Introduction
As society has developed in China, the average age
of the population has increased considerably. Consequently, the
incidence of cerebrovascular disease has increased, and appears to
be affecting younger individuals (1). Epidemiological survey results in recent
years have demonstrated that cerebrovascular disease accounts for
the second highest mortality rates in China, second only to
malignant tumors (2). As for the
survivors, ~75% of patients with cerebrovascular disease lose the
capacity to work, to varying degrees and >40% may be severely
disabled (3,4). Therefore, cerebrovascular disease is
associated with high morbidity and mortality, causing serious harm
to human health and quality of life. Cerebral infarction accounts
for >70% of all cerebral vascular diseases, therefore research
on cerebral infarction is urgently required (5). Acute cerebral infarction (ACI) refers
to disturbance in the blood supply to brain tissue due to stenosis
or occlusion of the intracranial or extracranial arteries, which
leads to ischemic necrosis or cerebromalacia due to focal brain
tissue ischemia and hypoxia (6).
Cells in the central area of severe ischemia do not survive severe
ischemia and hypoxia, which is the irreversible process (7,8).
Previous studies have demonstrated that regional blood flow in the
area surrounding the cerebral infarction, which is named the
penumbra, reduced and exhibited a low perfusion state (5,9).
Therefore, the brain cells were apoptotic and if they could be
protected in a timely manner, the occurrence of apoptosis may be
reduced and the process of cell death may be simultaneously
delayed. Nerve cell apoptosis in the ischemic penumbra has been
demonstrated to be an important type of cell death during ischemic
cerebrovascular disease (10).
Various stress factors, including HIF-1α, are produced during
apoptosis. These stress factors have been demonstrated to combine
with their corresponding target gene to regulate the molecular
pathways of hypoxia-responsive genes, which is an important
mechanism for the maintenance of mammalian homeostasis under
hypoxic conditions (11,12). The HIF-1α signal transduction pathway
may improve blood supply to cerebral tissue and reduce
hypoxia-induced ischemic injury. Therefore, HIF-1α may be a novel
molecular target for neuroprotective therapy following ischemic
brain injury, and may suggest a novel direction for the research of
neuroprotective drugs. Previous studies have demonstrated the
credibility and clinical significance of this hypothesis (13,14) In
the present study, rat models of ACI were established in order to
observe the expression of HIF-1α in the brain tissue of rats during
the early stage of ACI, and to investigate the underlying
mechanism.
Materials and methods
Model preparation
A total of 64 male Sprague Dawley rats, weighing
200–220 g and aged 2 months, were purchased from Henan Provincial
Experimental Animal Center (Zhengzhou, China). All rats were
maintained in a temperature (22±1°C) and humidity (60%) controlled
environment, with a 12 h light/dark cycle and ad libitum
access to food and water. Rat models of middle cerebral artery
infarction were established according to the suture-occluded
method. Rats were fasted for 12 h prior to the surgery. Rats were
anesthetized with 0.3 ml/100 g chloral hydrate (10%; Sangon Biotech
Co., Ltd., Shanghai, China) via intraperitoneal injection. Briefly,
an incision was made along the midline of the neck and the external
carotid arteries were double ligated (5/0 ligature; Beijing Dingguo
Biotechnology Co., Ltd., Beijing, China) and amputated ~3 mm from
the bifurcation of the common carotid artery. A small incision was
made in the common carotid artery. The head end of a fishing line
was burned into a sphere (diameter, ~0.205 mm), and slowly inserted
into the incision to 18–20 mm, until resistance was detected. The
clamp on the arteries was simultaneously released and the fishing
line and common carotid artery were ligated together and sutured.
Following this procedure, the rats exhibited listlessness,
ipsolateral Horner's disease, contralateral forelimb droop,
adduction, internal rotation and spontaneously circling to the
affected side 2 h after the surgery. These symptoms indicated that
modeling was successful (n=32) and rats without these symptoms were
eliminated from the present study. The blood vessels of rats in the
sham group were separated but not occluded. Rats in the surgery and
sham groups were subdivided into eight groups, with four rats in
each group, according to the time point at which blood samples were
collected (30 min and 1, 3, 6, 12, 48, 24 and 72 h, respectively).
The present study was performed in strict accordance with the
recommendations outlined in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health (15). The protocol for the present study was
reviewed and approved by the Institutional Animal Care and Use
Committee of Henan University Huaihe Hospital (Kaifeng, China).
ELISA
Blood samples were collected from rats in the
surgery and sham groups at eight time points: 30 min and 1, 3, 6,
12, 48, 24 and 72 h, respectively. After standing at room
temperature for 1 h, the whole blood was centrifuged at 3,000 × g
for 15 min and the liquid supernatant was extracted for ELISA
detection. ELISA was performed according to the protocol outlined
in the HIF-1α ELISA kit (Bioco Laibo Technology Co., Ltd, Beijing,
China) to assess the levels of HIF-1α in both groups at the various
time points. Three holes were used for each sample and standard.
Optical density values were measured at 492 nm using a microplate
reader (Miltiskan MK3; Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Immunohistochemistry
Brain tissues in all groups were perfused with 4%
paraformaldehyde (Sangon Biotech Co., Ltd.) following blood
collection. Specimens were continuously cut into 0.3 µm sections
and embedded by paraffin. The samples were adhered to the glass
slides, treated by polylysine (Sangon Biotech Co., Ltd.) and
incubated at 60°C for 1 h prior to treatment with xylene and 100,
95, 80 and 75% ethanol, successively. Subsequently, the samples
were washed three times with distilled water and immersed in 3%
H2O2 for 10 min to remove the endogenous
hydrogen peroxide enzyme. Following washing three times with
phosphate-buffered saline with Tween 20 (PBST; Sangon Biotech Co.,
Ltd.) for 5 min, citric acid buffer (Sangon Biotech Co., Ltd.) was
added and the sample was microwaved at moderate heat for 3 min, and
subsequently cooled. This procedure was repeated twice for antigen
retrieval. Samples were washed three times with PBST for 5 min and
incubated with 5% bovine serum albumin at room temperature for 30
min. Following washing three times with PBST for 5 min, the samples
were incubated with HIF-1α monoclonal antibody (1:100; cat. no.
BM0912; Boster Biotechnology Co., Wuhan, China) at 4°C overnight.
The samples were washed three times with PBST for 5 min and
incubated with HRP-conjugated goat anti-mouse secondary antibody
(1:500; cat. no. CSB-SA11952m; Qianjian Biotechnology Co.,
Shanghai, China) at room temperature for 1 h. Subsequently, the
samples were washed three times with PBST for 5 min, stained with
3,3′-diaminobenzidine (Sangon Biotech Co., Ltd.), followed by
staining with hematoxylin (Boster Biotechnology Co.),
differentiated with 0.1% hydrochloric acid, and retuned to blue
with PBS. Routine dehydration and transparency using a gradient
method were conducted. Neutral resin (Boster Biotechnology Co.) was
used for sealing. Cells were deemed to be positively stained if
their nuclei were brown/yellow and part of cytoplasm was stained,
otherwise the cells were deemed negative. Five fields were randomly
selected under high magnification (magnification, ×400; AX80;
Olympus Corporation, Tokyo, Japan) and 200 nerve cells were
assessed for positive staining. The HIF-1α label index (LI) was
calculated as follows: LI=positive staining cell number/total
number of nerve cells × 100%, LI >5% was indicative of positive
expression of HIF-1α.
Terminal deoxynucleotidyl transferase
(TdT) dUTP nick-end labeling (TUNEL)
Brain tissue sections from rats in all groups were
dewaxed and washed three times with PBST for 5 min. TUNEL was
performed according to manufacturer's protocol (Roche Diagnostics
GmbH, Basel, Switzerland). A total of 10 fields surrounding the
infarction and peripheric regions were randomly selected and
analyzed under a microscope (magnification, ×400; AX80; Olympus
Corporation). The brain cell apoptosis index was calculated as the
proportion of apoptotic cells in the total cells.
Statistical analysis
All data were analyzed with SPSS 17.0 statistical
software (SPSS, Inc., Chicago, IL, USA). Measurement data were
expressed as the mean ± standard deviation and were compared
between multiple groups using analysis of variance. Comparison
between groups was conducted according to the least significant
difference method. P<0.05 was considered to indicate a
statistically significant difference.
Results
Serum HIF-1α expression increases
during ACI, peaking at 12 h
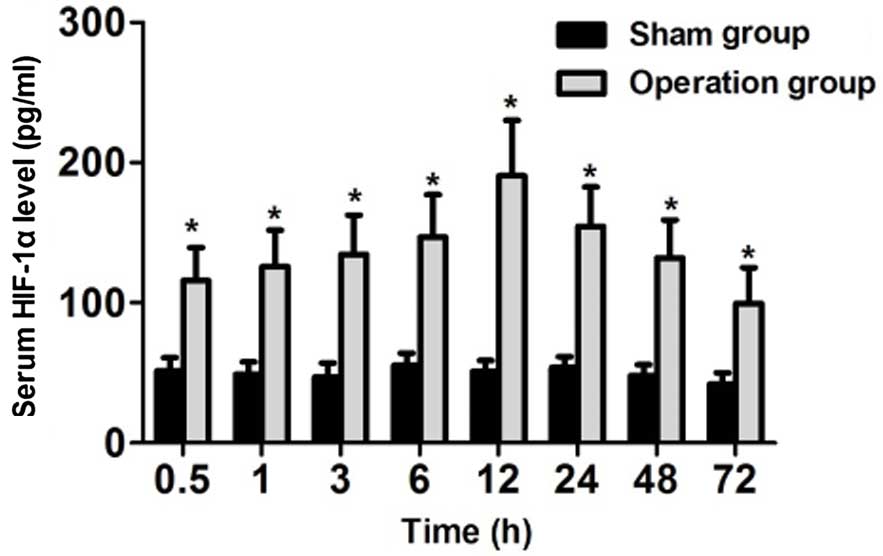
As shown in Fig. 1,
serum concentrations of HIF-1α were significantly higher in the
rats of the surgery group, as compared with the sham group
(P<0.05), at all eight of the time points, including 30 min and
1, 3, 6, 12, 24, 48 and 72 h. Rats in the surgery group exhibited
dynamic changes in serum HIF-1α concentration. HIF-1α concentration
levels began to increase during the 30 min postoperative period,
and peaked at 12 h. Significant differences in the concentration of
HIF-1α were detected between the surgery and sham groups, and among
all the time points in the surgery group in 12 h (P<0.05).
Although serum HIF-1α concentration gradually decreased in the
surgery group between 12 and 72 h, HIF-1α concentration remained
significantly higher than the sham group (P<0.05). In
conclusion, serum HIF-1α expression levels peaked at 12 h after
acute cerebral infarction, and decreased after this point.
HIF-1α expression in brain tissue
increases during ACI, peaking at 12 h
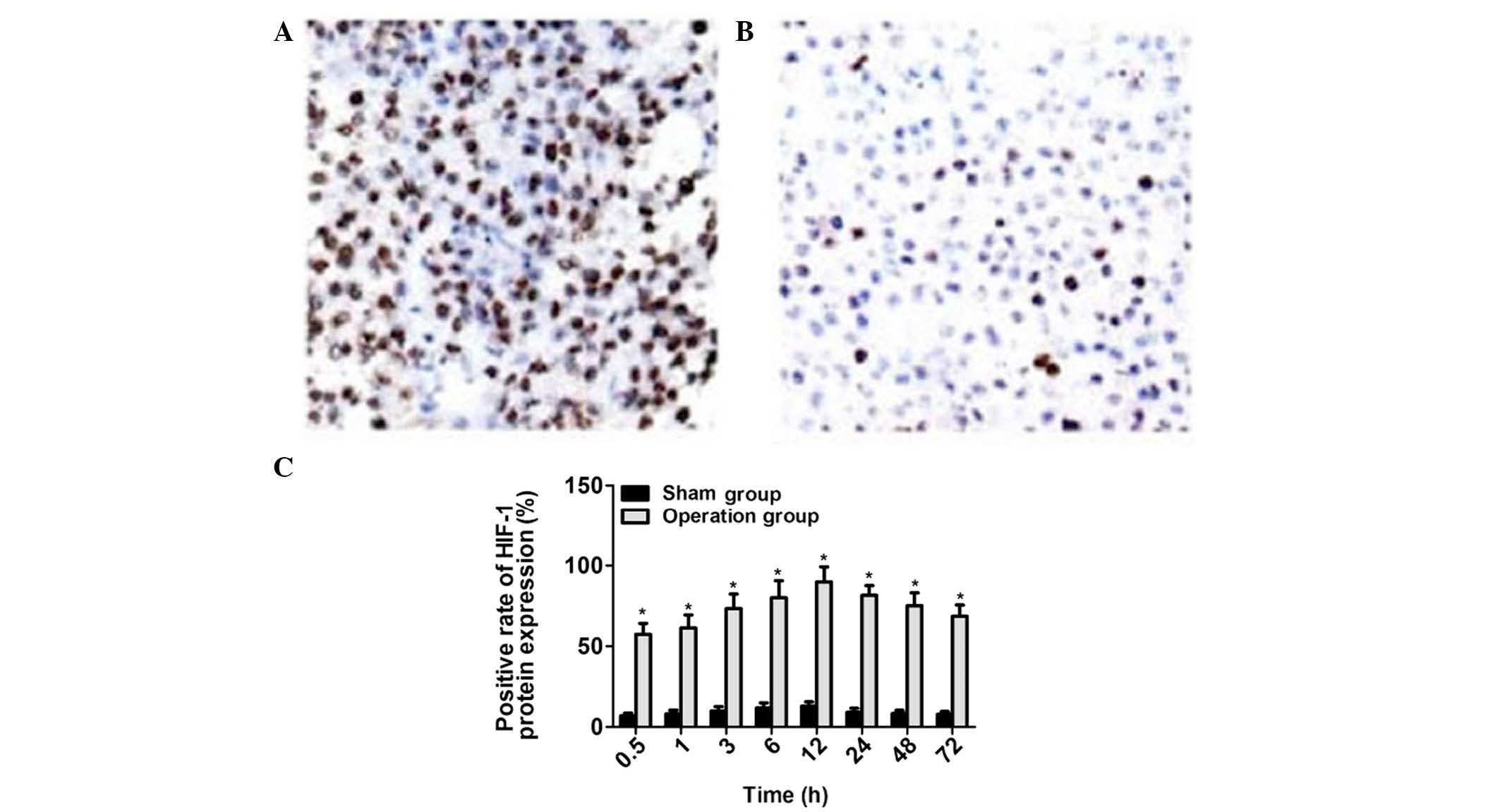
Immunohistochemical analysis demonstrated that
HIF-1α was predominantly expressed in the nuclei of nerve cells,
and was also expressed in the cytoplasm. HIF-1α was successfully
detected in the rats of the surgery group (Fig. 2A), whereas no positive expression of
HIF-1α was detected in the rats of the sham group (Fig. 2B). Within the brain tissue of rats in
the surgery group, HIF-1α expression was highest at 12 h
post-surgery, with the proportion of HIF-1α-positive cells
gradually decreasing in a time-dependent manner. Significant
differences in the proportion of HIF-1α-positive cells were
detected between the surgery and sham groups, and among the various
time points within the surgery group (P<0.05; Fig. 2C). In conclusion, HIF-1α expression
levels in brain tissue peaked at 12 h after acute cerebral
infarction, and decreased after this point.
During ACI, apoptosis increases in a
time-dependent manner, peaking at 12 h
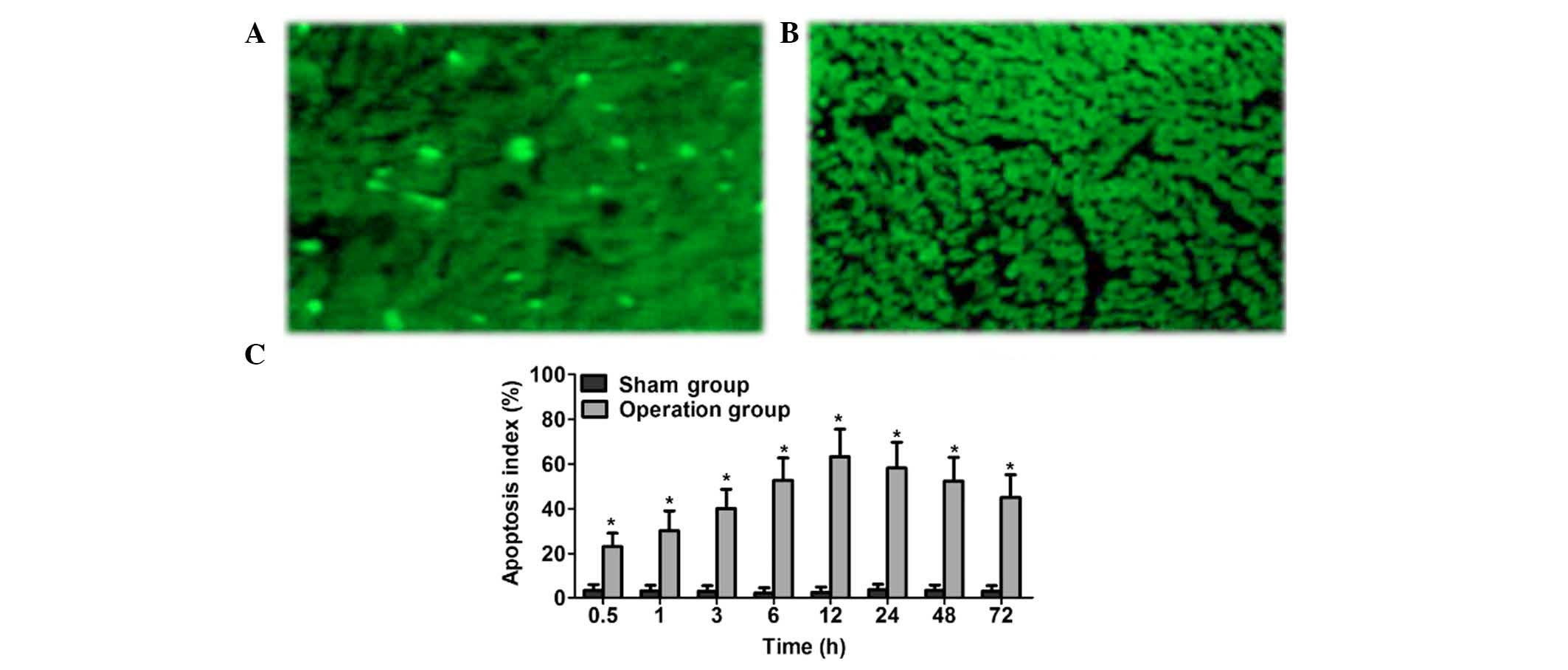
TUNEL staining demonstrated that, as compared with
the sham group, volume of apoptotic cells in the surgery group was
reduced and they were triangular or fan-shaped (Figs. 3A and B). Furthermore, the Nissl's
body was not detectable, nucleus pycnosis was deeply stained and
nuclear chromatin was condensed. The chromatin condensed around the
nucleus and appeared as crescent, annular or nuclear debris.
Apoptosis-negative cell nuclei were not colored. The proportion of
apoptotic cells in the brain tissue of rats in the sham group was
markedly reduced, as compared with the surgery group, which
exhibited obvious cell apoptosis. In the surgery group, the
proportion of apoptotic cells increased in a time-dependent manner,
peaking at 12 h (P<0.05), and gradually decreasing thereafter.
Significant differences in the proportion of apoptotic cells were
detected between the surgery and sham groups, and among the various
time points within the surgery group (P<0.05) (Fig. 3C). In conclusion, HIF-1α expression
levels in brain tissue peaked at 12 h after acute cerebral
infarction, and decreased after this point.
Discussion
Patients with acute ischemic cerebrovascular disease
suffer from high rates of morbidity and mortality (16). The probability of patients
experiencing recurrent stroke within five years is ~20% in patients
who have already had a stroke, which seriously affects the quality
of life of these patients (17,18).
Therefore, investigating cerebral infarction pathogenesis,
prevention, timely diagnosis and treatment following disease is
likely to significantly reduce the incidence of cerebral infarction
and improve the prognosis of patients with ACI. The ‘reducible
penumbra’ and ‘therapeutic time window’ ACI theories describe a
period within 3–6 h of hyperacute cerebral infarction where the
focal ischemic brain tissue is in the reversible stage (19). These theories suggest that, if timely
arteriovenous thrombolysis was conducted within this period, the
ischemic brain tissue may be completely recovered. Nerve cell
apoptosis in ischemic penumbra has been demonstrated to be an
important cell death mode in patients with ischemic cerebrovascular
disease, which also confirmed the existence of a substance (yet to
be elucidated) which could alleviate the cell apoptosis in early
ACI (20,21).
HIF-1α is a transcription regulator which has been
demonstrated to be widely distributed in mammals under hypoxic
conditions (22). Hypoxia is an
important pathophysiological factor of cerebral infarction and
HIF-1α has shown to be associated with the response of mammalian
cells to ischemic hypoxia (23). The
present study demonstrated that HIF-1α is associated with the
pathophysiological process of cerebral infarction, and as such has
an influence over cell apoptosis. Under normoxic conditions, HIF-1α
is vulnerable to degradation. This degradation process is blocked
under hypoxic conditions, resulting in the accumulation of HIF-1α
(24). Under hypoxic conditions,
HIF-1α is capable of regulating anaerobic metabolism by inducing
the expression of its target gene, and promoting angiogenesis and
an increase of erythropoietin, which ensures that the hypoxic
tissues and cells maintain at a certain oxygen concentration and
tolerate the hypoxic environment (25,26).
Previous studies have demonstrated that, when cerebral ischemia
occurs, HIF-1α-induced gene expression may promote reperfusion of
the ischemic penumbra area, thus improving glucose transport, which
helps to mediate the ability of cells to tolerate hypoxia and has
an important protective role for ischemic and hypoxic neurons
(27,28). Following mild transient ischemia and
hypoxia, HIF-1α expression levels are increased, which induces
ischemic and hypoxic tolerance and demonstrates the neuroprotective
effect of HIF-1α against ischemia and hypoxia. Chen et al
(29) have previously established a
rat model of focal cerebral infarction using the modified suture
and occlusion of the right middle cerebral artery method. The
concentrations of HIF-1α and VEGF were detected in blood using the
ELISA method. The results indicated that HIF-1α concentration
levels began to increase during hyperacute cerebral infarction and
peaked within 12 h, which has an important role on a series of
hypoxic responses after cerebral infarction. VEGF concentration
levels began to increase within 30 min, peaked within 24 h, which
indicated that VEGF was also involved in the high expression of
cerebral infarction. HIF-1α and VEGF were found to be significantly
correlated.
Notably, the expression of HIF-1α has been
demonstrated to promote nerve cell apoptosis after long-term severe
ischemia and hypoxia (30). HIF-1α
induced nerve cell toxicity and aggravated ischemic and hypoxic
injury of cerebral infarction. Hua et al (31) have previously established a rat model
of focal cerebral infarction and observed the effects of
recombinant human erythropoietin (RhEPO) on the protein expression
of HIF-1α and neuroprotective effects following cerebral infarction
using nerve score, 2,3,5-triphenyltetrazolium chloride staining and
immunohistochemistry. The results demonstrated that RhEPO was
capable of reducing HIF-1α protein expression following cerebral
ischemic infarction. Furthermore, the results also indicated that
earlier intervention induced a greater reduction in the expression
of HIF-1α and recovered neurological function to a greater degree.
Therefore, to intensify the research on HIF-1α in early ACI is
particularly important.
In conclusion, the results of the present study
indicate that serum HIF-1α expression levels in brain tissue peak
at 12 h after acute cerebral infarction, and decreased after this
time point. This may be a result of the up-regulation of HIF-1α to
protect hypoxia ischemic brain tissue. High expression levels of
HIF-1α regulate cytokines expressing VEGF in areas of hypoxia
ischemia, promote the formation of new blood vessels, and,
therefore, serve a role in the protection of brain tissue in early
acute cerebral infarction.
References
|
1
|
Wang J, An Z, Li B, Yang L, Tu J, Gu H,
Zhan C, Liu B, Su TC and Ning X: Increasing stroke incidence and
prevalence of risk factors in a low-income Chinese population.
Neurology. 84:374–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang H, Ju Z, Wang N, Zhang YF, Xu T and
Zhang YH: Pulse pressure and in-hospital mortality and morbidity in
acute stroke patients. Zhong Hua Gao Xue Ya Za Zhi. 16:633–636.
2008.(In Chinese).
|
|
3
|
Wang SS, Wang JJ, Wang PX and Chen R:
Determinants of fatigue after first-ever ischemic stroke during
acute phase. PLoS One. 9:e1100372014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wira CR III, Rivers E, Martinez-Capolino
C, Silver B, Iyer G, Sherwin R and Lewandowski C: Cardiac
complications in acute ischemic stroke. West J Emerg Med.
12:414–420. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J: Clinical characteristics observed in
elderly patients with cerebral infarction. Zhong Guo Shi Yong Shen
Jing Ji Bing Za Zhi. 14:59–60. 2011.(In Chinese).
|
|
6
|
Fang H, Song B, Cheng B, Wong KS, Xu YM,
Ho SS and Chen XY: Compensatory patterns of collateral flow in
stroke patients with unilateral and bilateral carotid stenosis. BMC
Neurol. 16:392016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saqqur M, Ibrahim M, Butcher K, Khan K,
Emery D, Manawadu D, Derksen C, Schwindt B and Shuaib A:
Transcranial Doppler and cerebral augmentation in acute ischemic
stroke. J Neuroimaging. 23:460–465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwata T: Current and future prospects of
endovascular treatment for acute ischemic stroke. Rinsho
Shinkeigaku. 54:1200–1202. 2014.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan CG and Guo S: Effect of Ginkgo biloba
extract on acute cerebral infarction rats apoptosis genes. Zhong
Guo Yi Yuan Yao Xue Za Zhi. 35:902–907. 2015.(In Chinese).
|
|
10
|
Teasell R, Rice D, Richardson M, Campbell
N, Madady M, Hussein N, Murie-Fernandez M and Page S: The next
revolution in stroke care. Expert Rev Neurother. 14:1307–1314.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li L, Candelario KM, Thomas K, Wang R,
Wright K, Messier A and Cunningham LA: Hypoxia inducible factor-1α
(HIF-1α) is required for neural stem cell maintenance and vascular
stability in the adult mouse SVZ. J Neurosci. 34:16713–16719. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen L, Pei H, Zhu S, Zhu J and Shi R:
Expression and significance of hypoxia-inducible factor-1α in lung
tissues of obesity-asthma rat. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
30:1262–1265. 2014.(In Chinese). PubMed/NCBI
|
|
13
|
Salva E, Turan SO, Eren F and Akbuğa J:
The enhancement of gene silencing efficiency with chitosan-coated
liposome formulations of siRNAs targeting HIF-1α and VEGF. Int J
Pharm. 478:147–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen C, Ostrowski RP, Zhou C, Tang J and
Zhang JH: Suppression of hypoxia-inducible factor-1alpha and its
downstream genes reduces acute hyperglycemia-enhanced hemorrhagic
transformation in a rat model of cerebral ischemia. J Neurosci Res.
88:2046–2055. 2010.PubMed/NCBI
|
|
15
|
Institute of Laboratory Animal Resources
(US). Committee on Care, Use of Laboratory Animals, and National
Institutes of Health (US). Division of Research Resources: Guide
for the care and use of laboratory animals (8th). National
Academies Press. (Washington, DC). 2011.
|
|
16
|
Walcott BP, Boehm KM, Stapleton CJ, Mehta
BP, Nahed BV and Ogilvy CS: Retrievable stent thrombectomy in the
treatment of acute ischemic stroke: Analysis of a revolutionizing
treatment technique. J Clin Neurosci. 20:1346–1349. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rahme R, Abruzzo TA and Ringer AJ: Acute
ischemic stroke in the setting of cervical carotid occlusion: A
proposed management strategy. World Neurosurg. 76(Suppl 6):
S60–S65. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amar AP: Brain and vascular imaging of
acute stroke. World Neurosurg. 76(Suppl 6): S3–S8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu Y, Sun Y, Mao XO, Jin KL and Greenberg
DA: Expression of poly(C)-binding proteins is differentially
regulated by hypoxia and ischemia in cortical neurons.
Neuroscience. 110:191–198. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Donohue A, McLaughlin C, Crowe M and
Horgan F: Clinical guideline adherence by physiotherapists working
in acute stroke care. Ir Med J. 107:287–289. 2014.PubMed/NCBI
|
|
21
|
El-Koussy M, Schroth G, Brekenfeld C and
Arnold M: Imaging of acute ischemic stroke. Eur Neurol. 72:309–316.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bentovim L, Amarilio R and Zelzer E: HIF1α
is a central regulator of collagen hydroxylation and secretion
under hypoxia during bone development. Development. 139:4473–4483.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma S, Kapahi R, Sambyal V, Guleria K,
Manjari M, Sudan M, Uppal MS and Singh NR: No association of
hypoxia inducible factor-1α gene polymorphisms with breast cancer
in north-west indians. Asian Pac J Cancer Prev. 15:9973–9978. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tennant DA, Frezza C, MacKenzie ED, Nguyen
QD, Zheng L, Selak MA, Roberts DL, Dive C, Watson DG, Aboagye EO
and Gottlieb E: Reactivating HIF prolyl hydroxylases under hypoxia
results in metabolic catastrophe and cell death. Oncogene.
28:4009–4021. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng J, Huang Q, Wang Y, Shen P, Guan F,
Li J, Huang H and Shi C: Hypoxia-inducible factor-1alpha regulates
autophagy to activate hepatic stellate cells. Biochem Biophys Res
Commun. 454:328–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Botlagunta M: Neuronal pentraxin 1
expression is regulated by hypoxia inducible factor-1α. Biochem
Biophys Res Commun. 456:662–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu Q, Liang X, Chen D, Chen Y, Doycheva D,
Tang J, Tang J and Zhang JH: Delayed hyperbaric oxygen therapy
promotes neurogenesis through reactive oxygen
species/hypoxia-inducible factor-1α/β-catenin pathway in middle
cerebral artery occlusion rats. Stroke. 45:1807–1814. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma Y, Lovekamp-Swan T, Bekele W, Dohi A
and Schreihofer DA: Hypoxia-inducible factor and vascular
endothelial growth factor are targets of dietary soy during acute
stroke in female rats. Endocrinology. 154:1589–1597. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen L, Chen SH and Chen JY: Change
characteristics and relation study of serum HIF-1, VEGF factor on
acute stage cerebral infarction in SD rats. Leshan Shi Fan Xue Yuan
Xue Bao. 27:13–18. 2012.(In Chinese).
|
|
30
|
Panchision DM: The role of oxygen in
regulating neural stem cells in development and disease. J Cell
Physiol. 220:562–568. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hua W, Zhou M, Liu W and Qi JP: Effect of
RhEPO to hypoxia-inducible factor-1α after cerebral ischemia. Ha Er
Bin Yi Ke Da Xue Xue Bao. 47:216–219. 2013.(In Chinese).
|