Introduction
Ankylosing spondylitis (AS) is a chronic
inflammatory disease characterized by syndesmophytes and ankylosis
(1). AS affects ~0.22% of the
population in China, which is similar to the prevalence of AS in
Europe and America (2). Heterotopic
ossification (HO), manifesting as tendon and ligament ossification,
is the primary cause leading to disability in AS. Fibroblasts are
the principal cell-type in ligament tissues, and are closely
associated with HO in AS patients (3). Core-binding factor alpha 1 (Cbfα1) is
the osteogenic marker gene of fibroblasts, and a prior study has
suggested that Cbfα1 expression is regulated by bone morphogenetic
protein (BMP) signaling pathways (4). Among the BMPs, BMP-2 has been
demonstrated to have the most marked osteogenic capacity (5,6).
Notably, serum levels of BMP-2 in AS patients with spinal fusion is
higher than healthy controls or AS patients without spinal fusion
(7). In in vitro studies,
BMP-2 has been shown to induce osteogenic differentiation of
fibroblasts (5,6). However, following connexin 43 (Cx43)
knockout, BMP-2 is unable to induce osteogenic differentiation
(8). Cx43 is widely expressed in
fibroblasts and serves a crucial function in the ossification and
differentiation of fibroblasts (9).
At present, there is no entirely satisfactory
therapeutic strategy for AS, although nonsteroidal
anti-inflammatory drugs (NSAIDs) have been considered as the
cornerstone treatment options (10,11).
However, the side effects of long-term use of NSAIDs include
gastrointestinal discomfort and the risk of cardiovascular
incidence (12). Biological agents,
such as tumor necrosis factor (TNF) inhibitor, were developed
during the first decade of 21st century, and are typically
recommended for the management of AS (13). However, TNF inhibitor agents cannot
lead to the improvement of the modified stokes AS spinal cord of AS
patients, since these drugs are unable to suppress new bone
formation in such patients (14).
Therefore, the identification of safer and more effective drugs or
therapeutic strategies with the inhibitory effects on new bone
formation are required.
Traditional Chinese medicine has been applied to the
treatment of rheumatic diseases for thousands years, and may
provide an alternative choice for patients with AS.
Bushen-Qiangdu-Zhilv (BQZ) decoction was established in
Rheumatology (15). In a recent
clinical study, a modified form of BQZ was demonstrated to be more
effective than sulfasalazine, a typical DMARD, for treating AS by
relieving clinical symptoms and inflammatory activity indicators in
AS patients (15). However, the
mechanisms underlying the effects of BQZ remain largely unclear. In
a previous report, we showed that the extracts from BQZ may induce
significant anti-inflammatory effects by suppressing TNF-α and
interleukin-1 expression (16). The
aim of the present study was to determine whether BQZ could have
the effects on HO and new bone formation in AS.
Materials and methods
Cell lines and cell culture
Primary cells were obtained from 5 male
Sprague-Dawley rats (age, 2–4 weeks). Rats were provided by the
Animal Experimental Center of Guangzhou University of Chinese
Medicine (Guangzhou, China). Fibroblast cell lines were established
from rat ligament tissues from the hip joint. The tissues were
aseptically collected and cut into small pieces. The pieces were
washed several times in 0.01 M sterile phosphate-buffered saline
(PBS; pH 7.4; Hyclone, Logan, UT, USA) then seeded into a
25-cm3 flasks (Corning, NY, USA). Primary cell cultures
were grown in Dulbecco's modified Eagle's medium (DMEM; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) with 20% fetal bovine
serum (FBS) and 1% penicillin-streptomycin (both from Hyclone) at
37°C in a humidified atmosphere containing 5% CO2. When
the cells reached 80% confluence, they were digested with
EDTA-trypsin (0.25%; Thermo Fisher Scientific, Inc.,) and completed
the passage with the proportion of 1:1 for the first generation.
For the second and third generations, cells were passaged with the
proportion of 1:2 or 1:3, and DMEM with 10% FBS were used in the
sub-culture. Fibroblasts at the third passage were used in the
experiments outlined below.
Preparation of the freeze-dried BQZ
powder
As previous described (16), BQZ is a mixture composed of 22
species of herbal plant. All components, purchased from Kangei
Pharmaceutical Co., Ltd. (Guangzhou, China), were identified by the
authors Professor Yi-Ting He and Dr Xiao-Hong He. A weight of 319 g
of BQZ was boiled in 1.4 L ultrapure water in a Chinese medicine
decocting pot (Guangzhou Wen Xin Electronics Co. Ltd., Guangzhou,
China) for 2 h, yielding 500 ml of solution. The solution was
frozen at −80°C for 24 h, and subsequently lyophilized for 24 h
using a freeze-dryer (Christ Alpha 2–4 LSCplus; Marin Christ
Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany). Finally,
the solution was freeze-dried, powdered and stored at 4°C in
tightly sealed 25-ml centrifuge tubes (Yancheng Great Wall Glass
Instruments Manufacturer Co., Ltd., Yancheng, China) sealed with
parafilm. The final yield of the material was 18% (57.4 g).
MTS analysis
The effect of BQZ on fibroblast proliferation was
detected using a CellTiter 96® AQueous One Solution Cell
Proliferation Assay (Promega Corporation, Madison, WI, USA),
according to the manufacturer's instructions. Cells were seeded at
a density of 105/ml into 96-well plates and incubated
overnight for attaching. The cells were divided into 7 groups with
5 wells for every group. BQZ with a concentration of 0 µg/ml
(control group), 25 µg/ml, 50 µg/ml, 100 µg/ml, 200 µg/ml, 500
µg/ml and 750 µg/ml were added into the 7 groups respectively. A
total of 5 groups were set with BMP-2 treated with 0, 12, 24, 48 or
72 h. Following treatment with graded levels of BQZ, the control
and treated cells were incubated for 48 h. Following the addition
of 20 µl MTS to each micro well, the plates were read at 492 nm
using a microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The growth inhibition rate was calculated using the
formula: Inhibition rate = [1 - (OD450 treatment /
OD450 control)] × 100%.
Western blot analysis
Cells were washed with ice-cold PBS, collected and
homogenized with radioimmunoprecipitation assay lysis buffer (Wuhan
Boster Biological Technology, Ltd., Wuhan, China) containing 1X
PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS and 1 mM
phenylmethylsulfonyl fluoride (Beyotime Institute of Biotechnology,
Beijing, China). Total protein was extracted and measured using a
bovine serum albumin protein assay kit (Bio-Rad Laboratories,
Inc.). Equal quantities of protein (20 µg) were boiled for 10 min,
separated using a 10% SDS-PAGE, and transferred to polyvinylidene
difluoride membranes (both from EMD Millipore, Billerica, MA, USA).
Subsequently, the membranes were blocked with 5% non-fat milk for 1
h. Next, they were incubated overnight at 4°C with the appropriate
primary antibody subsequent to washing with PBS containing 1% Tween
20, followed by exposure to the secondary antibody at room
temperature for 1 h. The following antibodies were used for the
western blot analysis: Rabbit polyclonal antibodies against
polyclonal rabbit Cx43 (1:500; 3512S), polyclonal pCx43 (1:500;
3511S) and monoclonal rabbit Cbfα1 (1:500; 12556S) were obtained
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Rabbit
polyclonal antibody against monoclonal rabbit GAPDH (1:1,000; 2118)
was obtained from Cell Signaling Technology, Inc. (Beverly, MA,
USA). Secondary antibody, affinity purified goat anti-rabbit (IgG
H&L), was also purchased from Cell Signaling Technology, Inc.
Specific proteins were detected by using an enhanced
chemiluminescence detection system (Clarity™ ECL Western Blotting
Substrate; Bio-Rad Laboratories, Inc.) to the membranes.
Densitometry was performed using Image Lab version 4.1 software
(Bio-Rad Laboratories, Inc.).
Immunohistochemical assay
In the present study, two groups (A and B) were set.
The reagents, including the 3% H2O2 blocking
buffer, primary and secondary antibodies were from the SV0002
two-step immunohistochemical detection kit (Wuhan Boster Biological
Technology, Ltd., Wuhan, China). The cells of the two groups were
fixed with 4% paraformaldehyde for 10 min. Next, the cells were
incubated in 3% H2O2 for 15 min and then in
blocking buffer for 1 h at room temperature. Next, the primary
antibody (1:250; anti-vimentin) was added to the cells of group A
followed by incubation for 3 h at room temperature. The cells were
rinsed thrice for 5 min each time in PBS with gentle agitation. The
conjugate HRP secondary antibody was then applied to the cells of
groups A and B and incubated for 1 h at room temperature. The cells
were then rinsed thrice for 2 min each time with TBS. The blot was
developed with a chromogen (DBA chromogenic reagent kit; Wuhan
Boster Biological Technology, Ltd.) for 10 min at room temperature
and it was rinsed with distilled water thrice for 5 min each time.
It was counterstained with hematoxylin, dehydrated, cleared and
then observed with a DMI3000 inverted fluorescence microscope
(Leica Microsystems GmbH, Wetzlar, Germany).
Statistical analysis
Data are presented as the mean ± standard deviation
from experiments repeated at least twice. Student's t-test was used
to compare the difference between two groups. One-way analysis of
variance followed by Dunnett's test was employed for comparisons
among more than two groups. Statistical analyses were conducted
using SPSS software, version 11.6 (SPSS, Inc., Chicago, IL, USA). A
two-tailed P-value of <0.05 was considered to indicate a
statistically significant difference.
Ethics statement
Animal work in this study was conducted in strict
accordance with the United States Institute of Animal Research
guidelines for the care and use of laboratory animals (17), and was approved by the Institutional
Animal Care and Use Committee, Guangzhou University of Chinese
Medicine (Ethic no: 0088139; Guangzhou, China). Animals were
sacrificed by decapitation under anesthesia consisting of 0.5 ml
ketamine (100 mg/ml; Shanghai Civi Chemical Technology Co., Ltd.,
Shanghai, China) combined with 0.05 ml xylazine (20 mg/ml; Shanghai
Civi Chemical Technology Co., Ltd.) at a dosage of 0.55 ml/100 g
body weight.
Results
Cellular growth, morphological
character and cell source identification
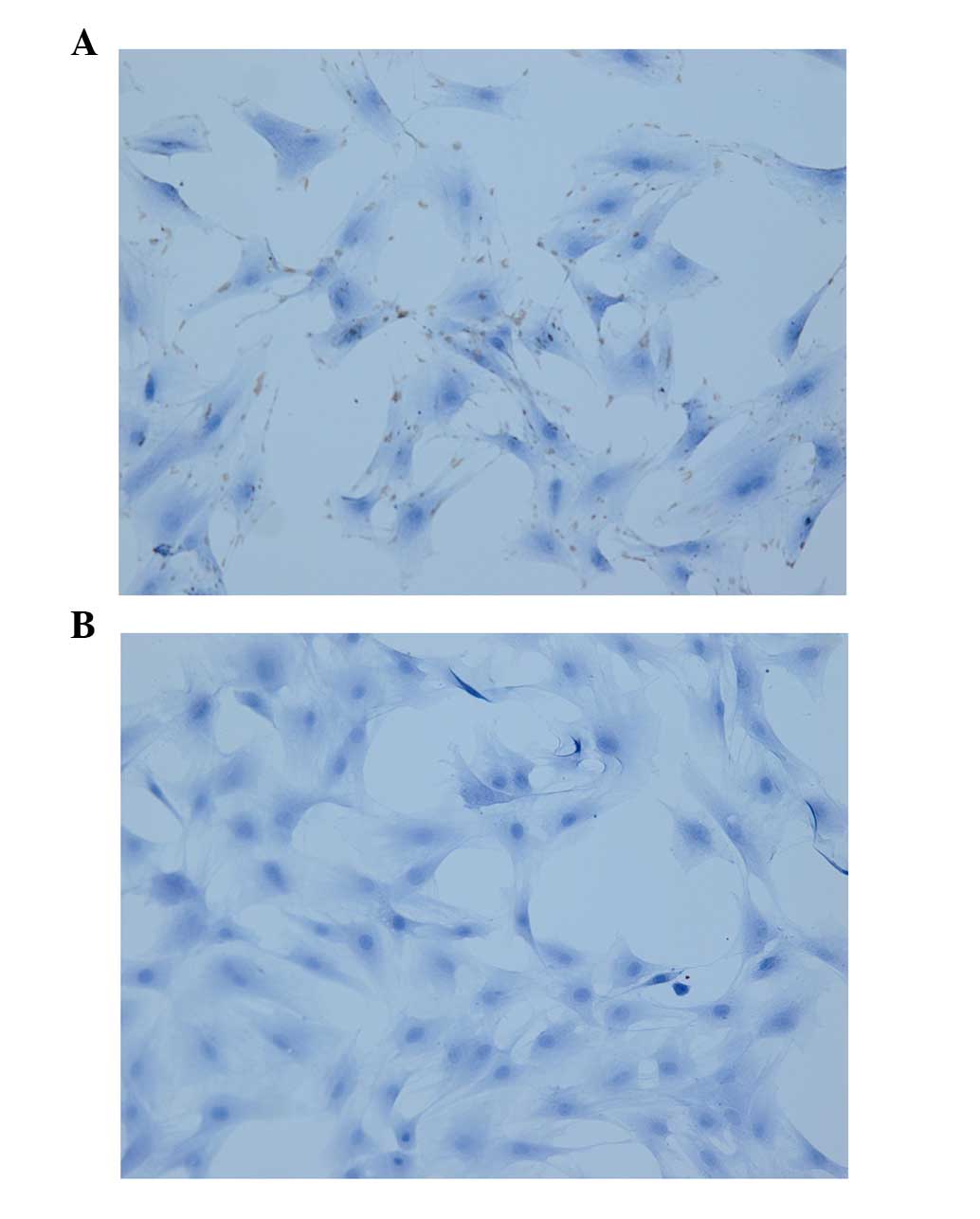
In the primary culture, cells emerged from tissue
pieces between days 5 and 7. After day 8, the fibroblasts encircled
the tissue pieces and grew in a stellate pattern, forming
outgrowth. After day 11, the cells thrived and grew to ~50%
confluence, with a small space among the tissue pieces. After day
13 the cells proliferated without any space among the pieces, but
had not adhered to each other. After day 15, the cells adhered to
each other. At passages, the cells grew stably, in net-like
formation at a low density, in bundle-like or whorl-like formation
at a high density. The cells were spindle-shaped, with a plump cell
body, even cytoplasm, round nucleus and a clear nucleolus. Cells
were positive for staining with a monoclonal antibody against
vimentin, confirming that the cells were fibroblasts derived from
the mesoderm (Fig. 1).
BQZ inhibited proliferation of
fibroblast cells
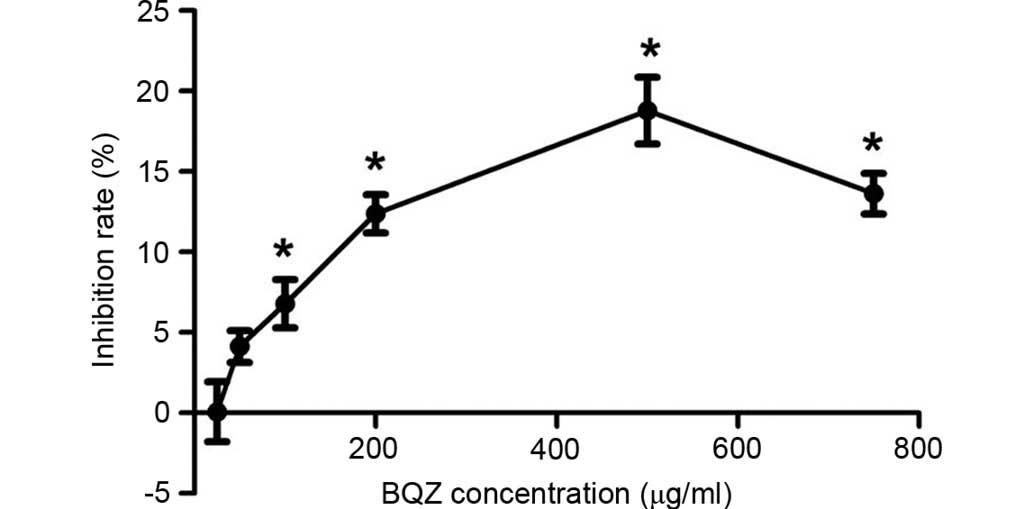
The inhibitory effect of BQZ on fibroblast cells
proliferation was measured by the MTS assay (Fig. 2). The mean of inhibition rate for
each group was 0.07% (25 µg/ml), 4.13% (50 µg/ml), 6.79% (100
µg/ml), 12.38% (200 µg/ml), 18.79% (500 µg/ml) and 13.63% (750
µg/ml). BQZ at concentrations of 100, 200, 500 and 750 µg/ml could
significantly inhibit the proliferation of fibroblast cells
(P<0.05). As BQZ at concentration of 500 µg/ml led to the
highest inhibition rate, even higher than that of 750 µg/ml, it was
used in the high-dose group in the subsequent experiments. In
addition, the concentrations of 100 µg/ml and 200 µg/ml were
employed in the low-dose group and middle-dose group respectively
in the next experiments.
Effect of BQZ on protein expression of
Cbfα1 and Cx43/pCx43 in fibroblast cells without BMP-2
treatment
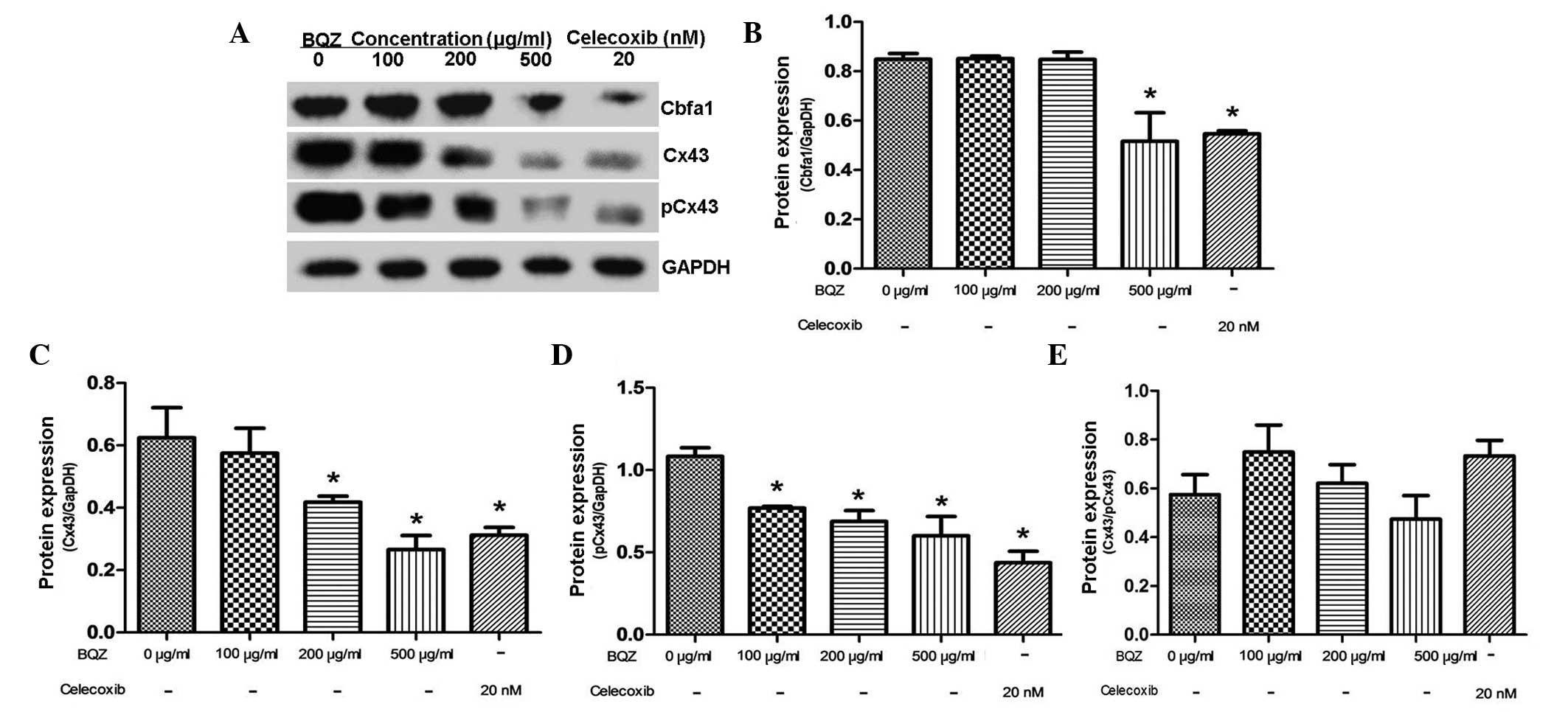
After treatment with different concentrations of BQZ
for 48 h, the protein expression levels of Cbfα1 and Cx43/pCx43
were detected by western blot analysis. Fig. 3A shows that BQZ could inhibit the
protein expression of Cbfα1 and Cx43/pCx43. Fig. 3B demonstrates that, after treating
the cells with 500 µg/ml BQZ, the level of Cbfα1 protein was
decreased by 39.2%. However, treatment with 100 and 200 µg/ml BQZ
produced a negligible effect on Cbfα1 expression. Cx43 protein
expression was decreased by 33.1 and 57.4% respectively after
treatment with 200 and 500 µg/ml BQZ, and such effects were similar
to celecoxib treatment (P>0.05) (Fig.
3C). Moreover, the protein expression of pCx43 was
significantly decreased by 28.9, 36.5 and 44.6% at concentrations
of 100, 200 and 500 µg/ml BQZ, respectively (Fig. 3D). Celecoxib treatment served as the
additional control in all groups, and showed that the inhibitory
effects of BQZ on the protein expression of Cbfα1 and Cx43/pCx43
were similar to celecoxib treatment. Expression of Cbfα1 and
Cx43/pCx43 in the 500 µg/ml BQZ group showed no significant
difference compared with the celecoxib group (P>0.05).
BMP-2 increased the protein expression
of Cbfα1 and Cx43 in fibroblast cells
BMP-2 is considered to serve a crucial role in
osteoblast differentiation and the subsequent bone formation by
stimulating Cbfα1 (18), which is
essential for osteoblast differentiation and bone formation
(19). A previous reports
demonstrated that fibroblasts could differentiate into osteogenic
cells in response to BMP-2 at concentration of 200 µg/ml (20). Therefore, 200 µg/ml BMP-2 was
utilized in the present study to construct in vitro model
for new bone formation of AS patients.
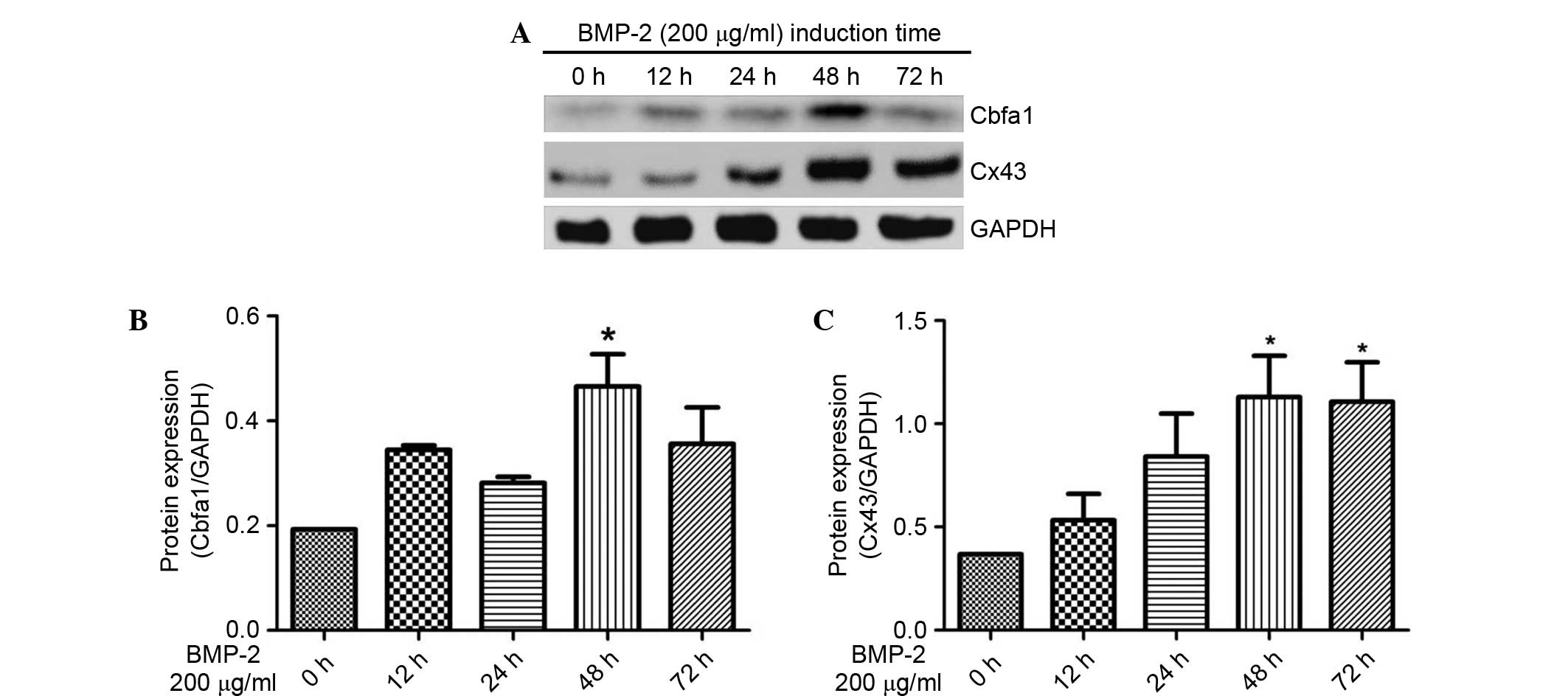
Following treatment with 200 µg/ml recombinant human
BMP-2 (rhBMP-2) for 12–72 h, the expression levels of Cbfα1 and
Cx43 protein were determined using western blot experiments. As
shown in Fig. 4A, Cbfα1 and Cx43
were increased with rhBMP-2 induction. The densitometric analysis
of the western blots shows that 200 µg/ml rhBMP-2 significantly
increased the expression of Cbfα1 protein at 48 h by 141.1%
compared with at 0 h (P<0.05) (Fig.
4B). In addition, the expression of Cx43 protein was increased
by 206.8 and 200.7% at 48 and 72 h, respectively, with stimulation
by 200 µg/m rhBMP-2 compared with at 0 h (P<0.05) (Fig. 4C). Therefore, it appears that 48 h is
an appropriate induction period for osteoblast differentiation and
was used in the following experiments.
Effect of BQZ on protein expression of
Cbfα1 and Cx43/pCx43 in fibroblast cells stimulated with
rhBMP-2
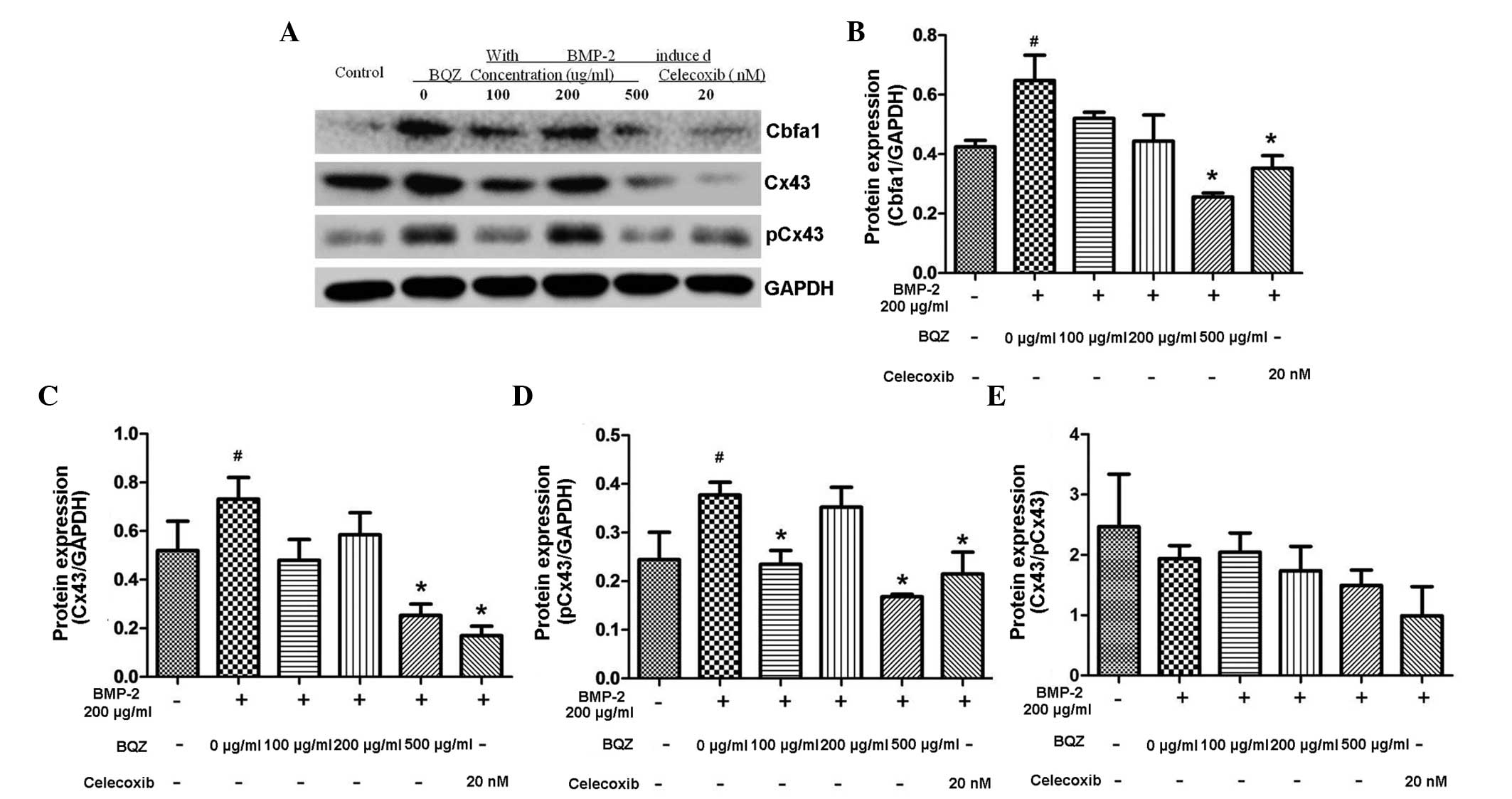
Following treatment with graded levels of BQZ for 30
min before 48 h stimulation with rhBMP-2, the expression of Cbfα1
and Cx43/pCx43 protein in fibroblasts was detected by western blot
analysis. Fig. 5A shows that the
protein levels of Cbfα1, Cx43 and pCx43 were increased by rhBMP-2
stimulation, which is in line with the data shown in Fig. 4, and that the effect was inhibited by
pre-treatment of BQZ. Fig. 5B and C
show that 500 µg/ml BQZ significantly decreased the Cbfα1 and Cx43
protein expression by 60.6 and 65.4% respectively, in the presence
of rhBMP-2 (P<0.05). Furthermore, no significant difference was
detected between the 500 µg/ml BQZ group compared to the celecoxib
group (P>0.05). pCx43 protein expression significantly decreased
by 37.7 and 55.4% after treatment with 100 and 500 µg/ml BQZ,
respectively, compared with the 0 µg/ml BQZ group (P<0.05).
Celecoxib treatment served as the additional control in all groups,
and showed that the effects of BQZ were similar to those of
celecoxib.
Discussion
Heterotopic ossification (HO) in connective tissue,
such as ossification of tendons and ligament, is the characteristic
pathological associated with AS (1).
Fibroblasts are crucial cells in connective tissue and play an
important role in the HO pathological process (3). Moreover, fibroblasts have the potential
of expressing osteogenic marker genes and differentiating into
osteoblasts (21). A prior study
showed that osteoblasts overexpress two genes, namely Cbfα1 and
osteocalcin (OC), in comparison with fibroblasts (22). Cbfα1 is a key transcription factor
and regulator of osteogenic differentiation, which may also
regulate the expression of other osteogenic differentiation marker
genes and osteogenic phenotype related genes such as alkaline
phosphatase (ALP), OC, type I collagen, thereby enhancing the
expression of osteogenic phenotypes to promote osteogenic
differentiation and bone formation (23–25).
Thus, the increased expression of Cbfα1 protein in fibroblasts is
usually considered to be a sign of the osteogenic differentiation
of fibroblasts, whereas the decreased expression of Cbfα1 may
indicate the suppression of osteogenic differentiation (20).
Cbfα1 protein expression has been demonstrated to be
markedly increased in fibroblasts compared to the fibroblasts
without BMP-2 induction, which are differentiated into osteogenic
cells in response to BMP-2 (20).
Notably, it has been reported that the expression of Cbfα1 is
regulated by the BMP signal pathway (26). The present study also confirmed that
BMP-2 is able to induce the expression of Cbfα1, resulting in the
osteogenic differentiation of rat fibroblasts.
Connexin 43 (Cx43), a major gap junction (GJ)
protein widely expressed in fibroblasts, plays a critical role in
osteogenic differentiation (8). Loss
of Cx43 may result in decreased bone formation and resorption,
which indicates that Cx43 is vital to normal bone metabolism
(27). A previous study showed that
Cx43 signaling pathway is involved in the osteogenic
differentiation of fibroblasts (28). Alteration of the expression of the
gap junction protein Cx43 modulates osteogenic marker gene
expression (29). In addition, the
cellular communication mediated by Cx43 is crucial for normal
osteogenic differentiation induced by BMP-2 (6). The present study indicated that BMP-2
is able to induce the protein expression of Cbfα1 in fibroblasts,
in addition to increasing the protein level of Cx43. Therefore,
Cx43 may be involved in the HO pathological change of AS by
interfering with the BMP-2-Cbfα1 pathway.
BQZ is a widely used traditional Chinese medicinal
formula with a long history of application in the treatment of AS,
and is speculated to be effective in relieving clinical symptoms
and signs (15). BQZ is effective
not only for inflammation control, but also for ossification delay.
Our previous study presented the anti-inflammatory effects of BQZ,
this indicating potentially mechanisms underlying its anti-AS
effects (16). In the present study,
potential anti-ossifying effect of BQZ was investigated.
Firstly, BZQ was shown to inhibit the proliferation
of rat fibroblasts in a dose-dependent manner (P<0.05).
Furthermore, BQZ decreased the expression of Cbfα1 protein, an
osteoblast marker (22), which
indicated that BQZ could suppress the osteogenic differentiation of
fibroblasts.
In addition, the protein expression levels of Cx43
and pCx43 were suppressed by BQZ. These results indicated that BQZ
may affect the osteogenic differentiation of fibroblasts by
regulating Cx43. Overproduction of BMP-2 was noted in AS patients
(30). BMP-2 is secreted mainly by
osteoblasts, and the high levels of BMP-2 is AS patients may lead
to new bone formation by stimulating the osteogenic differentiation
of fibroblasts (31). Thus,
fibroblasts in the present study were stimulated with rhBMP-2 to
simulate a physiological state comparable to AS. The results
demonstrated that BQZ was able to decrease the level of Cbfα1 and
Cx43/pCx43 in rat fibroblast cells stimulated with rhBMP-2.
The anti-inflammatory chemical celecoxib is a
first-line drug option for patients with AS (11). Numerous lines of evidence have
indicated that celecoxib may slow radiographic progression in
patients with AS, by suppressing fibroblast proliferation and
collagen expression (10,32). Notably, BQZ showed similar effects to
those of celecoxib regarding the inhibition of Cbfα1 and Cx43/pCx43
protein expression. These results may elucidate in part the
mechanisms underlying the anti-AS effect of BQZ, and may lead to
the discovery of novel drugs adding to the available therapeutic
interventions for AS patients.
The study demonstrated that BQZ is able to decrease
the protein levels of Cx43/pCx43 and Cbfα1 in fibroblasts in the
presence or absence of rhBMP-2. These results indicate that BQZ may
exert anti-AS effects via the suppression of the osteogenic
differentiation of fibroblasts by regulating Cx43.
Overall, the present results indicate the anti-AS
effect of BQZ. Thus, this study may provide an experimental basis
for the identification of novel anti-ossification drugs which
inhibit fibroblast proliferation and osteogenic differentiation.
However, it remains unclear whether selective Cx43 blockers could
inhibit Cbfα1 expression in a similar manner to BQZ. Furthermore,
it is not clear which downstream signal transduction pathway(s) of
Cx43 are necessary for the BMP-2/Cbfα1 pathway regulation, and
whether BQZ may exhibit similar effects in vivo. We intend
to validate the present approach in rat models in further
studies.
Acknowledgements
This study was supported by National Natural Science
Foundation of China (grant no. 81273736).
Glossary
Abbreviations
Abbreviations:
|
BQZ
|
Bushen-Qiangdu-Zhilv decoction
|
|
AS
|
ankylosing spondylitis
|
|
HO
|
heterotopic ossification
|
|
Cx43
|
Connexin43
|
|
rhBMP-2
|
recombinant human bone morphogenetic
protein-2
|
|
Cbfα1
|
core binding factor alpha1
|
|
pCx43
|
phosphorylated Connexin43
|
|
BMPs
|
bone morphogenetic proteins
|
|
NSAIDs
|
nonsteroidal anti-inflammatory
drugs
|
|
TNF
|
anti-tumor necrosis factors
|
|
PBS
|
phosphate-buffered saline
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
GJ
|
gap junction
|
References
|
1
|
Wendling D and Claudepierre P: New bone
formation in axial spondyloarthritis. Joint Bone Spine. 80:454–458.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiang YJ and Dai SM: Prevalence of
rheumatic diseases and disability in China. Rheumatol Int.
29:481–490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu F, Cui Y, Zhou X, Zhang X and Han J:
Osteogenic differentiation of human ligament fibroblasts induced by
conditioned medium of osteoclast-like cells. Biosci Trends.
5:46–51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nishimura R, Hata K, Harris SE, Ikeda F
and Yoneda T: Core-binding factor alpha 1 (Cbfa1) induces
osteoblastic differentiation of C2C12 cells without interactions
with Smad1 and Smad5. Bone. 31:303–312. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kopf J, Petersen A, Duda GN and Knaus P:
BMP2 and mechanical loading cooperatively regulate immediate early
signalling events in the BMP pathway. BMC Biol. 10:372012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang J and Wang JH: BMP-2 mediates
PGE(2)-induced reduction of proliferation and osteogenic
differentiation of human tendon stem cells. J Orthop Res. 30:47–52.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen HA, Chen CH, Lin YJ, Chen PC, Chen
WS, Lu CL and Chou CT: Association of bone morphogenetic proteins
with spinal fusion in ankylosing spondylitis. J Rheumatol.
37:2126–2132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hashida Y, Nakahama K, Shimizu K, Akiyama
M, Harada K and Morita I: Communication-dependent mineralization of
osteoblasts via gap junctions. Bone. 61:19–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang HS, Lu XH, Chen DY, Yuan W, Yang LL,
He HL and Chen Y: Upregulated expression of connexin43 in spinal
ligament fibroblasts derived from patients presenting ossification
of the posterior longitudinal ligament. Spine (Phila Pa 1976).
36:2267–2274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haroon N, Kim TH and Inman RD: NSAIDs and
radiographic progression in ankylosing spondylitis Bagging big game
with small arms? Ann Rheum Dis. 71:1593–1595. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Braun J, van den Berg R, Baraliakos X,
Boehm H, Burgos-Vargas R, Collantes-Estevez E, Dagfinrud H,
Dijkmans B, Dougados M, Emery P, et al: 2010 update of the
ASAS/EULAR recommendations for the management of ankylosing
spondylitis. Ann Rheum Dis. 70:896–904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kristensen LE, Jakobsen AK, Askling J,
Nielsson F and Jacobsson LT: Safety of etoricoxib, celecoxib, and
nonselective Nonsteroidal Antiinflammatory Drugs in Ankylosing
spondylitis and other Spondyloarthritis patients: A Swedish
national population-based cohort study. Arthritis Care Res
(Hoboken). 67:1137–1149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Escalas C, Trijau S and Dougados M:
Evaluation of the treatment effect of NSAIDs/TNF blockers according
to different domains in ankylosing spondylitis: Results of a
meta-analysis. Rheumatology (Oxford). 49:1317–1325. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baraliakos X, Haibel H, Listing J, Sieper
J and Braun J: Continuous long-term anti-TNF therapy does not lead
to an increase in the rate of new bone formation over 8 years in
patients with ankylosing spondylitis. Ann Rheum Dis. 73:710–715.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang N, Zhang YZ, Tao QW and Yan XP:
Treatment of ankylosing spondylitis by modified bushen zhuanggu
recipe: A clinical observation. Zhongguo Zhong Xi Yi Jie He Za Zhi.
33:1611–1616. 2013.(In Chinese). PubMed/NCBI
|
|
16
|
Huang RY, Lin JH, He XH, Li X, Lu CL, Zhou
YY, Cai J and He YT: Anti-inflammatory activity of extracts of
Bushen-Qiangdu-Zhilv decoction, a Chinese medicinal formula, in
M1-polarized RAW264.7. BMC Complement Altern Med. 14:2682014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garber JC BRBJ: Guide for the care and use
of laboratory animals. United State: National Academies Press.
2011.PubMed/NCBI
|
|
18
|
Men T, Piao SH and Teng CB: Regulation of
differentiation of mesenchymal stem cells by the Hippo pathway
effectors TAZ/YAP. Yi Chuan. 35:1283–1290. 2013.(In Chinese).
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zouani OF, Rami L, Lei Y and Durrieu MC:
Insights into the osteoblast precursor differentiation towards
mature osteoblasts induced by continuous BMP-2 signaling. Biol
Open. 2:872–881. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Umehara K, Iimura T, Sakamoto K, Lin Z,
Kasugai S, Igarashi Y and Yamaguchi A: Canine oral mucosal
fibroblasts differentiate into osteoblastic cells in response to
BMP-2. Anat Rec (Hoboken). 295:1327–1335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rutherford RB, Moalli M, Franceschi RT,
Wang D, Gu K and Krebsbach PH: Bone morphogenetic
protein-transduced human fibroblasts convert to osteoblasts and
form bone in vivo. Tissue Eng. 8:441–452. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ducy P, Schinke T and Karsenty G: The
osteoblast: A sophisticated fibroblast under central surveillance.
Science. 289:1501–1504. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harada H, Tagashira S, Fujiwara M, Ogawa
S, Katsumata T, Yamaguchi A, Komori T and Nakatsuka M: Cbfa1
isoforms exert functional differences in osteoblast
differentiation. J Biol Chem. 274:6972–6978. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Banerjee C, Hiebert SW, Stein JL, Lian JB
and Stein GS: An AML-1 consensus sequence binds an
osteoblast-specific complex and transcriptionally activates the
osteocalcin gene. Proc Natl Acad Sci USA. 93:4968–4973. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kern B, Shen J, Starbuck M and Karsenty G:
Cbfa1 contributes to the osteoblast-specific expression of type I
collagen genes. J Biol Chem. 276:7101–7107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee MH, Javed A, Kim HJ, Shin HI,
Gutierrez S, Choi JY, Rosen V, Stein JL, van Wijnen AJ, Stein GS,
et al: Transient upregulation of CBFA1 in response to bone
morphogenetic protein-2 and transforming growth factor beta1 in
C2C12 myogenic cells coincides with suppression of the myogenic
phenotype but is not sufficient for osteoblast differentiation. J
Cell Biochem. 73:114–125. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Loiselle AE, Paul EM, Lewis GS and Donahue
HJ: Osteoblast and osteocyte-specific loss of Connexin43 results in
delayed bone formation and healing during murine fracture healing.
J Orthop Res. 31:147–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li S, Zhang H, Li S, Yang Y, Huo B and
Zhang D: Connexin43 and ERK regulate tension-induced signal
transduction in human periodontal ligament fibroblasts. J Orthop
Res. 33:1008–1014. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niger C, Lima F, Yoo DJ, Gupta RR, Buo AM,
Hebert C and Stains JP: The transcriptional activity of osterix
requires the recruitment of Sp1 to the osteocalcin proximal
promoter. Bone. 49:683–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park MC, Park YB and Lee SK: Relationship
of bone morphogenetic proteins to disease activity and radiographic
damage in patients with ankylosing spondylitis. Scand J Rheumatol.
37:200–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen MH, Chen HA, Chen WS, Chen MH, Tsai
CY and Chou CT: Upregulation of BMP-2 expression in peripheral
blood mononuclear cells by proinflammatory cytokines and
radiographic progression in ankylosing spondylitis. Mod Rheumatol.
25:913–918. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li F, Fan C, Zeng B, Zhang C, Chai Y, Liu
S and Ouyang Y: Celecoxib suppresses fibroblast proliferation and
collagen expression by inhibiting ERK1/2 and SMAD2/3
phosphorylation. Mol Med Rep. 5:827–831. 2012.PubMed/NCBI
|