intravenously) for 7 days for steroid-resistant
ARE. All patients with AREs were eventually reversed and there were
no recurrences.
Recovery of allograft function
No patients experienced hyperacute rejection
reaction. There were 10 cases of DGF in the recipients, with an
incidence of 30.3%. One patient with DGF developed severe bleeding
from the renal vein leading to graft loss at 14 days
post-transplantation due to Mycosis pilorum infection. Others
(9/10, 90.0%) recovered renal graft function to normal levels
within 9–32 days (13.3±6.5 days): 66.7% (6/9) by 14 days and 33.3%
(3/9) by 32 days.
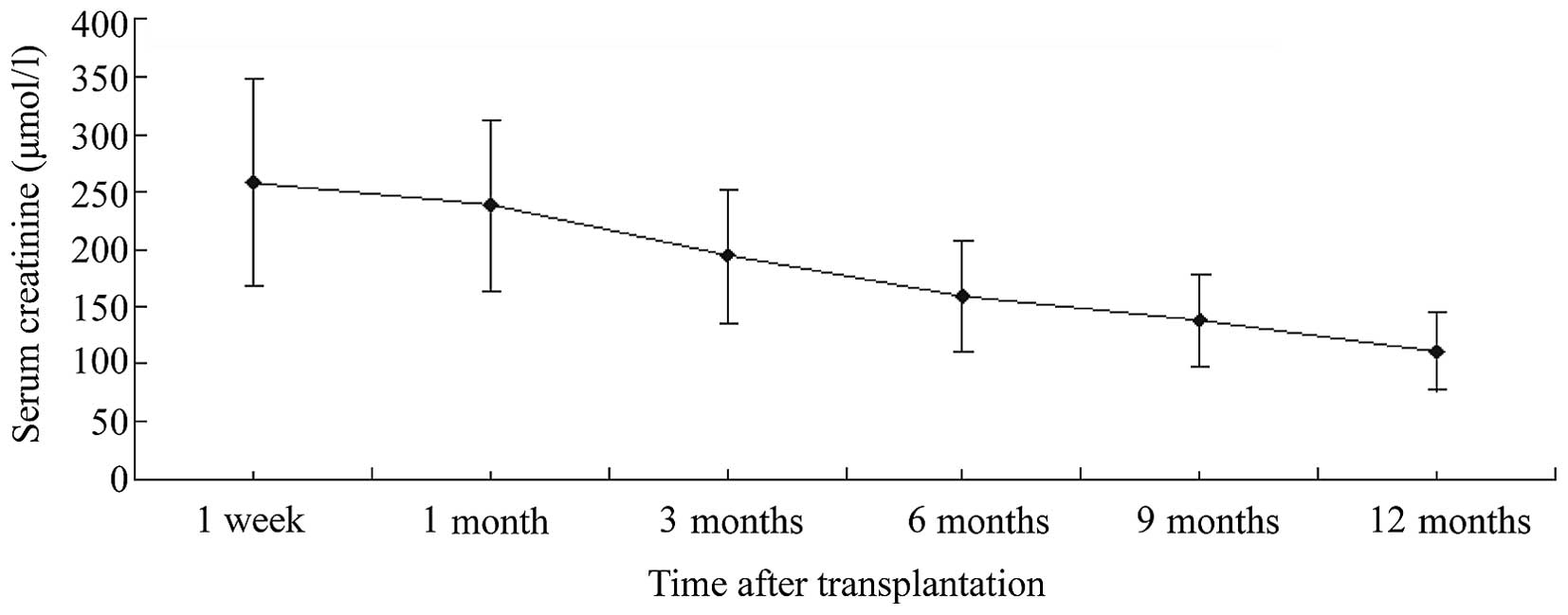
The mean sCr values at 1 week, and at 1, 3, 6, 9 and
12 months post-transplantation were 257.6 ± 89.3, 238.2 ± 74.1,
194.5 ± 57.5, 159.3 ± 48.6, 137.9 ± 40.2 and 110.8 ± 32.9 µmol/l,
respectively. These results showed a favorable trend to allograft
function recovery over time (Fig.
1).
Patient and allograft survival
By the end of the follow-up, 1 patient succumbed due
to Guillian-Barre syndrome at 3 months post-transplantation; at the
time of death, the transplant was functioning with a plasma Cr of
127 µmol. In addition, 1 patient experienced graft loss from severe
pulmonary infection; and one graft was lost from severe bleeding of
the renal vein due to Mycosis pilorum infection, as
mentioned above. Overall, 1-year patient and graft survival rates
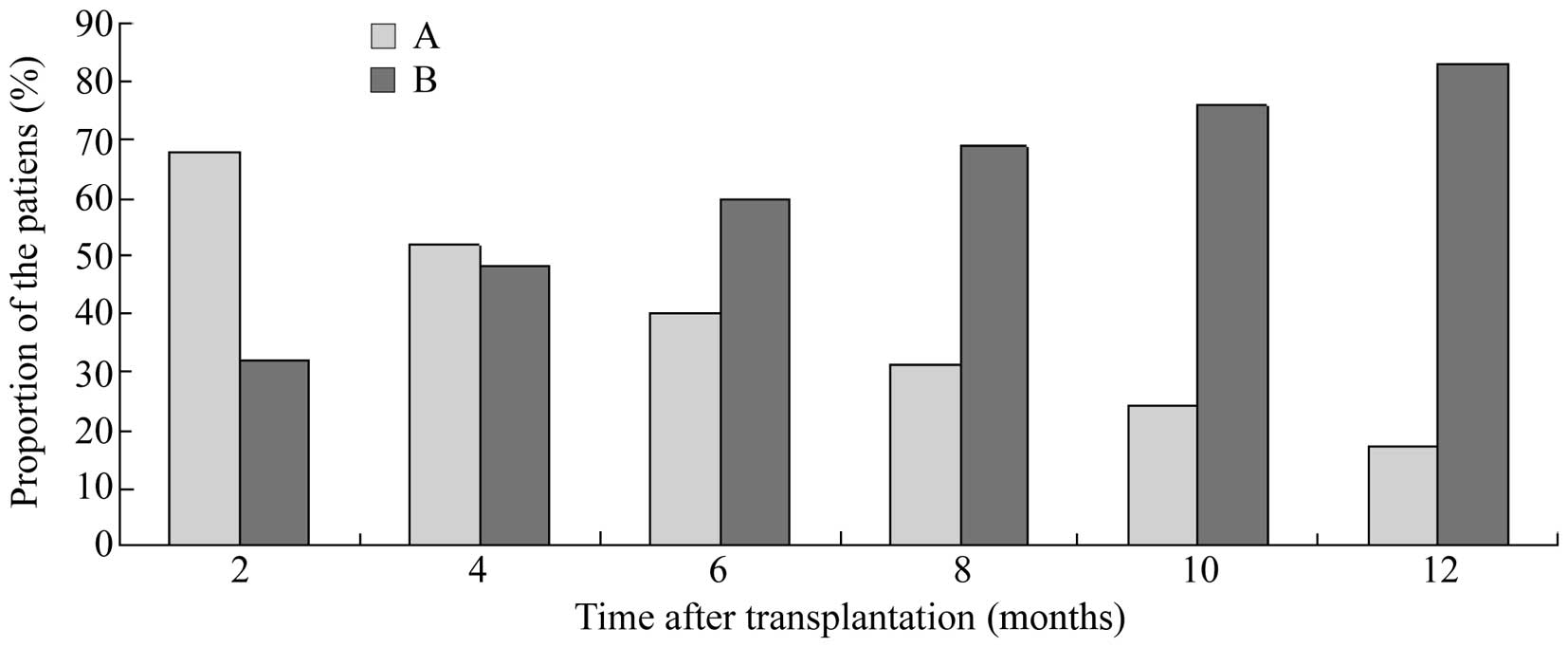
were 96.9 and 90.9%, respectively. Furthermore, when reduction of
sCr to <177.0 µmol/l was regarded as normalization of the renal
allograft, the results demonstrated that the proportion of patients
with normalization of the renal allograft gradually increased as
the time elapsed, as shown in Fig.
2.
Safety analyses
During the follow-up period, no patient developed a
malignancy. Infections occurred in 8 patients with an overall
incidence of 24.2%, including 6 cases of pulmonary infection, 1
case of urinary tract infection and 1 case of Mycosis
pilorum infection in the renal vein. No CMV infection was
observed. In addition, a total of 12 patients presented with other
adverse events, including increasing alanine aminotransferase
(ALT)/aspartate transaminase (AST) levels (>50 U/l) in 8
patients, hypertension (>140/90 mmHg) in 6 patients and
leukopenia (defined as WBC counts <3,500/µl) in 2 patients.
However, following careful examination and further evaluation,
increasing ALT/AST was considered to be associated with basiliximab
in only 2 patients, and they were recovered following appropriate
symptomatic treatment.
Discussion
Renal transplantation is the preferred treatment for
the majority of patients with ESRD because transplantation markedly
improves the length and quality of life for dialysis patients
(7). At the end of 2013, according
to data from the Chinese Renal Data Registration System, the number
of patients in mainland China receiving dialysis was 326,000
(280,000 hemodialysis and 46,000 peritoneal dialysis patients),
accounting for ~20% of the global ESRD population; however, the
donor supply for kidney transplantation in China is rather limited
(8). The discrepancy between renal
transplant supply and demand in China is expected to grow as the
population ages, as the prevalence of hypertension, diabetes
increases, and with the increasing number of patients whose
previous allograft loses function. Allografts from DCD donors are
increasingly being used (9). It has
been reported that long-term results equivalent to those with
deceased donors can be achieved using kidneys from DCD donors. To
further improve these results, the use of induction therapy
continues to be investigated in DCD kidney transplantation
(10).
Induction therapy in kidney transplantation is
considered to be important for reducing acute rejections, despite
the potential for adverse effects. Induction therapies include
polyclonal anti-lymphocyte antibodies, monoclonal anti-lymphocyte
antibodies and anti-IL-2 receptor monoclonal antibody (7). The chimeric IL-2 receptor antibody
basiliximab has been widely used as an effective, safe and
economical induction therapy for kidney transplantation.
Basiliximab is directed against the CD25 subunit, which prevents
acute cellular rejection (ACR) by inhibiting IL-2-driven T-cell
proliferation (11). Basiliximab is
one of the most commonly used induction agents due to the following
advantages: i) Evidence of reduced overall cost demonstrated by
pharmacoeconomic analysis; ii) ease of administration; iii)
short-term use; iv) no need for blood level monitoring; and v) lack
of major toxicity.
Studies have shown that induction therapy with
basiliximab leads to greater graft and patient survival rates
(12,13). In a large prospective
placebo-controlled trial of basiliximab in 700 adult renal
transplant patients in Europe, the result showed a significant
reduction in rejection in the first year after transplantation,
with a 32% reduction in biopsy-proven acute rejection in the first
6 months (from 44% with placebo to 30% with basiliximab) and a 57%
reduction in steroid-resistant rejection with the use of
basiliximab (1).
Similarly, a large meta-analysis reported a rate of
acute rejection of 40% in patients receiving placebo or no
induction, with a reduction in the risk of acute rejection of 34%
(relative risk, 0.66) using basiliximab (14). Thus, for every 100 patients treated
with IL-2 receptor antagonist, it was expected that 14 fewer
patients would experience acute rejection, and 7 patients would
need to be treated to prevent one rejection episode [number needed
to treat (NNT) acute rejection = 7]. An updated analysis found a
38% incidence of rejection in the placebo/no induction arm, and
suggested an NNT of 9 patients to prevent one episode of acute
rejection (15).
In the present study, 33 renal transplantations from
DCD donors using a single-dose basiliximab induction strategy were
evaluated. The 1-year graft survival rate was 90.9%, similar to
that observed by Ledinh et al (16). An intraoperative 20-mg single-dose of
basiliximab was used in the present study, which is lower than that
used by Matl et al (4), who
demonstrated that the efficacy, safety and tolerability of a 40-mg
single-dose basiliximab regimen were comparable to those of the
conventional 2 × 20 mg two-dose regimen. Since Chinese people may
be considered to have a smaller body size than westerners, this 20
mg single-dose regimen of basiliximab in DCD kidney transplantation
in Chinese patients is valid.
Infection is an important complication after
transplantation that could affect the life quality and survival of
recipients. Induction therapy in renal transplantation is prone to
increase the risk for infection. In the present study, 8 patients
developed infection, including 6 cases of pulmonary infection, 1
case of urinary tract infection and 1 case of Mycosis
pilorum infection in the renal vein. This infection rate
described in these DCD recipients is not specific to these
particular patients but is typical of patients in the
transplantation setting in China. Of note, for the recipient
diagnosed with Mycosis pilorum infection in the renal vein,
the donor was drowned in a muddy pool; and after further
investigation, it was found that the recipient receiving the other
kidney underwent graft nephrectomy for rupture and severe bleeding
of the allograft in the third postoperative week (in another
hospital). One patient succumbed to Guillain-Barré syndrome (GBS)
at 3 months post-transplantation. This patient's HLA loci matched
well with the donor who also died of GBS; they had only one HLA
mismatch. The 36-year-old patient had excellent renal function
after transplantation; but 10 weeks later, he was readmitted due to
progressive, symmetrical weakness of the lower limbs, paresthesia
and dysphagia, and in the next few days he developed acute
respiratory failure requiring mechanical ventilation. At the time
of his death, his transplant was functioning well. Our evaluation
of infectious complications did not show a significant difference
in incidence or its role as a cause of mortality/graft loss. These
data are consistent with the meta-analysis of Adu et al
(17).
During the study period, 10 patients experienced
DGF, a common complication in the immediate post-transplant period,
with an incidence of 30.3%. The incidence of DGF after
transplantation from DCD donors has been described as 25 to 90%
(18). Several studies have
documented that one of the potential problems with kidneys from DCD
donors is the potential for a higher rejection rate and a higher
risk for graft failure due to the higher incidence of DGF (19,20). In
addition, DGF leads to extended hospital stays, resulting in
increased costs (19). The commonly
accepted explanation for the increased risk of DGF in DCD kidney
transplantation is warm ischemia, which results in reperfusion
injury, acute tubular necrosis and graft dysfunction. The variable
period of warm ischemia that occurs prior to organ procurement is a
major disadvantage of DCD organ donation. While the interval from
asystole to cross-clamp and organ perfusion is readily determined,
warm ischemia occurring during the withdrawal phase prior to
asystole is much more difficult to quantify (21). In the present study, the duration of
warm ischemia was taken as being from the time of persistent
systolic blood pressure <60 mmHg to the time of organ
reperfusion with cold preservation solution. The outcome would be
improved by any intervention that could preserve the function of
the graft and reduce the incidence of DGF; improved methods for
organ recovery and reduction of drug toxicity may, therefore,
further improve the outcome of DCD kidney transplantation.
Basiliximab has been reported to be associated with reduced DGF
incidence, mainly when calcineurin inhibitor administration has
been reduced or delayed to avoid nephrotoxicity to ischemic kidneys
recovering from cold storage (22).
It has been reported that basiliximab does not cause
lymphocyte depletion, with an incidence of cytopenia lower than
that with depleting agents such as ATG or alemtuzumab, and partial
humanization structurally decreases the development of inhibitory
antibodies (23). It is administered
peripherally and is less expensive than ATG in China. The incidence
of infusion reactions is also lower with basiliximab than with
depleting agents (24). In the
present study cohort, increasing ALT/AST was considered to be
associated with basiliximab in only 2 patients, and they both
recovered satisfactorily. This clinical study demonstrated that the
application of single-dose of basiliximab as an induction therapy
in DCD kidney transplantation is very well tolerated.
The shortcomings of the present study include it
being a single-center evaluation, with a relatively small
population and short duration of study, as well there being as lack
of comparison between single-dose and conventional two-dose
basiliximab regimens in DCD kidney transplantation. Furthermore, in
future studies, pre-operative evaluation of the match of the donor
and recipient requires further improvement, including construction
of better inclusion criteria and exclusion criteria, in order to
reduce the number of mismatches between the donor and
recipient.
The present study of single-dose of basiliximab as
an induction therapy in DCD kidney transplantation showed that
favorable clinical outcomes within 1 year were achieved in terms of
graft survival and function. The single-dose regimen in a larger
population as well as in comparison with a conventional two-dose
regimen in DCD kidney transplant recipients requires further
investigation.
Acknowledgements
The authors would like to thank Mr. Jianfeng Zhu
(Department of Clinical Laboratory, Yinzhou No. 2 Hospital, Ningbo,
China) for his excellent laboratory management.
References
|
1
|
Clark G, Walsh G, Deshpande P and Koffman
G: Improved efficacy of basiliximab over antilymphocyte globulin
induction therapy in paediatric renal transplantation. Nephrol Dial
Transplant. 17:1304–1309. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrer F, Machado S, Alves R, Macário F,
Bastos C, Roseiro A and Mota A: Induction with basiliximab in renal
transplantation. Transplant Proc. 42:467–470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jorge S, Guerra J, Silva S, Santana A,
Mil-Homens C and Prata MM: Induction immunosuppressive therapy in
renal transplantation: Does basiliximab make the difference?
Transplant Proc. 40:693–696. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matl I, Bachleda P, Michalsky R, Navratil
P, Lao M, Treska V, Prestele H, Matthisson M and Korn A:
Basiliximab can be administered safely and effectively in a single
dose on day 1 postrenal transplantation in patients receiving
triple therapy with azathioprine. Transplant Proc. 33:3205–3206.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gravel MT, Arenas JD, Chenault R II, Magee
JC, Rudich S, Maraschio M, DebRoy M, Miller W and Punch JD: Kidney
transplantation from organ donors following cardiopulmonary death
using extracorporeal membrane oxygenation support. Ann Transplant.
9:57–58. 2004.PubMed/NCBI
|
|
6
|
Solez K and Racusen LC: The Banff
classification revisited. Kidney Int. 83:201–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ocampo C, Aristizabal A, Nieto J, Abadia
H, Angel W, Guzman C, Mena A, Vanegas J, Velez C, Aguirre C, et al:
Induction therapies in kidney transplantation: The experience of
hospital Pablo Tobon Uribe, Medellin, Colombia 2005–2010.
Transplant Proc. 43:3359–3363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu X and Yang X: Peritoneal dialysis in
China: Meeting the challenge of chronic kidney failure. Am J Kidney
Dis. 65:147–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wind J, van Mook WN, Willems ME and van
Heurn LW: Higher organ donation consent rates by relatives of
potential uncontrolled donors versus potential controlled donors
after death. Nephrol Dial Transplant. 27:4219–4223. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vandivort CS, Taber D, McGillicuddy JW,
Bratton C, Chavin K and Baliga P: Protocol based induction therapy
improves rejection rates, complications and transplant event costs
in adult kidney transplant recipients. J Surg Res. 186:6552014.
View Article : Google Scholar
|
|
11
|
Naderi GH, Mehraban D, Ganji MR,
Jafarpouriani M and Latif AH: The outcome of induction therapy with
monoclonal antibodies in kidney transplantation among Iranian
patients: A prospective study. Transplant Proc. 41:2768–2771. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gralla J and Wiseman AC: The impact of
IL2ra induction therapy in kidney transplantation using tacrolimus-
and mycophenolate-based immunosuppression. Transplantation.
90:639–644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heldal K, Thorarinsdottir S, Hartmann A,
Leivestad T, Reisæter AV, Foss AE and Midtvedt K: Induction with
interleukin-2 antagonist for transplantation of kidneys from older
deceased donors: an observational study. Transplant Res. 2:2–11.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Zhou P, Han M, Xue CB, Hu XP and Li
C: Basiliximab or antithymocyte globulin for induction therapy in
kidney transplantation: A meta-analysis. Transplant Proc.
42:1667–1670. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Webster AC, Playford EG, Higgins G,
Chapman JR and Craig J: Interleukin 2 receptor antagonists for
kidney transplant recipients. Cochrane Database Syst Rev: CD003897.
2004. View Article : Google Scholar
|
|
16
|
Ledinh H, Bonvoisin C, Weekers L, de
Roover A, Honoré P, Squifflet JP, Meurisse M and Detry O: Results
of kidney transplantation from donors after cardiac death.
Transplant Proc. 42:2407–2414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adu D, Cockwell P, Ives NJ, Shaw J and
Wheatley K: Interleukin-2 receptor monoclonal antibodies in renal
transplantation: Meta-analysis of randomized trials. BMJ.
326:7892003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Quiroga I, McShane P, Koo DD, Gray D,
Friend PJ, Fuggle S and Darby C: Major effects of delayed graft
function and cold ischaemia time on renal allograft survival.
Nephrol Dial Transplant. 21:1689–1696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Farney AC, Hines MH, al-Geizawi S, Rogers
J and Stratta RJ: Lessons learned from a single center's experience
with 134 donation after cardiac death donor kidney transplants. J
Am Coll Surg. 212:440–451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Snoeijs MGJ, van Ernest Heurn LW, van Mook
WNKA, Christiaans MH and van Hooff JP: Controlled donation after
cardiac death: A European perspective. Transplantation Reviews.
21:219–229. 2007. View Article : Google Scholar
|
|
21
|
Abt PL, Fisher CA and Singhal AK: Donation
after cardiac death in the US: History and use. J Am Coll Surg.
203:208–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wagner SJ and Brennan DC: Induction
therapy in renal transplant recipients: How convincing is the
current evidence? Drugs. 72:671–683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Favi E, Gargiulo A, Spagnoletti G, Salerno
MP, Silvestrini N, Valente I and Citterio F: Induction with
basiliximab plus thymoglobulin is effective and safe in old-for-old
renal transplantation: Six-month results of a prospective clinical
study. Transplant Proc. 42:1114–1117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haririan A, Morawski K, Sillix DH, El-Amm
JM, Garnick J, West MS, Granger DK, Migdal SD and Gruber SA:
Induction therapy with basiliximab versus thymoglobulin in
African-American kidney transplant recipients. Transplantation.
79:716–721. 2005. View Article : Google Scholar : PubMed/NCBI
|