Introduction
Infection is a common complication that occurs in
clinical trials (1). Since the
majority of patients of the Department of Orthopedics have open
wounds, there is a high risk of infection for these patients.
Infections may include complicated osteomyelitis, and can be
serious and even life threatening (2). In recent years, due to overuse and
misuse of antibiotics, certain pathogen bacteria have become
resistant to certain drugs. The development of antibiotic-resistant
bacteria and their distribution worldwide is the result of many
years of constant selection from human overuse and misuse of
antibiotics (2). Antibiotic
resistance, particularly the problems associated with
superbacteria, has become a major public health issue and a global
impediment for medical workers and investigators in the 21st
century.
Since patients in the Department of Orthopedics are
easily infected, antibacterial agents are key elements for the
treatment of open wounds of patients (3–5). To
analyze the distribution of drug-resistant pathogenic bacteria in
infected patients and to provide a guide to ameliorate the
appropriate use of antibiotics, we analyzed the distribution of
drug-resistant bacteria in 126 patients at the Department of
Orthopedics. Wound secretion samples were collected, the pathogen
bacteria isolated and cultured, and consequently drug-resistance
analyses were performed.
Patients and methods
General information
Between October 2013 and January 2015, 126 patients
were selected at the Department of Orthopedics, The Fourth
Affiliated Hospital of Harbin Medical University (Heilongjiang,
China). There were 72 male and 48 female patients with an age range
of 16–72 years and an average age of 39.4±10.2 years. Of the 126
patients, 32 cases had infectious arthritis, 48 cases had lower
limb open wound infection, 18 cases had soft issue abscess, 21
cases had postoperative infection and 7 cases had other types of
infection.
Identification of bacteria and drug
sensitivity test
Samples from the wound secretions and pus of
patients were taken in a sterile manner and immediately sent for
isolation and purification. The isolation and purification
protocols were based on The National Clinical Test Regulation of
Operation (6). For the
identification of pathogen bacteria an automated microorganism
identification instrument was used. The Kirby-Bauer antibiotic
testing agar diffusion method was used to evaluate bacterial
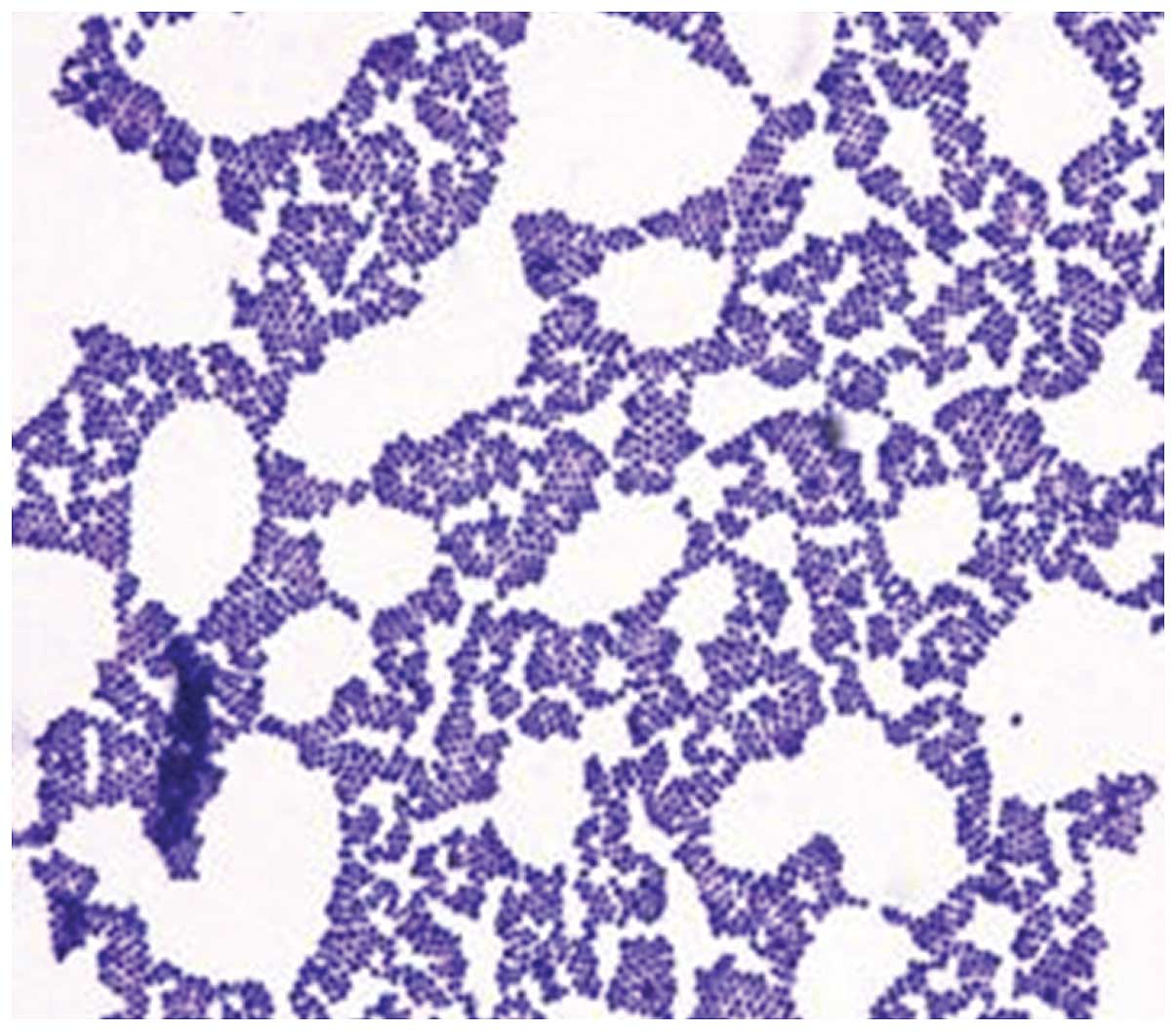
resistance. In this process, Staphylococcus aureus ATCC25923
(Fig. 1), Pseudomonas
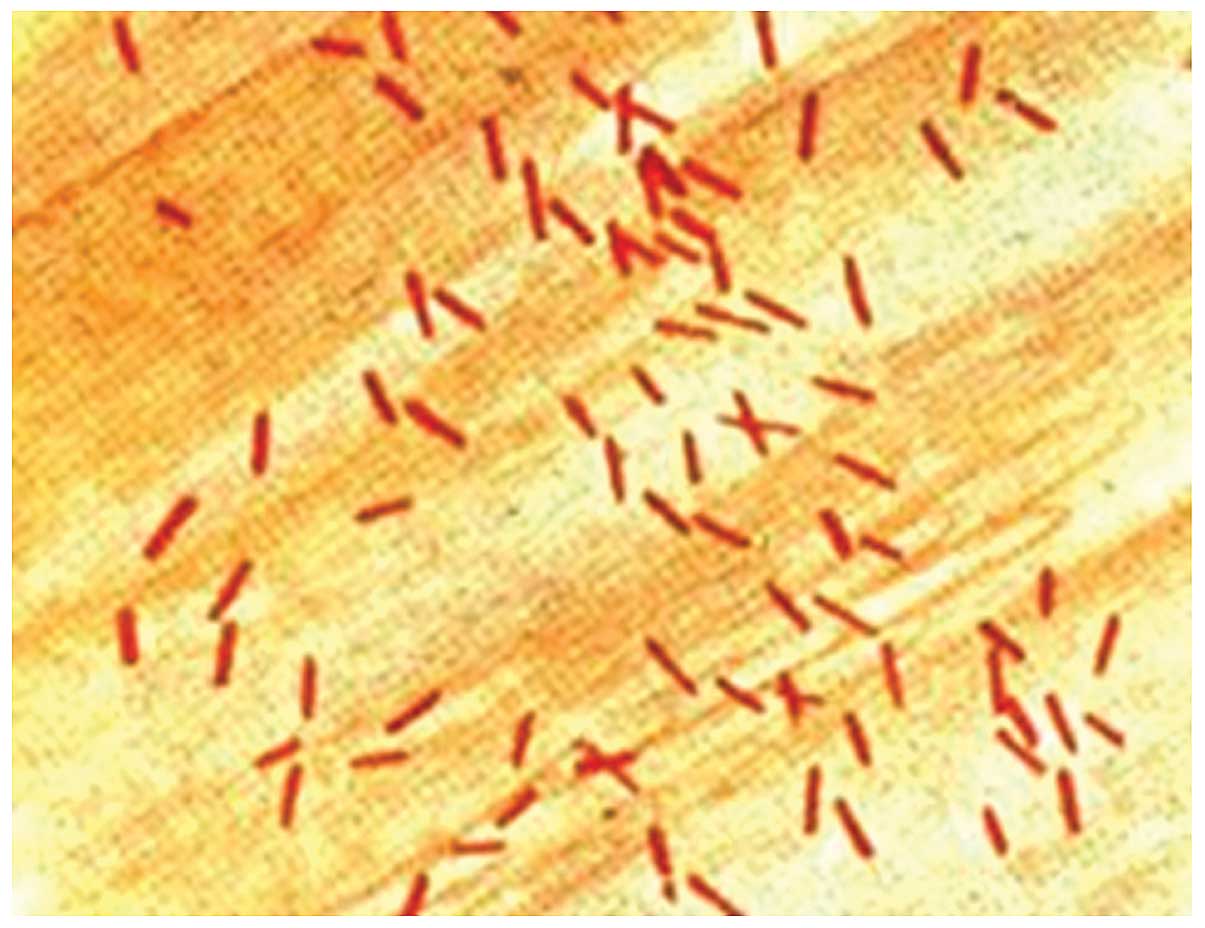
aeruginosa ATCC27853, Escherichia coli ATCC25922
(Fig. 2) and Klebsiella
pneumonia ATCC700603 were used as quality control bacteria.
Statistical analysis
Data were analyzed using SPSS 21.0 software (IBM
SPSS, Armonk, NY, USA). A t-test was used to make comparisons
between different groups. The size of the test was α=0.05.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Infection rate
Of the samples isolated from 126 patients, 118
samples (infection rate, 93.65%) had bacterial infections.
Pathogen distribution
Isolated pathogens included a variety of
gram-positive and -negative bacteria, with gram-negative bacteria
constituting the most abundant microorganisms. In total, 47
gram-positive (39.83%) and 71 gram-negative (60.17%) bacterial
strains were identified. In the gram-positive group, Staphylococcus
aureus, Staphylococcus epidermidis, Enterococcus sp.
and Staphylococcus pyogenes were identified. In the
gram-negative group Escherichia coli, Pseudomonas
aeruginosa, Klebsiella pneumonia, and Enterobacter
cloacae were identified. The most common bacteria were
Pseudomonas aeruginosa, Staphylococcus aureus,
Escherichia coli, Klebsiella pneumonia and
Staphylococcus epidermidis (Table
I).
 | Table I.Isolated pathogens and their
distribution. |
Table I.
Isolated pathogens and their
distribution.
| Pathogen
classification | No. of isolated
strains, strain | Composition ratio,
% |
|---|
| Gram-positive
bacteria | 47 | 39.83 |
|
Staphylococcus
aureus | 16 | 13.56 |
|
Staphylococcus
epidermidis | 12 | 10.16 |
|
Enterococcus
faecalis | 10 | 8.47 |
|
Staphylococcus
purulent |
6 | 5.08 |
|
Staphylococcus
haemolyticus |
4 | 3.39 |
| Gram-negative
bacteria | 71 | 60.17 |
|
Pseudomonas
aeruginosa | 20 | 16.95 |
|
Escherichia
coli | 15 | 12.71 |
| Klebsiella
pneumonia | 12 | 10.17 |
|
Acinetobacter
baumannii | 10 | 8.47 |
|
Bacillus
aerogenes |
5 | 4.24 |
|
Enterobacter
cloacae |
4 | 3.39 |
|
Others |
5 | 4.24 |
| Total | 118 | 100.0 |
Drug-resistant bacteria
The results of the drug resistance tests showed that
several strains were resistant to multiple antibacterial agents.
Pathogens such as Acinetobacter baumannii were resistant to
the majority of antibacterial agents used in those tests.
Gram-positive bacteria were sensitive to vancomycin (7), while almost all of the bacteria were
entirely resistant to commonly used antibiotics, such as penicillin
and erythromycin. Gram-negative bacteria were sensitive to
meropenem and imipenem, while almost completely resistant to
sulbactam and ampicillin. We selected typical gram-positive and
-negative bacteria (Staphylococcus aureus, Staphylococcus
epidermidis, Enterococcus faecalis, Pseudomonas
aeruginosa, Escherichia coli, Klebsiella
pneumonia and Acinetobacter baumannii) to test their
resistance to antibacterial agents (Tables II and III).
 | Table II.Drug-resistance test results for
gram-positive bacteria [n (%)]. |
Table II.
Drug-resistance test results for
gram-positive bacteria [n (%)].
| Antibacterial
agents | Staphylococcus
aureus 16 | Staphylococcus
epidermidis 12 | Enterococcus
faecalis 10 |
|---|
| Penicillin, n
(%) | 14 (87.5) | 9 (75) | 8 (80) |
| Erythromycin, n
(%) | 15 (93.75) | 10 (83.33) | 9 (90) |
| Gentamicin, n
(%) | 4 (25) | 8 (66.67) | 7 (70) |
| Rifampicin, n
(%) | 6 (37.5) | 7 (58.33) | 4 (40) |
| Levofloxacin, n
(%) | 9 (56.25) | 7 (58.33) | 5 (50) |
| Ciprofloxacin, n
(%) | 6 (37.5) | 7 (58.33) | 3 (30) |
| Ampicillin/sulbactam,
n (%) | 10 (62.5) | 8 (66.67) | 4 (40) |
| Amoxicillin, n
(%) | 3 (18.75) | 8 (66.67) | 4 (40) |
| Cefazolin, n (%) | 4 (25) | 9 (75) | 5 (50) |
| Ceftriaxone, n
(%) | 3 (18.75) | 8 (66.67) | 4 (40) |
| Vancomycin, n
(%) | 0 | 0 | 0 |
 | Table III.Drug-resistance test results for
gram-negative bacteria [n (%)]. |
Table III.
Drug-resistance test results for
gram-negative bacteria [n (%)].
| Antibacterial
agents | Pseudomonas
aeruginosa | Escherichia
coli | klebsiella
pneumonia | Acinetobacter
baumannii |
|---|
| Sulbactam, n (%) | 18 (90) | 9 (60) | 8(66.67) | 8 (80) |
| Ampicillin, n
(%) | 19 (95) | 10 (66.67) | 9 (75) | 9 (90) |
| Gentamicin, n
(%) | 7 (35) | 8 (53.33) | 7 (58.33) | 4 (40) |
| Ceftazidime, n
(%) | 9 (45) | 7 (46.67) | 4 (33.33) | 4 (40) |
| Cefepime, n (%) | 12 (60) | 7 (46.67) | 5 (41.67) | 2 (20) |
| Ceftriaxone, n
(%) | 9 (45) | 7 (46.67) | 3 (25) | 3 (30) |
| Cefazolin, n (%) | 13 (65) | 8 (53.33) | 4 (33.33) | 2 (20) |
| Ciprofloxacin, n
(%) | 6 (30) | 8 (53.33) | 4 (33.33) | 5 (50) |
| Norfloxacin, n
(%) | 7 (35) | 9 (60) | 5 (41.67) | 4 (40) |
| Levofloxacin, n
(%) | 6 (30) | 8 (53.33) | 4 (33.33) | 4 (40) |
| Meropenem, n
(%) | 1 (5) | 0 | 0 | 0 |
| Imipenem | 0 | 0 | 0 | 0 |
Discussion
The overuse of antibiotics has led to the emergence
of several drug-resistant bacteria that render the combat against
bacterial infections more difficult. The harm caused by these
drug-resistant bacteria has become more severe due to the lack of
appropriate antibacterial agents in our arsenal (8). In order to achieve a better
understanding regarding the drug resistance phenomenon, we need to
expand our knowledge of the proper use of antibacterial agents
(9). We investigated the occurrence
of drug-resistant bacteria in patients at the Department of
Orthopedics and analyzed the distribution of pathogens and
antibacterial agents. We successfully isolated bacterial pathogens
from 118 patients, and identified that those patients were
primarily infected with Pseudomonas aeruginosa,
Escherichia coli and Klebsiella pneumoniae. Of the
gram-positive bacteria, Staphylococcus aureus,
Staphylococcus epidermidis and Enterococcus faecalis
had the highest infection rates. These results were consistent with
the existing literature (10). Drug
sensitivity test results revealed that most gram-negative bacteria
were not sensitive to sulbactam and ampicillin, but were sensitive
to meropenem and imipenem. Gram-negative bacteria were resistant to
penicillin and erythromycin, but sensitive to vancomycin (11). Drug resistance continuously increased
(12–14), thus, the work of clinical workers
became more cumbersome.
Results obtained from previous studies (15–18)
revealed that: i) Most patients had open wounds that were easily
infected; ii) external wounds caused immune disorders, thereby
causing alterations in intestinal flora and leading to internal
infections; iii) errors during clinical treatment, non-sterile
manipulation and the failure to treat the wounds in a timely manner
potentially increased the risk of infection; and iv) failure to
sterilize the wound also increased the risk of infection.
Since their identification, antibiotics have been
crucial agents in the battle against infectious diseases caused by
bacteria. Antimicrobial therapy has been an important reason for
the significant increase of average life expectancy in the 20th
century (19). The increase of
drug-resistant bacteria poses a serious threat to public health and
the economy. The emergence of superbacteria has indicated that the
available pool of antibacterial agents are to be appropriately used
and that the appropriate antibacterial agent to which the pathogens
are sensitive should be utilized (19). We should only consider the use of
newer and more potent antibacterial agents in severe infections
caused by multi-resistant bacteria.
The most effective manner in which to prevent
infection is thorough correct debridement prior to surgery and
timely sterilization thereafter (20). The use of antibiotics should be
guided by patients' etiological tests (21).
In conclusion, we identified that the existing risk
factors in the Department of Orthopedics were complex and any
non-standard procedures may cause bacterial infection.
Additionally, there were evident dissimilarities among infectious
bacteria with regard to their sensitivity to various antibacterial
agents. Manipulation techniques during the treatment process are to
be performed in a sterile manner and the use of antibacterial
agents should be strictly in accordance with the results of drug
sensitivity tests to provide effective etiologic information and a
treatment plan for clinical trials and to reduce the risk of
infection by multi-resistant bacteria.
References
|
1
|
Dong L, Zhang XY, Liu ZY, Jiang FL, Zhang
J and Wu YP: Analysis of Distribution and Drug Resistance of
Pathogens in the Infection Inpatients from Orthopedic Department of
Our Hospital during 2011–2013. China Pharm. 2:195–197. 2015.
|
|
2
|
Ramos-Luces O, Molina-Guillén N,
Pillkahn-Díaz W, Moreno-Rodríguez J, Vieira-Rodríguez A and
Gómez-León J: Surgical wound infection in general surgery. Cir Cir.
79:323–329. 2011.PubMed/NCBI
|
|
3
|
Wu XY, Ni KX and Li SB: Clinical
characteristics and resistance of wound surface infections causing
by Bacillus cereus in the department of orthopaedics. Zhongguo Gu
Shang. 26:753–756. 2013.(In Chinese). PubMed/NCBI
|
|
4
|
Bouza E, Burillo A, Munoz P, Cercenado E
and Rodríguez-Créixems M: Semiquantitative culture of open surgical
wounds for diagnosis of surgical site infection. Eur J Clin
Microbiol Infect Dis. 23:119–122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wysocki AB: Evaluating and managing open
skin wounds: Colonization versus infection. AACN Clin Issues.
13:382–397. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao X: Pathogen analysis and clinical
prevention and treatment of inhospital postoperative infection of
aging patients in department of orthopedics. Practical Clinical
Medicine. 19:145–147. 2015.(In Chinese).
|
|
7
|
Bagga B and Shenep JL: Management of
infections caused by vancomycin-resistant gram-positive bacteria.
Pediatr Infect Dis J. 29:662–664. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Neill AJ: New antibacterial agents for
treating infections caused by multi-drug resistant Gram-negative
bacteria. Expert Opin Investig Drugs. 17:297–302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ii K, Ichikawa S, Al-Dabbagh B, Bouhss A
and Matsuda A: Function-oriented synthesis of simplified
caprazamycins: Discovery of oxazolidine-containing uridine
derivatives as antibacterial agents against drug-resistant
bacteria. J Med Chem. 53:3793–3813. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li S and Ye G: Infection distribution of
Aureus and analysis on its drug resistance. J Chin Hosp Infect.
20:3045–3046. 2010.
|
|
11
|
Qiubao A and Aimin H: Clinical
distribution and drug resistance analysis of pseudomonas aeruginosa
in our hospital. Med Infant. 13:236–237. 2013.
|
|
12
|
Maltezou HC, Giakkoupi P, Maragos A,
Bolikas M, Raftopoulos V, Papahatzaki H, Vrouhos G, Liakou V and
Vatopoulos AC: Outbreak of infections due to KPC-2-producing
Klebsiella pneumoniae in a hospital in Crete (Greece). J
Infect. 58:213–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gregory CJ, Llata E, Stine N, Gould C,
Santiago LM, Vazquez GJ, Robledo IE, Srinivasan A, Goering RV and
Tomashek KM: Outbreak of carbapenem-resistant Klebsiella
pneumoniae in Puerto Rico associated with a novel carbapenemase
variant. Infect Control Hosp Epidemiol. 31:476–484. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meunier O: From the discovery of
antibiotics to emerging highly drug-resistant bacteria. Soins.
797:14–20. 2015.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Selasi GN, Nicholas A, Jeon H, Lee YC, Yoo
JR, Heo ST and Lee JC: Genetic basis of antimicrobial resistance
and clonal dynamics of carbapenem-resistant Acinetobacter
baumannii sequence type 191 in a Korean hospital. Infect Genet
Evol. 36:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kirkland KB, Briggs JP, Trivette SL,
Wilkinson WE and Sexton DJ: The impact of surgical-site infections
in the 1990s: Attributable mortality, excess length of
hospitalization, and extra costs. Infect Control Hosp Epidemiol.
20:725–730. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Olsen MA, Nepple JJ, Riew KD, Lenke LG,
Bridwell KH, Mayfield J and Fraser VJ: Risk factors for surgical
site infection following orthopaedic spinal operations. J Bone
Joint Surg Am. 90:62–69. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cole MR, Hobden JA and Warner IM:
Recycling antibiotics into GUMBOS: A new combination strategy to
combat multi-drug-resistant bacteria. Molecules. 20:6466–6487.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
ter Pull Gunne AF, Hosman AJ, Cohen DB,
Schuetz M, Habil D, van Laarhoven CJ and van Middendorp JJ: A
methodological systematic review on surgical site infections
following spinal surgery: part 1: risk factors. Spine.
37:2017–2033. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Olsen MA, Butler AM, Willers DM, Devkota
P, Gross GA and Fraser VJ: Risk factors for surgical site infection
after low transverse cesarean section. Infect Control Hosp
Epidemiol. 29:477–484; discussion 485–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferronato AE, Gilio AE, Ferraro AA, Paulis
M and Vieira SE: Etiological diagnosis reduces the use of
antibiotics in infants with bronchiolitis. Clinics (Sao Paulo).
67:1001–1006. 2012. View Article : Google Scholar : PubMed/NCBI
|