Introduction
Polyethylene terephthalate (PET) artificial
ligaments have been used for a number of years as biomedical
implants in humans, including in the ligament advanced
reinforcement system (LARS®; Surgical Implants and
Devices, Arc-sur-Tille, France) (1,2). With
non-degradable features, PET ligaments remain highly mechanically
stable for a long time. However, PET artificial ligaments also have
a hydrophobic character and chemical inertness, which limit the
healing with the surrounding bone following implantation in the
host bone (3,4). Instead of a normal tendon-bone
fibrocartilaginous insertion, a weak fibrous scar tissue band
occurs between the pure PET ligament graft and the host bone
(5). In a multicenter follow-up
study, Gao et al (6) observed
fibrous tissue between the PET artificial ligament graft and the
bone in the second-look arthroscopy of several failed cases. These
observations indicated that the surface property of the PET graft
played a crucial role in graft-bone healing in vivo.
Therefore, surface modification on the PET ligament is required in
biomedical fields to improve and promote the graft-bone healing of
PET artificial ligaments following implantation.
Currently, surface coating with calcium phosphate
(CaP) or hydroxyapatite (HAp) is widely applied as a surface
modification of metal implants in order to promote the graft-bone
healing (7–9). A CaP coating on grafts is able to
enhance osteoblast differentiation, provide an ideal environment
for osteoblast activity and stimulate bone formation at the
interface between the graft and bone. Bigi et al (10) used a biomimetic approach to obtain a
nanocrystalline HAp coating on metallic substrates and demonstrated
that the HAp coating on a metallic alloy was able to promote bone
ingrowth around grafts.
The attachment of a native anterior cruciate
ligament (ACL) on the bone can be divided into four zones
histologically, which include the ligament, unmineralized
fibrocartilage, mineralized fibrocartilage and bone, and this
composite structure can sustain a very high load without failure
(11). For a pure tendon or
artificial ligament graft, forming this type of mineralized
composite structure at the interface between graft and bone can be
a challenge. Thus, CaP has also been applied to enhance the tendon
healing of ACL grafts in the bone tunnel (12,13). In
an animal study (14),
CaP-hybridized tendons were shown to regenerate a direct
insertion-like formation of tendons, similar to that of a normal
healthy ACL insertion, at three weeks postoperatively. The coating
of CaP on a PET artificial ligament to enhance tendon-to-bone
insertion site formation has subsequently been attracting
increasing interest.
Previously, nanoscale HAp particles have been coated
on a PET artificial ligament to enhance graft-bone healing, and it
was demonstrated that the HAp coating had a positive effect on the
induction of graft healing within the bone tunnel (15). However, an agglomeration phenomenon
of these nano HAp particles on the PET graft was observed, and the
distribution of the coating was not as uniform as desired. In the
current study, apatite deposition via biomimetic mineralization
(BM) was attempted through a layer-by-layer (LBL) self assembling
organic template on the PET ligament. The aim of the present study,
therefore, was to modify the PET ligament with a nanoscale
biomimetic CaP coating and evaluate the effect of the coating in
vitro. The LBL organic template was hypothesized to induce a
nanoscale CaP coating deposition on the graft. Furthermore, this
CaP coating was hypothesized to stimulate the activity of MC3T3-E1
osteoblastic cells in vitro.
Materials and methods
Preparation of PET sheets
PET sheets from a LARS ligament were removed and
immersed in 75% alcohol solution for 4 h to eliminate any
impurities. The sheets were subsequently washed with copious
quantities of deionized water and dried under reduced pressure at
37°C for 24 h.
Preparation of LBL sheets via an LBL
self-assembly process
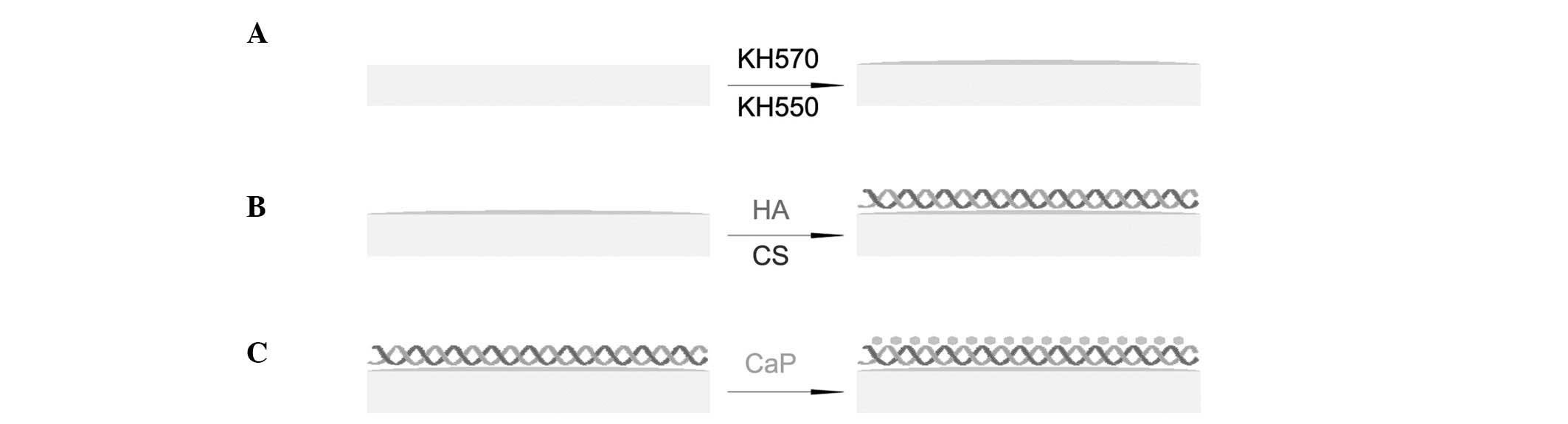
The γ-methacryloxypropyltrimethoxysilane (ACROS
Organics, Geel, Belgium) was grafted on the cleaned PET sheets
using plasma surface modification (CTP-2000 K plasma apparatus;
Nanjing Suman Electronics Co., Ltd. Nanjing, China). The silanized
ligament sheets were immersed in a 0.1 wt% ethanol solution of
3-aminopropyltriethoxysilane (ACROS Organics) for 4 h at 37°C
(Fig. 1A), treated with 1%
hydrochloric acid solution for 2 h at room temperature and
subsequently placed for 1 min into a solution comprising 0.1 wt%
acetic acid and 0.1 wt% chitosan (CS; Sigma-Aldrich, St. Louis, MO,
USA). The sheets were then dipped in 0.1 wt% hyaluronic acid
solution (CPN Spol s.r.o., Dolní Dobrouĉ, Czech Republic) for 1 min
(Fig. 1B). The deposition process
was repeated until 10 bilayers of CS and hyaluronic acid had been
prepared. Finally, the samples were dried at 37°C for 48 h.
BM
CaP solution was prepared by dissolving
CaCl2 (5 mmol), NaH2PO4 (2 mmol)
and NaHCO3 (1.5 mmol) salts into 1 litre demineralized
water. The LBL-modified sheets were soaked in the solution at 37°C
for three days to reproduce bone-like apatite on the ligament
sheets (Fig. 1C). The BM sheets were
subsequently washed gently with distilled water and dried at 30°C
under reduced pressure for 12 h.
Scanning electron microscopy
(SEM)
Surface morphologies of the PET sheets, with or
without modification, were imaged using SEM (VEGA3; Tescan Co.,
Ltd., Brno, Czech Republic). Briefly, small sections of the sheets
were vacuum-coated with gold (JS-1600; Beijing HTCY Technology,
Beijing, China), placed in the vacuum chamber of the SEM and viewed
at a 20-kV accelerating voltage.
Atomic force microscopy (AFM)
Morphologies of the modified-sheet fibers were
studied using AFM in tapping mode (Multimode Nanoscope; Veeco
Process Equipment, Inc., Camarillo, CA, USA) using a silicon tip
(NSC11/AIBS, Ultrasharp µmasch; MikroMasch, Lady's Island, SC, USA)
under ambient conditions. Scans of 1×1 µm areas on the fiber
surface were investigated.
Energy-dispersive X-ray spectroscopy
(EDX)
An EDX system (QUANTA X400; Bruker Corporation,
Ettlingen, Germany) was used to analyze the elements of the surface
modification and measure the ratio of calcium (Ca) and phosphorus
(P) on the surface of the mineralized sheets.
In vitro analysis with MC3T3-E1 mouse
osteoblastic cells
In vitro experiments were performed with an
MC3T3-E1 mouse osteoblastic cell line (Riken Cell Bank, Ibaraki,
Japan). The cells were grown in tissue culture polystyrene flasks
containing Dulbecco's modified Eagle's medium (Hyclone; GE
Healthcare, Logan, UT, USA), supplemented with 15% fetal bovine
serum, 300 mg/ml L-glutamine, 100 IU/ml penicillin and 25 mg/ml
streptomycin solution, at 37°C in a humidified atmosphere
containing 5% CO2. The CaP-coated sheets (BM group) and
the uncoated sheets (control group), with a 1-cm diameter, were
soaked in 70% ethanol for 30 min and left overnight in a
laminar-flow cabinet to dry. The sheets were washed twice with
sterile phosphate-buffered saline (PBS), transferred to a 24-well
untreated plate, and incubated at 37°C in a humid atmosphere with
5% CO2 for 4 h in basic cell culture medium. Following
the removal of the medium, cells at the logarithmic growth phase
were cultured at 5×104 cells/ml in the sheets. The
sheets were incubated at 37°C in a humidified CO2
atmosphere for a period of up to seven days.
Cell proliferation was assessed using a
methylthiazol tetrazolium (MTT) assay (Sigma-Aldrich). After the
cells had been cultured for 1, 3 and 7 days, they were mixed with
200 µl complete medium and 20 µl MTT solution (5 mg/ml in PBS) and
incubated at 37°C to form MTT formazan crystals. After 4 hours, 200
µl dimethyl sulfoxide (DMSO) was added, in order to dissolve the
formazan crystals. The solution was agitated until it became
homogeneous (~15 min on a shaker). The optical density (OD) was
measured at 570 nm against a DMSO solution blank using a microplate
reader (VICTOR X, PerkinElmer, Inc., Waltham, MA, USA). Three
parallel replicates were evaluated for each sample. In addition,
the morphology of the MC3T3-E1 cells cultured on the sheets was
observed by SEM (VEGA3; Tescan Co., Ltd.) at a 5-kV accelerating
voltage. Alkaline phosphatase (ALP) is an enzyme that can be used
to indicate the occurrence of active bone formation, since ALP is a
byproduct of osteoblast activity. Thus, ALP activity was assessed
biochemically on day seven using an ALP testing kit (Houbio Tech
Co., Ltd., Hong Kong, China). A 1,270-µl solution, containing 250
µl priming solution, 20 µl sample solution and 1,000 µl PBS, was
used for measuring the absorbance OD value of the
p-nitrophenol at 405 nm with a microplate reader (UV1000;
Shanghai Tian Mei Scientific Instrument Co., Ltd., Shanghai,
China).
Statistical analysis
Results are presented as the mean ± standard
deviation. A paired Student's t-test was used to determine
statistically significant differences among the groups. Statistical
analyses were conducted using Stata 10.0 software (StatCorp LP,
College Station, TX, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results and Discussion
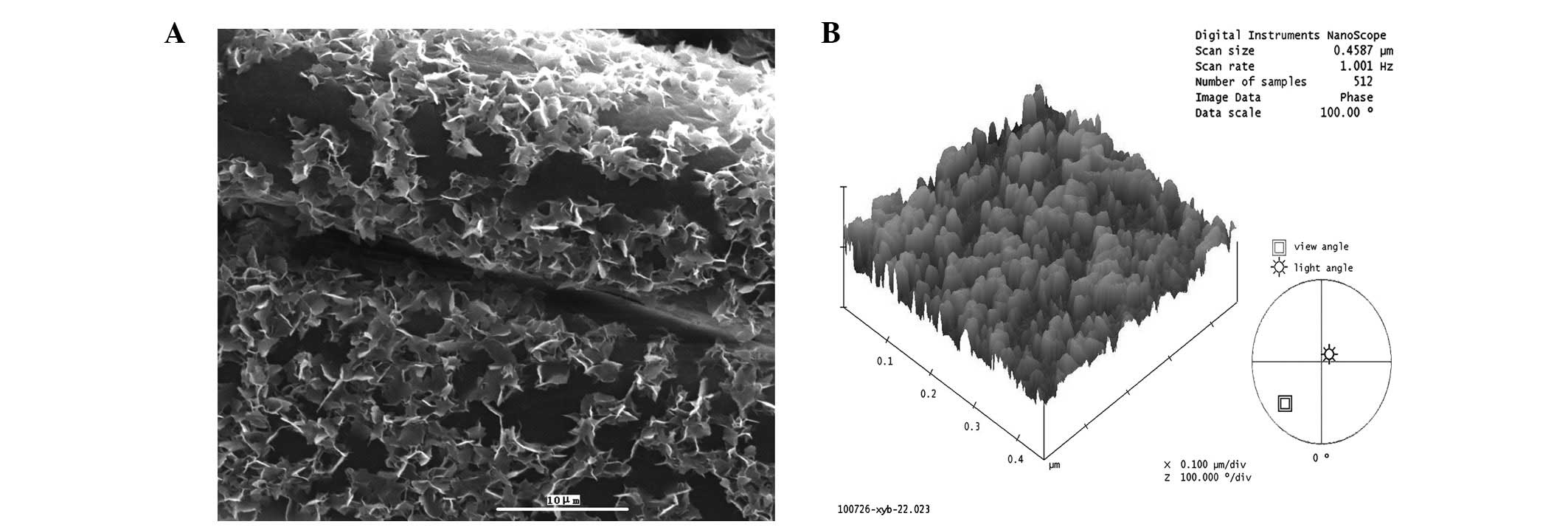
As shown in Fig. 2A,
the PET grafts were initially smooth and uniform and the LBL sheets
appeared smooth and dark. Following immersion in the CaP solution
for three days, numerous CaP particles were deposited on the
surface of the PET ligament fibers. In the present study, a
previously devised LBL procedure was applied to successfully coat
the PET sheets with an organic multilayered template of CS and
hyaluronic acid (16).
Representative three-dimensional renderings of the mineralized
fibers revealed that the fibers of the CaP group exhibited a
non-spiky plateau surface (Fig. 2B).
When CaP was deposited on the fibers, the particle size was ~50 nm
in diameter. These observations indicated that BM through an
organic template was a successful method for producing nanoscale
particles on PET fibers.
With numerous carboxylate (−COO−) and
amino (−NH3+) groups, this organic multilayered template
is able to regulate biomineral formation and overcome the
dispersion problem of CaP particles on PET fibers (17). In addition, the carboxylic groups
interact with the calcium ions of CaP, which enables the
multilayered template to effectively stimulate BM and induce the
deposition of crystalline CaP (18–20).
However, in contrast to other fast methods for biomimetic
deposition of nanocrystalline apatite, the current technique
requires approximately three days to deposit a uniform nanoscale
CaP coating on the PET ligament, which may be due to the chemical
and physical properties of the surface of the PET ligament.
Through elemental analysis using EDX, the quality
ratio of Ca and P was determined to be 1.60. Previously, the Ca/P
ratios of HAp, tricalcium phosphate and octacalcium phosphate have
been reported as 1.67, 1.50 and 1.33, respectively (21); therefore, it is conceivable that the
CaP crystalline deposition observed in the present study was a
mixture of several CaP crystals.
In the present study, mouse MC3T3-E1 preosteoblast
cells were used to investigate the proliferation, adhesion and
expression of bone-related protein, according to a previous report
(22). The cell proliferation
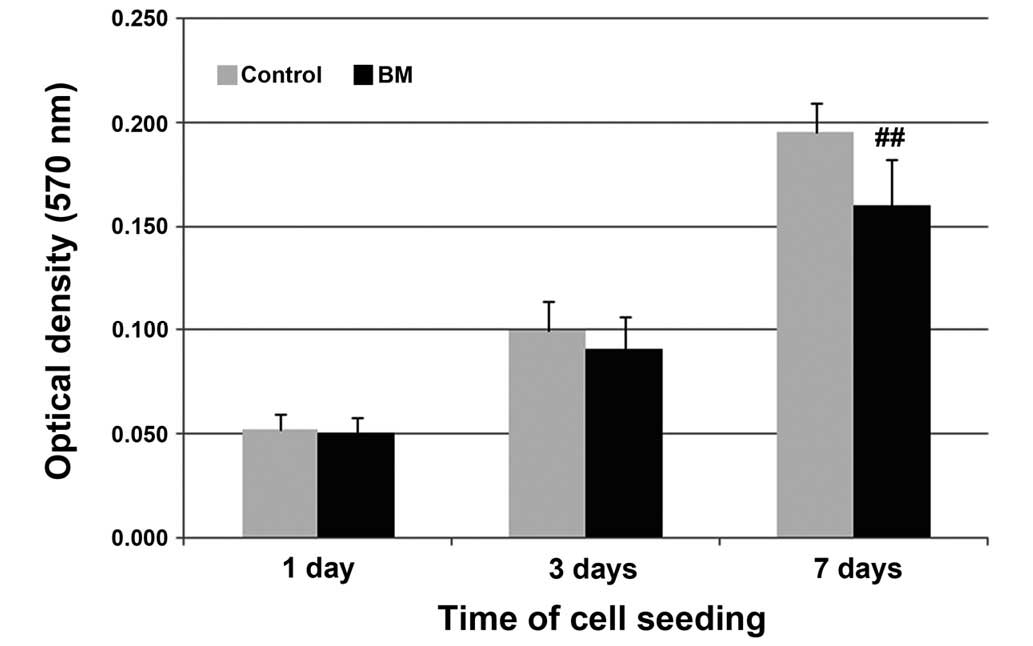
results were presented as OD values measured in an MTT assay
(Fig. 3). The OD value increased
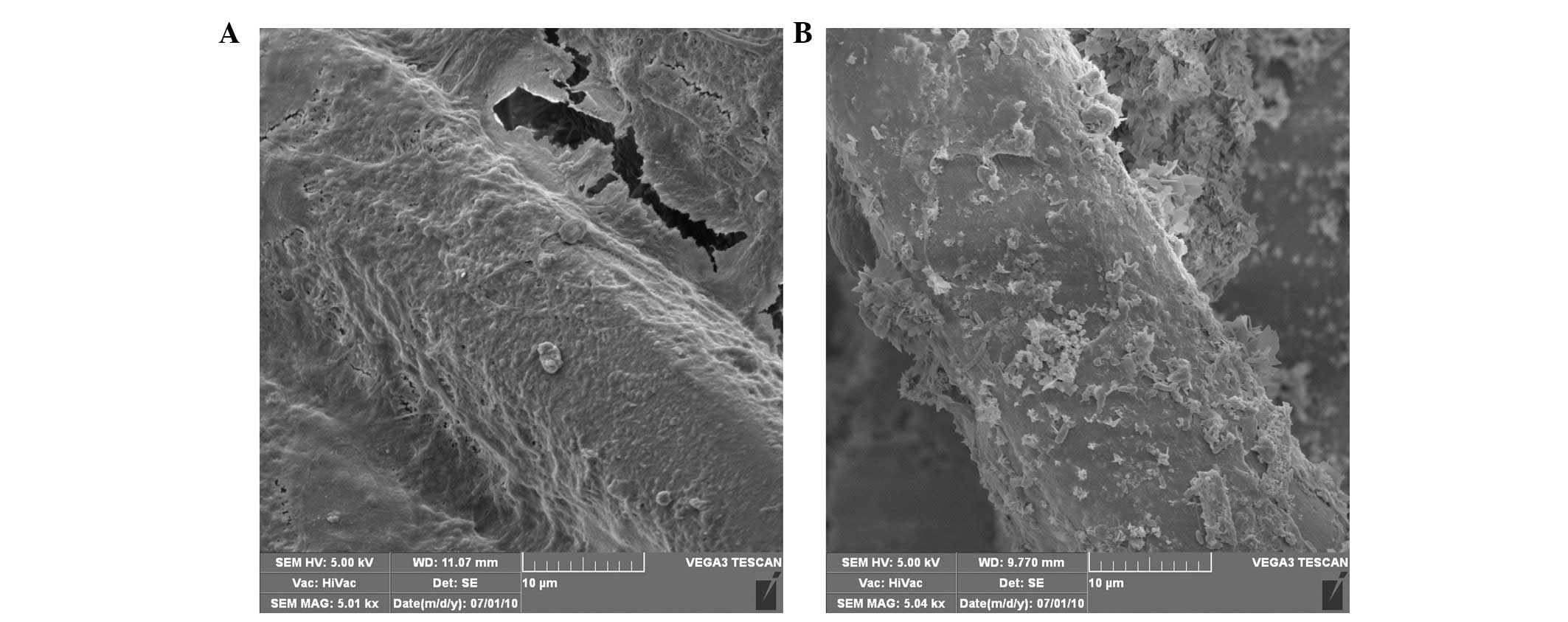
with time in the control and BM groups. In addition, SEM images
revealed that the MC3T3-E1 cells successfully attached and spread
on the PET and BM sheets after seven days of culture (Fig. 4), with a significant amount of
extracellular matrix covering the fibers of the sheets. Previous
studies have demonstrated that the biomimetic CaP layer supports
the proliferation and adhesion of osteoblasts for long culture
periods (23–25). Furthermore, MC3T3-E1 cells have been
demonstrated to preferentially adhere to and/or proliferate in
regions with a high CaP content (26). Notably, the BM group were observed to
have a lower optical density (0.160±0.022) compared with the
control group (0.196±0.014) after seven days of cell culture.
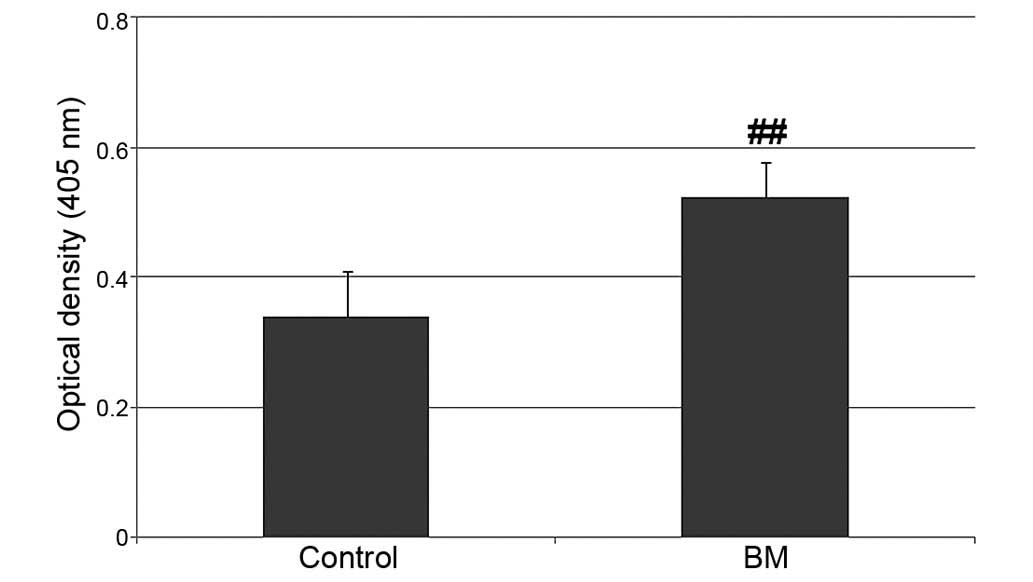
Considering that the BM group had a significantly higher ALP
activity compared with the control group (Fig. 5), the lower proliferation observed
may have been the result of the induction of differentiation
stimulated by the biomimetic CaP coating (27,28).
In conclusion, a biomimetic CaP coating was
successfully prepared on a PET artificial ligament in the present
study. The organic, multiple layers of hyaluronic acid and CS
functioned as an effective and efficient template to induce the
deposition of nanoscale CaP apatites. In addition, the biomimetic
CaP coating had a superior property with regard to the promotion of
osteoblast activity in vitro. However, further studies are
being undertaken to investigate the effects of different amounts of
this biomimetic CaP coating on the PET ligament surface in
vivo.
Acknowledgements
The study was supported by grants from the 973
Project (no. 2009CB930000) of the Ministry of Science and
Technology of China and the Nano Project of Shanghai Municipal
Science and Technology Commission (no. 1052nm03701).
References
|
1
|
Nau T, Lavoie P and Duval N: A new
generation of artificial ligaments in reconstruction of the
anterior cruciate ligament. Two-year follow-up of a randomised
trial. J Bone Joint Surg Br. 84:356–360. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Machotka Z, Scarborough I, Duncan W, Kumar
S and Perraton L: Anterior cruciate ligament repair with LARS
(ligament advanced reinforcement system): a systematic review.
Sports Med Arthrosc Rehabil Ther Technol. 2:292010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kock HJ, Stürmer KM, Letsch R and
Schmit-Neuerburg KP: Interface and biocompatibility of polyethylene
terephthalate knee ligament prostheses. A histological and
ultrastructural device retrieval analysis in failed synthetic
implants used for surgical repair of anterior cruciate ligaments.
Arch Orthop Trauma Surg. 114:1–7. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guidoin MF, Marois Y, Bejui J, Poddevin N,
King MW and Guidoin R: Analysis of retrieved polymer fiber based
replacements for the ACL. Biomaterials. 21:2461–2474. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li H, Chen S, Wu Y, et al: Enhancement of
the osseointegration of a polyethylene terephthalate artificial
ligament graft in a bone tunnel using 58S bioglass. Int Orthop.
36:191–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao K, Chen S, Wang L, et al: Anterior
cruciate ligament reconstruction with LARS artificial ligament: a
multicenter study with 3- to 5-year follow-up. Arthroscopy.
26:515–523. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu L, Pan F, Yu G, Yang L, Zhang E and
Yang K: In vitro and in vivo evaluation of the surface bioactivity
of a calcium phosphate coated magnesium alloy. Biomaterials.
30:1512–1523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goyenvalle E, Aguado E, Nguyen JM, et al:
Osteointegration of femoral stem prostheses with a bilayered
calcium phosphate coating. Biomaterials. 27:1119–1128. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Jonge LT, Leeuwenburgh SC, van den
Beucken JJ, et al: The osteogenic effect of electrosprayed
nanoscale collagen/calcium phosphate coatings on titanium.
Biomaterials. 31:2461–2469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bigi A, Fini M, Bracci B, et al: The
response of bone to nanocrystalline hydroxyapatite-coated
Ti13Nb11Zr alloy in an animal model. Biomaterials. 29:1730–1736.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lui P, Zhang P, Chan K and Qin L: Biology
and augmentation of tendon-bone insertion repair. J Orthop Surg
Res. 5:592010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baxter FR, Bach JS, Detrez F, et al:
Augmentation of bone tunnel healing in anterior cruciate ligament
grafts: application of calcium phosphates and other materials. J
Tissue Eng. 2010:7123702010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mutsuzaki H, Sakane M, Fujie H, Hattori S,
Kobayashi H and Ochiai N: Effect of calcium phosphate-hybridized
tendon graft on biomechanical behavior in anterior cruciate
ligament reconstruction in a goat model: novel technique for
improving tendon-bone healing. Am J Sports Med. 39:1059–1066. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mutsuzaki H, Sakane M, Nakajima H, et al:
Calcium-phosphate-hybridized tendon directly promotes regeneration
of tendon-bone insertion. J Biomed Mater Res A. 70:319–327. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Ge Y, Wu Y, et al: Hydroxyapatite
coating enhances polyethylene terephthalate artificial ligament
graft osseointegration in the bone tunnel. Int Orthop.
35:1561–1567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li H, Ge Y, Zhang P, Wu L and Chen S: The
effect of layer-by-layer chitosan-hyaluronic acid coating on
graft-to-bone healing of a poly(ethylene terephthalate) artificial
ligament. J Biomater Sci Polym Ed. 23:425–438. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Supová M: Problem of hydroxyapatite
dispersion in polymer matrices: a review. J Mater Sci Mater Med.
20:1201–1213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boanini E, Torricelli P, Gazzano M,
Giardino R and Bigi A: Nanocomposites of hydroxyapatite with
aspartic acid and glutamic acid and their interaction with
osteoblast-like cells. Biomaterials. 27:4428–4433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyazaki T, Ohtsuki C, Akioka Y, et al:
Apatite deposition on polyamide films containing carboxyl group in
a biomimetic solution. J Mater Sci Mater Med. 14:569–574. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang LJ, Liu HG, Feng XS, et al:
Mineralization mechanism of calcium phosphates under three kinds of
Langmuir monolayers. Langmuir. 20:2243–2249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barrère F, van Blitterswijk CA and de
Groot K: Bone regeneration: molecular and cellular interactions
with calcium phosphate ceramics. Int J Nanomedicine. 1:317–332.
2006.PubMed/NCBI
|
|
22
|
Ku Y, Chung CP and Jang JH: The effect of
the surface modification of titanium using a recombinant fragment
of fibronectin and vitronectin on cell behavior. Biomaterials.
26:5153–5157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Xie J, Yuan X and Xia Y: Coating
electrospun poly(epsilon-caprolactone) fibers with gelatin and
calcium phosphate and their use as biomimetic scaffolds for bone
tissue engineering. Langmuir. 24:14145–14150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Araujo JV, Martins A, Leonor IB, Pinho ED,
Reis RL and Neves NM: Surface controlled biomimetic coating of
polycaprolactone nanofiber meshes to be used as bone extracellular
matrix analogues. J Biomater Sci Polym Ed. 19:1261–1278. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arafat MT, Lam CX, Ekaputra AK, Wong SY,
Li X and Gibson I: Biomimetic composite coating on rapid prototyped
scaffolds for bone tissue engineering. Acta Biomater. 7:809–820.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Xie J, Lipner J, Yuan X, Thomopoulos
S and Xia Y: Nanofiber scaffolds with gradations in mineral content
for mimicking the tendon-to-bone insertion site. Nano Lett.
9:2763–2768. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu X, Smith LA, Hu J and Ma PX:
Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone
tissue engineering. Biomaterials. 30:2252–2258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mavis B, Demirtas TT, Gümüşderelioĝlu M,
Gündüz G and Colak U: Synthesis, characterization and osteoblastic
activity of polycaprolactone nanofibers coated with biomimetic
calcium phosphate. Acta Biomater. 5:3098–3111. 2009. View Article : Google Scholar : PubMed/NCBI
|