The gastric mucosa is lined by a single layer of
epithelial cells that is supported by delicate elements of loose
connective tissue underlaid by a thin layer of smooth muscle
fibers. In many individuals, the gastric epithelium is exposed not
only to its own acidic and enzymatic secretions, but also to
duodenal bile, highly prevalent Helicobacter pylori (H.
pylori), frequently used non-steroidal anti-inflammatory drugs
(NSAIDs) and alcohol intake (1).
Therefore, gastric mucosal damage is very common and may evolve
into gastric ulcers in many patients. If not treated adequately, a
gastric ulcer may lead to serious complications, such as
perforation and bleeding, or may progress toward gastric cancer
with substantial morbidity and mortality rates (2–4).
Inhibition of acid secretion using proton pump inhibitors and
eradication of H. pylori by treatment with clarithromycin,
amoxicillin and metronidazole, are currently the most widely used
therapeutic regimens for gastric ulcer (5). However, with the side effects of these
therapeutic agents (6,7), the emerging resistance of H.
pylori to antibiotics (8,9), and the
high recurrence rate of gastric ulcer (10–12),
efforts are being directed toward the identification of new
therapeutic modalities.
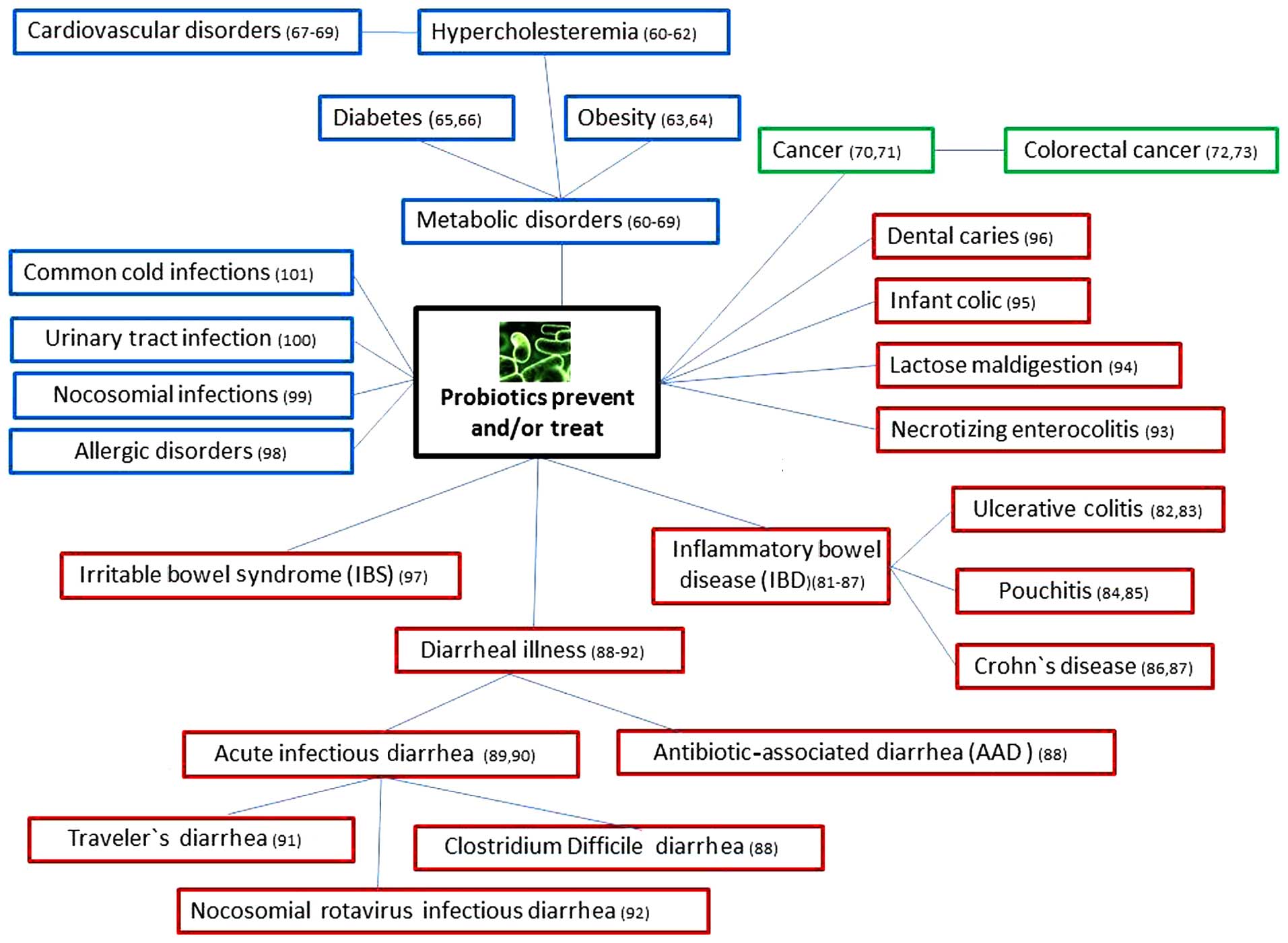
With the increase of their popularity of use in the
prevention and treatment of a number of systemic and
gastrointestinal diseases (Fig. 1),
probiotics have attracted the attention of numerous cell biologists
and clinicians who are interested in exploring their effects on
gastric ulcers and H. pylori. Even though the number of
clinical studies investigating the impact of probiotics on gastric
ulcer is relatively low, a number of experimental studies have
generated promising results. The present review aims to summarize
the available data concerning the potential role of probiotics in
the prevention and healing of gastric ulcer.
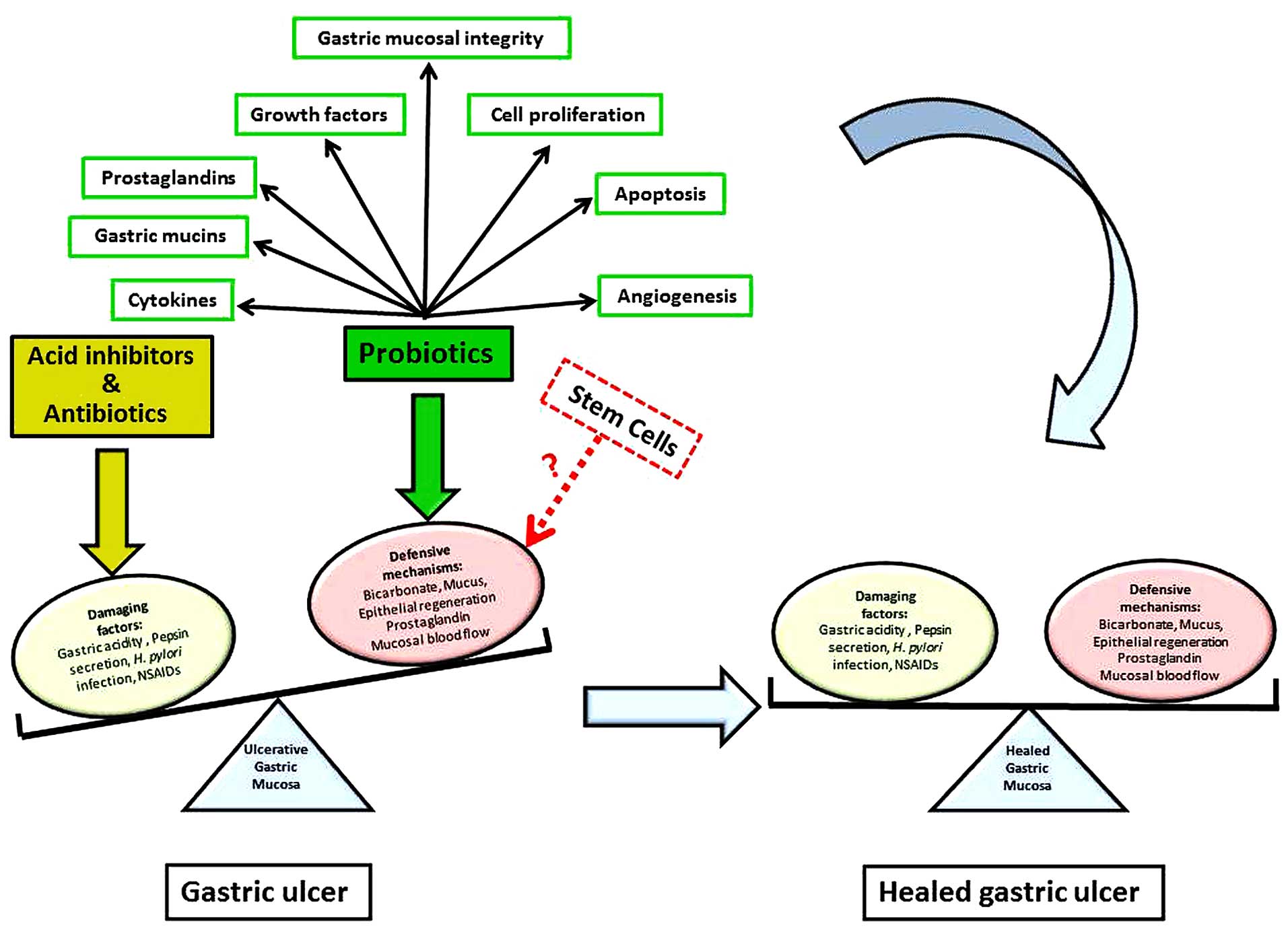
Gastric ulcer is one of the most common and serious
chronic diseases of the upper gastrointestinal tract. The
prevalence of gastric ulcer is 2.4% in the Western population
(13) and may be up to 6.1% in Asia
(14). Despite advancements in
anti-ulcer therapy, the recurrence rate remains high (10–12,15). A
gastric ulcer is a localized deep necrotic lesion involving the
entire mucosal thickness and the muscularis mucosa (16). It is generally considered that these
ulcers develop from an imbalance between mucosal defensive
mechanisms and damaging factors at the luminal surface of the
stomach (1). In developing
countries, the high prevalence of H. pylori, long-term
frequent use of NSAIDs, and cigarette smoking represent the major
risk factors involved in ulcer development (17,18).
Ulcerogenesis starts by disruption of the protective
mucous layer formed by the epithelial cells. Enhanced secretion of
acid and pepsin by parietal and zymogenic cells may contribute to
damage of the mucous layer (1).
Smoking contributes to ulcer formation by upregulating the
production of the proton pump and, therefore, acid secretion
(19). Damage to the mucous layer
may lead to peeling of the surface epithelium and exposure of the
endothelial cells of capillaries in the underlying connective
tissue. Once capillaries are damaged, oxygen and nutrients will be
deficient. As a consequence, hypoxic necrosis will occur in deep
glandular cells, namely stem/progenitor cells, mucous neck cells,
zymogenic cells, enteroendocrine cells and parietal cells.
Moreover, damaged macrophages, mast cells and endothelial cells
release vasoactive agents and pro-inflammatory mediators that
worsen the mucosal microcirculation. Epithelia and connective
tissue necrosis eventually lead to the formation of ulcers
(1,20).
Healing of gastric ulcer involves an orchestrated
array of different mechanisms that work together to correct the
imbalance between damaging and defensive factors in the stomach
(Fig. 2). Healing occurs by
repairing the mucosal defect with epithelial cells and connective
tissue elements, which involves the production of extracellular
matrix, cell proliferation, migration, differentiation and gland
reconstruction. These events are controlled by many factors,
including epidermal growth factor, hepatocyte growth factor,
insulin-like growth factor 1, trefoil factors, cyclooxygenase
2-generated prostaglandin, and several cytokines in a spatially and
temporally coordinated manner (21).
Healing also requires angiogenesis, which is triggered by hypoxia
and involves vascular endothelial growth factor, fibroblast growth
factor and angiopoietins (22). In
addition to local mucosal cells from viable tissue at the ulcer
edge, a study demonstrated that bone marrow-derived stem and
progenitor cells are attracted to the site of injury and contribute
to the regeneration of epithelial and connective tissue components
(23). It has been proposed that the
proliferation of these stem cells is followed by their commitment
to different pathways and differentiation into parietal, surface
mucous, mucous neck and zymogenic cells (24). Mucous neck cells are thought to be
also involved in the healing of gastric ulcer (25,26).
They synthesize and secrete trefoil factor 2, which downregulates
acid secretion by parietal cells and, therefore, promotes mucosal
healing (26).
Cell therapy may have some potential applications in
gastric ulcer treatment. When bone marrow mesenchymal stem cells
were injected (locally or intravenously) in rat models of gastric
ulcer, they were found to promote ulcer healing (27,28).
However, the involved mechanisms are not known and this stem cell
injection method remains to be evaluated. Other studies have
demonstrated the possibility of gastric tissue engineering with the
formation of all cell lineages or only mucous cells using freshly
isolated gastric organoids, isolated gastric stem cells or gastric
stem cell line (29–31). These promising studies require
further evaluation and testing in animal models of gastric
ulcers.
Numerous studies have indicated that probiotics can
be used for the treatment of gastric ulcers. The idea of using
probiotics arose from the study conducted by Elliott et al
in 1998 (32). In a rat model of
acetic acid-induced gastric ulcer, colonization of gram-negative
bacteria occurred rapidly at the site of the ulcer and
significantly impaired ulcer healing. However, colonization by
gram-positive bacteria promoted ulcer healing. Notably,
administration of the exogenous probiotic strain
Lactobacillus accelerated ulcer healing (32).
Historically, the concept of probiotics began around
1900 by the Nobel laureate Elie Metchnikoff who discovered that the
consumption of live bacteria (Lactobacillus bulgaricus) in
yogurt or fermented milk improves the biological features of the
gastrointestinal tract (33,34). The Food and Agriculture Organization
and the International Scientific Association for Probiotics and
Prebiotics define probiotics as live microorganisms which, when
administered in adequate amounts, confer a health benefit on the
host (35).
Several studies have revealed a number of beneficial
effects of certain lactobacilli, such as the suppression of
pathogenic bacteria in the gut and inhibition of allergic,
inflammatory and neoplastic changes (38–41).
Furthermore, it has been shown that lactobacilli are particularly
useful in promoting gastric ulcer healing in rats, when
administered as an individual probiotic strain, such as
Lactobacillus rhamnosus GG (42), Lactobacillus gasseri OLL2716
(43,44), or Lactobacillus acidophilus
(45,46) or as a probiotic mixture, VSL#3
(47). Lactobacillus
rhamnosus GG increases the cellular proliferation to apoptosis
ratio and therefore promotes regeneration of epithelial cells,
particularly at the ulcer margins (42,48). In
clinical studies, a probiotic mixture was demonstrated to be better
than a single strain for improving the characteristics of
indigenous microflora (47,49). In addition to bacteria, certain
yeasts, such as Saccharomyces boulardii, have been
investigated and have shown potential therapeutic effects in a rat
model of ibuprofen-induced gastric ulcer (50,51).
This yeast has neuraminidase activity, which removes sialic acid
residues from the apical membranes of gastric epithelial cells. The
loss of sialic acid prevents the adhesin-mediated binding of H.
pylori to the epithelial cells (52).
To date, >13,438 research articles on probiotics
have appeared in PubMed and ~1,422 articles were published during
2015 alone. Many of these articles report invaluable results
demonstrating the effects of probiotics on the gastrointestinal
tract using in vitro studies, animal models and
healthy/unhealthy volunteers. The main gastrointestinal disorder
targeted by probiotic research is irritable bowel syndrome
(53–55). However, studies assessing the effects
of probiotics on gastric ulcers are relatively limited. This could
be due to the adverse physiological conditions of the host, such as
an acidic environment, digestive enzymes, bile acids and mechanical
stress that attenuate the survival and growth of certain
probiotics. To overcome these conditions, a high dose of multiple
probiotics has been administered (47,56,57), and
probiotics packaged into a suitable delivery system have been
developed (45,46).
The beneficial effects of probiotics depend mainly
on their ability to survive the acidic conditions and the
hydrolytic enzymes and bile content in the stomach and duodenum
(37). Several studies have shown
that the strength of acidity, length of exposure and strain of
probiotic are major factors affecting their survival (58–60).
Among probiotic strains, lactic acid bacteria such as
Lactobacillus and Bifidobacterium exhibit a great
ability to survive gastric transit and, therefore, are extensively
used in many pharmaceutical and dairy probiotic products.
The reason underlying the survival of some probiotic
strains in the stomach has been attributed to F-type ATPase. This
bacterial membrane-bound ATP synthase is responsible for generating
a constant gradient between extra- and intracellular pH for
protection against acidic conditions (65). So, in an acidic environment, the
F0F1-ATPase is upregulated and generates a
proton motive force via proton expulsion and, therefore, increases
the intracellular pH (66). It has
been reported that Lactobacillus acidophilus has a high
cytoplasmic buffering capacity, which allows changes in cytoplasmic
pH and stability in acidic conditions (67). Glucose enhances the survival of
lactobacilli in acidic conditions because glycolysis provides ATP
to F0F1-ATPase, and thereby enables proton
exclusion (68,69).
To overcome the inability of some probiotics to
survive, microencapsulated or coated probiotic strains have been
developed (70–72). Recently, Villena and coworkers
designed gastro-resistant tablets containing Lactobacillus
fermentum CECT5716 using sodium alginate (73). Calcium alginate beads have also been
proposed to protect the delivery of viable probiotic strains in the
gastrointestinal tract (74,75) and have even been used to treat cold
restraint-induced gastric ulcers (46).
In addition to microencapsulation, coating and food
supplements, the use of non-living probiotic strains could also
contribute to overcoming the problem of acid-sensitive probiotic
strains not surviving in the stomach. Even though some viable
probiotic strains do not survive gastric transit, their dead forms
remain beneficial (76). Substantial
evidence from in vitro and animal studies has shown that
both live and dead probiotics can act as biological response
modifiers (76–78). Nonviable probiotics are now known as
‘paraprobiotics’ or ‘ghost probiotics’ (79).
The prophylactic and therapeutic effects of
probiotics in some gastrointestinal and non-gastrointestinal
diseases are summarized in Fig. 1.
In addition to their conventional benefits for gastrointestinal
functions, probiotics have shown potential therapeutic effects in
some metabolic diseases, such as hyperlipidemia or
hypercholesterolemia (85–89), obesity (90,91) and
diabetes (92,93). Therefore, the use of probiotics may
contribute to a reduced risk of atherosclerosis (94) and hypertension (95,96).
In the last few decades, several studies have
suggested a potential role for probiotics in cancer prevention and
therapy (97). Data have shown
specific alterations of the gut microbial composition (dysbiosis)
in patients with colon cancer (98).
Induction of colon cancer in rats using 1,2-dimethylhydrazine is
associated with significant dysbiosis, which could be inhibited by
the oral administration of Lactobacillus salivarius Ren,
leading to effective suppression of colon carcinogenesis (99). In mice, treatment with the probiotics
Clostridium butyricum and Bacillus subtilis has been
found to inhibit the development of 1,2-dimethylhydrazine-induced
colorectal cancer (100). As for
gastric cancer, little is known about the possible association
between probiotics and carcinogenesis. However, some in
vitro studies have demonstrated very promising
anti-proliferative and pro-apoptotic effects of probiotics on
gastric cancer cells (84,101–104).
Moreover, clinical studies have provided evidence for the possible
effects of probiotics in preventing the toxic effects of
chemotherapy and radiation therapy in cancer patients (105,106).
The possible use of probiotics as supplements or
even alternatives to oral antibiotic therapy has been suggested,
especially with increasing cases of resistance to antibiotics. When
frequently and unspecifically used, antibiotics may not only induce
resistance, but also harm the gastrointestinal microflora. In these
cases, the administration of probiotics may restore the normal
microflora, compete with the pathogenic resistant bacteria and,
therefore, help patients to recover (107,108).
Novel approaches have been used to design some
genetically modified probiotic strains with specific capabilities
for the delivery of anti-inflammatory cytokines, vaccines and
anti-pathogenic molecules (109–111).
Engineered Lactococcus lactis strains were produced as live
mucosal vaccines for a large number of antigens derived from
bacteria, viruses and parasites (112). In addition, recombinant strains of
Lactococcus lactis were used to produce the rotavirus
spike-protein subunit VP8 that can prevent rotavirus infection
(113). The future use of
probiotics as vectors targeted to gastrointestinal mucosal lesions
is promising. This new targeted drug delivery approach using
probiotics is known as ‘pharmabiotics’ (35).
There are data suggesting that probiotics could be
useful for gastrointestinal colic, acute infectious diarrhea,
inflammatory bowel syndrome, antibiotic-associated diarrhea,
travelers' diarrhea, lactose malabsorption and inflammatory bowel
diseases (85,86). However, the data available regarding
the possible association between probiotic administration and
gastric ulcer healing and prevention are limited.
Over the last two decades, the use of probiotics in
the management of gastric ulcer has been investigated in a number
of studies. Promising results for studies exploring both
prophylactic (Table I) and
therapeutic (Table II) effects of
probiotics have been obtained. The studies concerning the roles of
probiotics in gastric ulcer healing reported in the literature were
mainly conducted in rats. These studies were based on the use of
either individual probiotic strains, such as Lactobacillus
rhamnosus GG (42,48), Lactobacillus gasseri OLL2716
(44), Lactobacillus
acidophilus (45,46), Escherichia coli Nissle 1917
(114), Bifidobacterium
animalis VKL/VKB (115),
Bifidobacterium bifidum/brevis (116) and Saccharomyces boulardii
(51), or a mixture of probiotic
strains, such as VSL#3 (47). A
number of studies have reported that probiotics not only inhibit
the development of acute gastric mucosal lesions, but also
accelerate the process of healing of induced gastric ulcers
(42,44,47). The
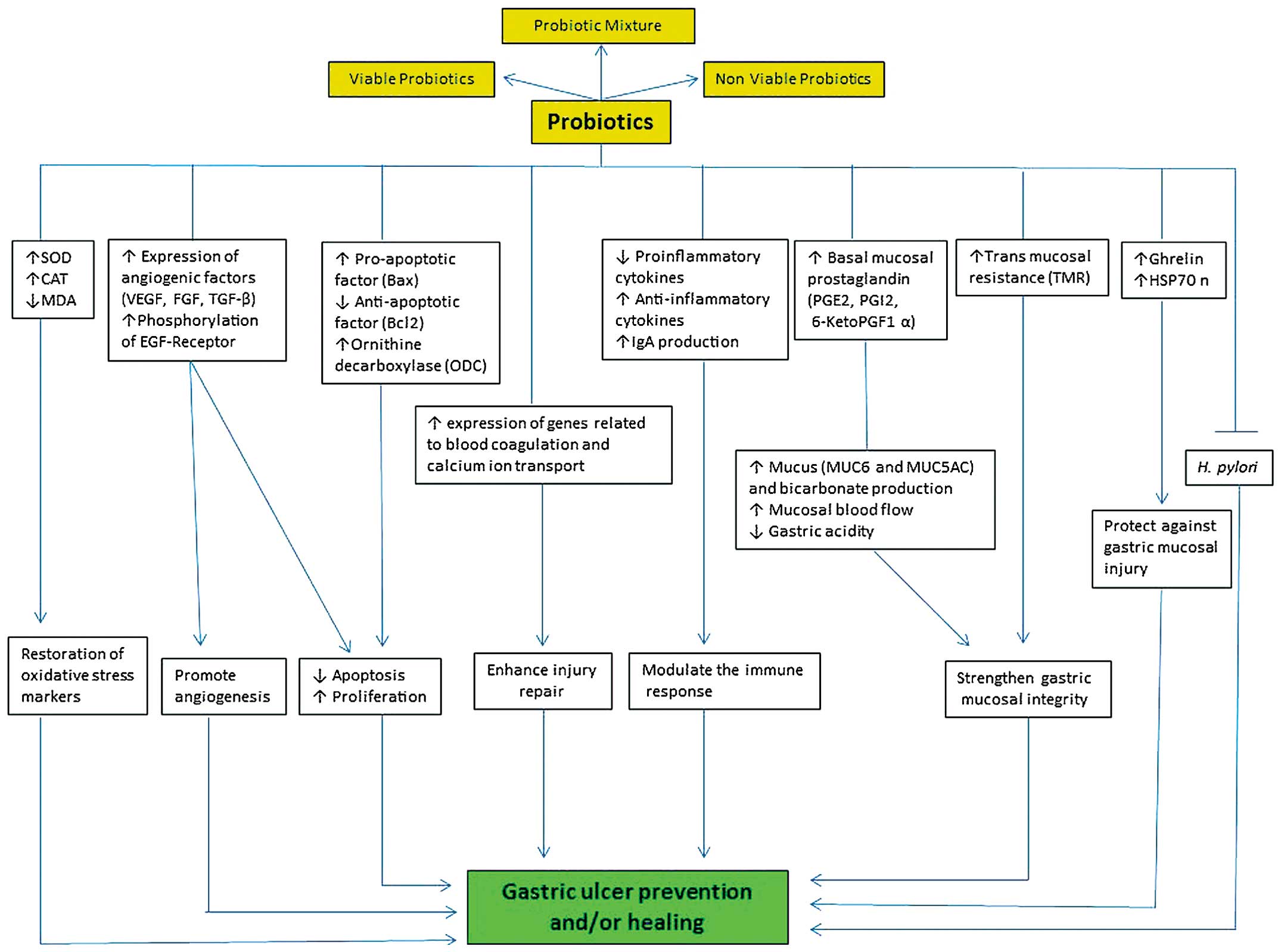
effects of probiotics on gastric ulcer are attributed to several
cellular and molecular mechanisms (Fig.
3).
In a normal stomach, the mucosal integrity is
maintained by three main barriers (117,118).
i) The preepithelial barrier is made of a
mucus-bicarbonate-phospholipid layer located between the gastric
lumen and the epithelium. ii) The epithelial barrier characterized
by a) a continuous sheet of surface epithelial cells connected by
tight junctions and generating different secretory products
including trefoil factors, prostaglandins, and heat shock proteins,
and b) continuous cell renewal accomplished by proliferation of
stem/progenitor cells and regulated by different mechanisms
involving growth factors, prostaglandins, gastrin and the
anti-apoptotic protein survivin. iii) The subepithelial barrier
composed of a) microcirculation through capillaries maintained by
the continuous generation of prostaglandins, nitric oxide and
hydrogen sulfide that protect endothelial cells from injury and
prevent aggregation of platelets and leukocytes, and b) sensory
innervations that regulate the mucosal blood flow (117,118).
When one or more of the above listed defensive
mechanisms is altered, the gastric mucosal barrier is disrupted and
a gastric ulcer may develop. The beneficial effects for probiotics
on the gastrointestinal mucosa may occur via two main mechanisms
(119–121). i) Antagonistic action achieved
through lactic acid or antimicrobial compounds that inhibit the
growth of pathogenic bacteria (122,123)
or by competing for the available nutrients and growth factors and,
therefore, inhibit the growth of pathogens or block their adhesion
to gastric epithelial cells (124,125).
ii) Immunomodulatory activity which involves the induction of
phagocytosis, secretion of immunoglobulin A (IgA), activation of
natural killer cells, stimulation of protective cytokines,
downregulation of proinflammatory cytokines, and modulation of T
cell responses (Th1 induction and Th2 attenuation) (126–129).
Probiotics can also protect the integrity of the
gastric mucosal barrier by upregulating prostaglandin, mucous
secretion, tight junction protein expression and cell
proliferation, and by inhibiting apoptosis (43,48,130–132).
In rats, the administration of Bifidobacterium bifidum BF-1
or Bifidobacterium animalis VKL and VKB has been found to
protect the gastric mucosa through either preventing the mucous
barrier from degradation (115) or
increasing gastric mucous production (133). The probiotic mixture VSL#3 protects
the epithelial barrier and upregulates the expression of tight
junction proteins (occludin and zonula occludens-1) in vivo
and in vitro via the activation of p38 or mitogen-activated
protein (MAP) kinase and extracellular signal-regulated kinase
(ERK) signaling pathways (134).
Mennigen et al demonstrated that probiotics can strengthen
the gastric mucosal barrier by inhibiting the redistribution and
expression of tight junction proteins and blocking apoptosis
(135). The probiotic strains
Lactobacillus gasseri OLL2716, Lactobacillus
rhamnosus GG and Escherichia coli Nissle 1917 are able
to protect the altered gastric mucosal barrier (43,48,114).
In humans, Gotteland et al found that pretreatment with
Lactobacillus GG protected against indomethacin-induced
disruption of the gastric mucosal barrier (131).
Recently, three mouse models of induced gastric
ulcers using alcohol, restraint cold stress and pyloric ligation
were investigated. Pretreatment of these mice with the probiotic
bacterial strain Clostridium butyricum alleviated the
histopathological changes, specifically, the infiltration of
inflammatory cells and gastric mucosal damage (136). Moreover, the same study showed that
this bacterium alleviated oxidative stress damage by inhibiting the
activity of superoxide dismutase and catalases and decreasing
malondialdehyde levels. These results were similar to those
obtained with omeprazole pretreatment (136).
Prostaglandins are involved in the ulcer healing
process by inhibiting acid secretion, stimulating the production of
mucus, bicarbonate and phospholipids, increasing blood flow and
accelerating epithelial restitution (119). Therefore, prostaglandins are also
thought to be a target for the prophylactic effect of probiotics in
gastric ulcers (43,48,114).
Ethanol-induced gastric mucosal lesions in rats were prevented by
pretreatment with the probiotic strain Lactobacillus
rhamnosus GG through the upregulation of prostaglandin E2
(48). The effectiveness of the
probiotic strain Escherichia coli Nissle 1917 in preventing
stress-induced ulcers in rats has also been reported. This effect
was achieved through induction of mucosal anti-inflammatory
cytokines, synthesis of gastric mucosal protective factors (ghrelin
and heat shock protein 70), enhancement of gastric
microcirculation, and involvement of prostaglandins and nitric
oxide (114).
Uchida and Karakazu demonstrated that pretreatment
of rats with LG21 yogurt containing Lactobacillus gasseri
OLL2716 significantly inhibited the formation of acetic
acid-induced gastric ulcers in a dose dependent manner. This effect
was mediated by increasing the production of mucosal prostaglandin
E2/I2. Notably, the gastro-protective effect of prostaglandin was
attenuated by pretreatment of the rats with indomethacin, which is
known to inhibit prostaglandin (43). A few years later, the same authors
demonstrated that the administration of the same Lactobacillus
gasseri OLL2716 yogurt for 10 days significantly accelerated
the healing of chronic gastric ulcers through the stimulation of
prostaglandin production (44).
However, yogurt containing gamma-ray-exposed Lactobacillus
gasseri OLL2716 increased the generation of prostaglandin
without affecting the healing of the acetic acid-induced gastric
ulcers. These findings indicate that the increased production of
prostaglandin does not necessarily explain the therapeutic effect
of LG21 yogurt on ulcer healing (44). Recently, pretreatment with the
probiotic Clostridium butyricum in mouse models of induced
gastric ulcer caused a reduction in the level of
6-keto-prostaglandin F1α, the stable metabolite of prostaglandin I2
(136).
Aside from the probiotic bacteria itself, the
polysaccharide fractions can also exert a gastroprotective effect
against gastric ulcers. Nagaoka et al demonstrated that
polysaccharides of Bifidobacterium breve YIT4014 and 4043I
and Bifidobacterium bifidum YIT4007 were able to repair and
protect the mucosa of rats against acetic acid- and ethanol-induced
gastric ulcers and erosions. The polysaccharides of these probiotic
mixtures were found to increase the expression of growth factors
such as fibroblast growth factor and epidermal growth factor in
addition to 6-ketoprostaglandin F1 (116).
Recent studies on stress-induced gastric mucosal
lesions demonstrated that using a mixture of probiotics
(Lactobacillus, Lactococcus, Bifidobacterium,
Propionibacterium and Acetobacter) enhanced ulcer
healing by restoring the balance between pro- and anti-oxidants in
the gastric mucosa (137). In
addition, probiotic mixtures (comprising Bifidobacterium
animalis VKL and VKB with or without Lactobacillus casei
IMVB-7280) enhanced the recovery of stress hormones
(adrenocorticotropin and corticosterone), decreased proinflammatory
cytokines and increased anti-inflammatory cytokines (138). Moreover, pretreatment with
Clostridium byturicum attenuated the elevation of
proinflammatory factors (IL-1β, TNF-α and leukotriene B4) in mice
with induced gastric ulcer (136).
Probiotics are not only effective against gastric
ulcers induced by acetic acid, ethanol or stress, but also play
important roles in the prevention or treatment of ulcers induced by
NSAIDs, such as aspirin or indomethacin (139). In aspirin-treated rats, TNF
upregulates neutrophil-derived superoxide leading to oxygen
radical-mediated tissue damage (140,141).
Thus, this pro-inflammatory cytokine is an ideal target for
protection against gastric ulcer. In this context, using a rat
model of asprin-or ethanol-induced gastric mucosal damage, it was
found that using a probiotic mixture of 13 bacterial strains
composed of four strains of Lactobacillus fermentum
(BB16–75, AK2–8, AK5–22, AK6–26), three strains of Lactobacillus
plantarum (AA17–73, AK7–28, AK8–31B) and six strains of
Enterococcus faecium (AB6–21, AB16–68, AK4–120, AK7–31,
BK9–40, BK13–54) caused a reduction in mucosal damage scores, lipid
peroxidation, malondialdehyde content and pro-inflammatory cytokine
levels. In addition, these probiotics also induced an increase in
mucosal secretory IgA production and the stabilization of mucosal
mast cells (142,143).
Mucus is a cohesive mixture of ~95% water, 5% mucin
glycoprotein molecules, salts, immunoglobulins, cellular and serum
macromolecules, and trefoil peptides (144,145).
Mucus on the luminal surface of gastric mucosa forms two main
layers: The outer loosely adherent mucus and the inner firmly
adherent mucus. The former plays a role in binding luminal noxious
agents, absorbing nitrite and releasing nitric oxide. The latter is
important for protection against digestive enzymes and corrosive
acid (146).
There are several mucin genes encoding secreted and
transmembrane mucins, such as MUC1, MUC2, MUC3, MUC4, MUC5AC,
MUC5B, MUC6–8, MUC11, MUC12 and MUC16. The stomach has two distinct
cell types secreting different mucins: Surface mucus cells
secreting MUC5AC and mucus neck cells secreting MUC6 (147). Transmembrane mucins (MUC1, MUC4 and
MUC16) are mainly involved in signal transduction and cell adhesion
phenomena (148). Another
noteworthy class of secretory proteins is the trefoil peptides.
These are produced and secreted together with mucins, and thus are
present in fairly high concentrations in the mucous gel layer and
in the mucosal epithelial cells (145). They are intimately associated with
mucus to improve protection against noxious agents. They are
upregulated during mucosal injury and have been implicated in
promoting cell migration and the repair process (149–151).
Some studies have shown that probiotics promote
mucous secretion. Treatment of colonic epithelial Caco-2 cells or
colorectal HT29 cells with probiotics increased the expression of
mucins (154–156). Administration of VSL#3 to rats for
7 days was enough to induce a 60-fold increase in MUC2 expression
and its concomitant secretion (157). Probiotics can also adhere to mucus
via specific binding proteins and eventually modulate the immune
system for protection against pathogens (158,159).
In the stomach, the available studies on the effects of probiotics
on mucous production have demonstrated different results.
Pretreatment with Bifidobacterium BF-1 upregulates MUC5AC
gene expression and enhances the production of mucus by surface
mucous cells in rats with acute gastric lesions induced by
acid/ethanol (133). However, the
expression of MUC5AC was moderately upregulated or unchanged,
respectively, in VSL#3- or Lactobacillus rhamnosus
GG-treated rats with ethanol-induced gastric mucosal lesions
(47,48). Even though the MUC5AC gene is
responsible for the most abundantly produced mucin in the normal
mucosa, Lam and colleagues reported that pretreatment of rats with
Lactobacillus rhamnosus GG caused upregulation of MUC6 mRNA
expression (specific for mucous neck cells) in gastric mucosal
lesions induced by ethanol (48).
Moreover, it was demonstrated that pretreatment with a probiotic
mixture (Bifidobacterium animalis VKL and VKB) in rats with
stress-induced gastric mucosal erosion and ulcer, prevented
degradation of the mucous layer (115).
Perpetual cell renewal is an important epithelial
factor required for the maintenance of the gastric mucosal barrier.
The dynamics and cells involved in this physiological phenomenon
have been defined in rodents and humans (160,161).
Several factors and cell types in the corpus region of the stomach
are involved in the regulation of this renewal process including
enteroendocrine cells (Karam and Al-Menhali, unpublished data) and
parietal cells (162). Some studies
have also explored the effects of probiotics on cellular
proliferation and apoptosis. Pretreatment of rats with
Lactobacillus rhamnosus GG significantly reduced the number of
apoptotic cells in ethanol-induced gastric mucosal lesions
(48). The reduction of apoptosis is
controlled by upregulation of the anti-apoptotic protein, B cell
lymphoma 2 (42). Further
investigations revealed that the same Lactobacillus probiotic
strain not only inhibits the apoptosis of gastric mucosal cells,
but also stimulates gastric cell proliferation, which is mediated
by ornithine decarboxylase (42).
Induction of angiogenesis is one of the most
important effects of probiotics on gastric ulcers (42,47).
Vascular endothelial growth factor is required to stimulate the
formation of granulation tissue and development of new microvessels
(163). Administration of the
probiotic mixture VSL#3, composed of eight probiotic strains: four
Lactobacilli (acidophilus, bulgaricus, casei and plantarum), three
Bifidobacteria (breve, infantis and longum) and Streptococcus
accelerates gastric ulcer healing in a rat model by upregulating
the expression and production of vascular endothelial growth
factor. This ulcer-healing effect was confirmed using neutralizing
antibodies (47). In another study,
administration of the probiotic strain Lactobacillus rhamnosus GG
to rats with acetic acid-induced gastric ulcers led to a
significant increase in the number of blood microvessels (42). Notably, this angiogenic effect was
observed only at the edge of damaged gastric mucosa and not in the
surrounding normal tissues. Therefore, Lactobacillus rhamnosus GG
is a potential therapeutic agent for promoting vascularization and
gastric ulcer healing and requires further clinical
investigation.
Since the harsh physiological conditions in the
stomach may interfere with the colonization of some probiotic
strains, efforts have been directed toward packing probiotics into
a suitable delivery system. A novel synbiotic approach using
Lactobacillus acidophilus encapsulated with ginger extract
(45) or loaded in alginate floating
beads (46), significantly enhanced
the healing of gastric stress-induced ulcers in rats. This was
evidenced by the restoration of various biochemical (lipid
peroxidation, catalase and superoxide dismutase), physiological
(mucous content) and histological (ulcer index and hemorrhagic
streaks) changes. Moreover, histopathological studies have
indicated that the administration of Lactobacillus
acidophilus encapsulated with ginger extract leads to complete
recovery from gastric ulcer with no signs of inflammation or
mucosal damage visible at the ulcer edge (45,46).
The effects of probiotics on angiogenesis are not
restricted to bacterial strains. Yeast, such as Saccharomyces
boulardii, has been reported to have potential in the treatment
and prevention of gastric ulcer induced by ibuprofen in rats
(51). More recently, it was
demonstrated using DNA microarray that thioredoxin derived from
edible yeast, Saccharomyces cerevisiae, can protect the
gastric mucosa by up- or downregulating hundreds of genes involved
in the healing of the ulcerative mucosa induced by stress or
HCl/ethanol in rats (164).
For a long time, gastric ulcers were considered to
be a result of stress, improper diet and NSAID usage. However, the
discovery of H. pylori and its association with gastric
ulcers has changed the gastroenterological practice worldwide
(165). H. pylori can
uniquely survive and colonize in the harsh acidic environment of
the stomach for decades, leading to progressive inflammatory,
ulcerative and neoplastic changes (166). Among patients infected by H.
pylori, 10–20% may ultimately develop ulcers (16). Recent regression of ulcer incidence
is highly dependent on the worldwide eradication of H.
pylori (167).
In some countries, the marked rise in resistance to
clarithromycin has caused a steady decline in the efficiency of the
standard triple therapy (169,170).
To overcome this problem, new regimens including quadruple,
sequential, concomitant, dual and rescue therapies have been
introduced (168). However, the
development of resistance to antibiotics and their side effects has
caused poor patient compliance and, therefore, has limited their
applications (171).
During the last decade, numerous studies have
explored the possible use of probiotics to improve the protocol of
H. pylori eradication and to prevent its side effects
(172–176). The use of probiotics has also been
tested in asymptomatic H. pylori-infected patients and found
to lower the risk of gastric ulcer development (177). Kabir and co-workers were one of the
first groups to report that probiotic strain Lactobacillus
salivarus can prevent and eliminate H. pylori
colonization in the stomach of gnotobiotic BALB/c mice (178).
The immunological effects of probiotics include: i)
Maintaining the balance between pro- and anti-inflammatory
cytokines, which leads to recovery from gastritis (188), ii) downregulating the production of
IL-8 and TNF-α by producing conjugated linoleic acid that targets
the nuclear factor κB pathway (189,190),
iii) upregulating the anti-inflammatory suppressor of cytokine
signaling through activation of signal transducer and activator of
transcription (STAT)-1/STAT-3 transcription factors and
inactivation of Janus kinase 2 (191), and iv) enhancing gastric mucosal
barrier by stimulating IgA secretion and transport (181).
Gastric ulcers develop due to an imbalance between
damaging factors and the defense mechanisms of the gastric mucosa
(Fig. 2). The available studies in
the literature indicate that probiotics can accelerate the healing
of gastric ulcers via multiple mechanisms that involve both
damaging and defensive factors (Fig.
2). Even though only limited in vivo studies have
explored the impact of probiotics in gastric ulcer, some cellular
and molecular findings have suggested their protective and
therapeutic effects (Fig. 2).
Several studies also identified probiotic strains effective in
H. pylori eradication via immunological and
non-immunological mechanisms. Therefore, the use of probiotics in
the management of gastric ulcer appears promising and further
studies are required. Taking in consideration the probiotic
strains, dosage, commercial preparations and the heterogeneity of
patients, a combined clinical and basic science experimental
approach is likely to yield important strategies to optimize the
use of probiotics in health and disease (202).
This study is supported by the Emirates Foundation,
Grant no. 2010/146 and The Sheikh Hamdan Award, Grant no.
MRG50/2011-12.
|
1.
|
Tarnawski A, Ahluwalia A and Jones MK:
Gastric cytoprotection beyond prostaglandins: Cellular and
molecular mechanisms of gastroprotective and ulcer healing actions
of antacids. Curr Pharm Des. 19:126–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lau JY, Barkun A, Fan DM, Kuipers EJ, Yang
YS and Chan FK: Challenges in the management of acute peptic ulcer
bleeding. Lancet. 381:2033–2043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Hwang JJ, Lee DH, Lee AR, Yoon H, Shin CM,
Park YS and Kim N: Characteristics of gastric cancer in peptic
ulcer patients with Helicobacter pylori infection. World J
Gastroenterol. 21:4954–4960. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Taş İ, Ülger BV, Önder A, Kapan M and
Bozdağ Z: Risk factors influencing morbidity and mortality in
perforated peptic ulcer disease. Ulus Cerrahi Derg. 31:20–25.
2014.PubMed/NCBI
|
|
5.
|
Malfertheiner P: Helicobacter
pylori infection-management from a European perspective. Dig
Dis. 32:275–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Wallace JL, Syer S, Denou E, de Palma G,
Vong L, McKnight W, Jury J, Bolla M, Bercik P, Collins SM, et al:
Proton pump inhibitors exacerbate NSAID-induced small intestinal
injury by inducing dysbiosis. Gastroenterology. 141:1314–1322.e5.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Karam SM and Forte JG: Inhibiting gastric
H(+)-K(+)-ATPase activity by omeprazole promotes degeneration and
production of parietal cells. Am J Physiol. 266:G745–G758.
1994.PubMed/NCBI
|
|
8.
|
Ghotaslou R, Leylabadlo HE and Asl YM:
Prevalence of antibiotic resistance in Helicobacter pylori:
A recent literature review. World J Methodol. 5:164–174. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Howden CW, Chey WD and Vakil NB: Clinical
rationale for confirmation testing after treatment of
Helicobacter pylori infection: Implications of rising
antibiotic resistance. Gastroenterol Hepatol (NY). 10(7 Suppl 3):
S1–S19. 2014.
|
|
10.
|
Tarnawski A, Hollander D, Krause WJ,
Dabros W, Stachura J and Gergely H: ‘Healed’ experimental gastric
ulcers remain histologically and ultrastructurally abnormal. J Clin
Gastroenterol. 12(Suppl 1): S139–S147. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Watanabe T, Higuchi K, Tominaga K,
Fujiwara Y and Arakawa T: Acid regulates inflammatory response in a
rat model of induction of gastric ulcer recurrence by interleukin
1beta. Gut. 48:774–781. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Arakawa T, Watanabe T, Tanigawa T,
Tominaga K, Fujiwara Y and Morimoto K: Quality of ulcer healing in
gastrointestinal tract: Its pathophysiology and clinical relevance.
World J Gastroenterol. 18:4811–4822. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Groenen MJ, Kuipers EJ, Hansen BE and
Ouwendijk RJ: Incidence of duodenal ulcers and gastric ulcers in a
Western population: Back to where it started. Can J Gastroenterol.
23:604–608. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Sung JJ, Kuipers EJ and El-Serag HB:
Systematic review: The global incidence and prevalence of peptic
ulcer disease. Aliment Pharmacol Ther. 29:938–946. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Fujino S, Suzuki Y and Tanaka T:
Cost-benefit analysis of medicinal treatment for gastric ulcers.
Long-term model including healing and recurrence. Health Policy.
5:45–72. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Malfertheiner P, Chan FK and McColl KE:
Peptic ulcer disease. Lancet. 374:1449–1461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hirayama F, Takagi S, Kusuhara H, Iwao E,
Yokoyama Y and Ikeda Y: Induction of gastric ulcer and intestinal
metaplasia in mongolian gerbils infected with Helicobacter
pylori. J Gastroenterol. 31:755–757. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wang GZ, Huang GP, Yin GL, Zhou G, Guo CJ,
Xie CG, Jia BB and Wang JF: Aspirin can elicit the recurrence of
gastric ulcer induced with acetic acid in rats. Cell Physiol
Biochem. 20:205–212. 2007.PubMed/NCBI
|
|
19.
|
Hammadi M, Adi M, John R, Khoder GA and
Karam SM: Dysregulation of gastric H,K-ATPase by cigarette smoke
extract. World J Gastroenterol. 15:4016–4022. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Tarnawski AS: Cellular and molecular
mechanisms of gastrointestinal ulcer healing. Dig Dis Sci. 50(Suppl
1): S24–S33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Tarnawski A, Szabo IL, Husain SS and
Soreghan B: Regeneration of gastric mucosa during ulcer healing is
triggered by growth factors and signal transduction pathways. J
Physiol Paris. 95:337–344. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Tarnawski AS and Ahluwalia A: Molecular
mechanisms of epithelial regeneration and neovascularization during
healing of gastric and esophageal ulcers. Curr Med Chem. 19:16–27.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Okamoto R, Yajima T, Yamazaki M, Kanai T,
Mukai M, Okamoto S, Ikeda Y, Hibi T, Inazawa J and Watanabe M:
Damaged epithelia regenerated by bone marrow-derived cells in the
human gastrointestinal tract. Nat Med. 8:1011–1017. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Karam SM: Lineage commitment and
maturation of epithelial cells in the gut. Front Biosci.
4:D286–D298. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Helander HF and Poorkhalkali N: Parietal
cell density during gastric ulcer healing in the rat. Scand J
Gastroenterol. 39:20–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Farrell JJ, Taupin D, Koh TJ, Chen D, Zhao
CM, Podolsky DK and Wang TC: TFF2/SP-deficient mice show decreased
gastric proliferation, increased acid secretion, and increased
susceptibility to NSAID injury. J Clin Invest. 109:193–204. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Liu L, Chiu PW, Lam PK, Poon CC, Lam CC,
Ng EK and Lai PB: Effect of local injection of mesenchymal stem
cells on healing of sutured gastric perforation in an experimental
model. Br J Surg. 102:e158–e168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Chang Q, Yan L, Wang CZ, Zhang WH, Hu YZ
and Wu BY: In vivo transplantation of bone marrow mesenchymal stem
cells accelerates repair of injured gastric mucosa in rats. Chin
Med J (Engl). 125:1169–1174. 2012.PubMed/NCBI
|
|
29.
|
Maemura T, Shin M and Kinoshita M: Tissue
engineering of the stomach. J Surg Res. 183:285–295. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Sato T and Clevers H: SnapShot: Growing
organoids from stem cells. Cell. 161:1700–1700.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Pulikkot S, Greish YE, Mourad AH and Karam
SM: Establishment of a three-dimensional culture system of gastric
stem cells supporting mucous cell differentiation using
microfibrous polycaprolactone scaffolds. Cell Prolif. 47:553–563.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Elliott SN, Buret A, McKnight W, Miller MJ
and Wallace JL: Bacteria rapidly colonize and modulate healing of
gastric ulcers in rats. Am J Physiol. 275:G425–G432.
1998.PubMed/NCBI
|
|
33.
|
Podolsky SH: Metchnikoff and the
microbiome. Lancet. 380:1810–1811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Lilly DM and Stillwell RH: Probiotics:
Growth-promoting factors produced by microorganisms. Science.
147:747–748. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Hill C, Guarner F, Reid G, Gibson GR,
Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S,
et al: Expert consensus document. The International Scientific
Association for Probiotics and Prebiotics consensus statement on
the scope and appropriate use of the term probiotic. Nat Rev
Gastroenterol Hepatol. 11:506–514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Wallace TC, Guarner F, Madsen K, Cabana
MD, Gibson G, Hentges E and Sanders ME: Human gut microbiota and
its relationship to health and disease. Nutr Rev. 69:392–403. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Bezkorovainy A: Probiotics: Determinants
of survival and growth in the gut. AmJ Clin Nutr. 73(Suppl 2):
S399–S405. 2001.
|
|
38.
|
Isolauri E, Sütas Y, Kankaanpää P,
Arvilommi H and Salminen S: Probiotics: Effects on immunity. Am J
Clin Nutr. 73(Suppl 2): S444–S450. 2001.
|
|
39.
|
Hong WS, Chen YP and Chen MJ: The
antiallergic effect of kefir lactobacilli. J Food Sci.
75:H244–H253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Cain AM and Karpa KD: Clinical utility of
probiotics in inflammatory bowel disease. Altern Ther Health Med.
17:72–79. 2011.PubMed/NCBI
|
|
41.
|
Shyu PT, Oyong GG and Cabrera EC:
Cytotoxicity of probiotics from Philippine commercial dairy
products on cancer cells and the effect on expression of cfos and
cjun early apoptotic-promoting genes and interleukin-1β and tumor
necrosis factor-α proinflammatory cytokine genes. Biomed Res Int.
2014:4917402014. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Lam EK, Yu L, Wong HP, Wu WK, Shin VY, Tai
EK, So WH, Woo PC and Cho CH: Probiotic Lactobacillus
rhamnosus GG enhances gastric ulcer healing in rats. Eur J
Pharmacol. 565:171–179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Uchida M and Kurakazu K: Yogurt containing
Lactobacillus gasseri OLL2716 exerts gastroprotective action
against acute gastric lesion and antral ulcer in rats. J Pharmacol
Sci. 96:84–90. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Uchida M, Shimizu K and Kurakazu K: Yogurt
containing Lactobacillus gasseri OLL 2716 (LG21 yogurt)
accelerated the healing of acetic acid-induced gastric ulcer in
rats. Biosci Biotechnol Biochem. 74:1891–1894. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Singh PK and Kaur IP: Synbiotic (probiotic
and ginger extract) loaded floating beads: A novel therapeutic
option in an experimental paradigm of gastric ulcer. J Pharm
Pharmacol. 64:207–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Singh PK, Deol PK and Kaur IP: Entrapment
of Lactobacillus acidophilus into alginate beads for the
effective treatment of cold restraint stress induced gastric ulcer.
Food Funct. 3:83–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Dharmani P, De Simone C and Chadee K: The
probiotic mixture VSL#3 accelerates gastric ulcer healing by
stimulating vascular endothelial growth factor. PLoS One.
8:586712013. View Article : Google Scholar
|
|
48.
|
Lam EK, Tai EK, Koo MW, Wong HP, Wu WK, Yu
L, So WH, Woo PC and Cho CH: Enhancement of gastric mucosal
integrity by Lactobacillus rhamnosus GG. Life Sci.
80:2128–2136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Timmerman HM, Koning CJ, Mulder L,
Rombouts FM and Beynen AC: Monostrain, multistrain and multispecies
probiotics - A comparison of functionality and efficacy. Int J Food
Microbiol. 96:219–233. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Flatley EA, Wilde AM and Nailor MD:
Saccharomyces boulardii for the prevention of hospital onset
Clostridium difficile infection. J Gastrointestin Liver Dis.
24:21–24. 2015.PubMed/NCBI
|
|
51.
|
Girard P, Coppé MC, Pansart Y and
Gillardin JM: Gastroprotective effect of Saccharomyces
boulardii in a rat model of ibuprofen-induced gastric ulcer.
Pharmacology. 85:188–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Sakarya S and Gunay N: Saccharomyces
boulardii expresses neuraminidase activity selective for
α2,3-linked sialic acid that decreases Helicobacter pylori
adhesion to host cells. APMIS. 122:941–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Aragon G, Graham DB, Borum M and Doman DB:
Probiotic therapy for irritable bowel syndrome. Gastroenterol
Hepatol (NY). 6:39–44. 2010.
|
|
54.
|
Brenner DM, Moeller MJ, Chey WD and
Schoenfeld PS: The utility of probiotics in the treatment of
irritable bowel syndrome: A systematic review. Am J Gastroenterol.
104:1033–1050. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Moayyedi P, Ford AC, Talley NJ, Cremonini
F, Foxx-Orenstein AE, Brandt LJ and Quigley EM: The efficacy of
probiotics in the treatment of irritable bowel syndrome: A
systematic review. Gut. 59:325–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Ki Cha B, Mun Jung S, Hwan Choi C, Song
ID, Woong Lee H, Joon Kim H, Hyuk J, Kyung Chang S, Kim K, Chung WS
and Seo JG: The effect of a multispecies probiotic mixture on the
symptoms and fecal microbiota in diarrhea-dominant irritable bowel
syndrome: A randomized, double-blind, placebo-controlled trial. J
Clin Gastroenterol. 46:220–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Lyra A, Krogius-Kurikka L, Nikkilä J,
Malinen E, Kajander K, Kurikka K, Korpela R and Palva A: Effect of
a multispecies probiotic supplement on quantity of irritable bowel
syndrome-related intestinal microbial phylotypes. BMC
Gastroenterol. 10:1102010. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Fuochi V, Petronio GP, Lissandrello E and
Furneri PM: Evaluation of resistance to low pH and bile salts of
human Lactobacillus spp. isolates. Int J Immunopathol
Pharmacol. 28:426–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Pochart P, Marteau P, Bouhnik Y, Goderel
I, Bourlioux P and Rambaud JC: Survival of bifidobacteria ingested
via fermented milk during their passage through the human small
intestine: An in vivo study using intestinal perfusion. Am J Clin
Nutr. 55:78–80. 1992.PubMed/NCBI
|
|
60.
|
Lankaputhra WEV and Shah NP: Survival of
Lactobacillus acidophilus and Bifidobacterium in the
presence of acid and bile salts. Cult Dairy Prod J. 30:2–7.
1995.
|
|
61.
|
Costello M: Probiotic foods. Foodpro-93:
International Food Processing Machinery and Technology Exhibition
and Conference (Australia). Sydney Convention & Exhibition
Centre. July 12–14–1993.
|
|
62.
|
Clark PA, Cotton LN and Martin JH:
Selection of Bifidobacterium spp. for use as dietary
adjuncts in cultured dairy foods: II. Tolerance to simulated pH of
human stomachs. Cult Dairy Prod J. 28:11–14. 1993.
|
|
63.
|
Jacobsen CN, Rosenfeldt Nielsen V, Hayford
AE, Møller PL, Michaelsen KF, Paerregaard A, Sandström B, Tvede M
and Jakobsen M: Screening of probiotic activities of forty-seven
strains of Lactobacillus spp. by in vitro techniques and
evaluation of the colonization ability of five selected strains in
humans. Appl Environ Microbiol. 11:4949–4956. 1999.
|
|
64.
|
Lick S, Drescher K and Heller JK: Survival
of Lactobacillus delbrueckii subsp. bulgaricus and
Streptococcus thermophilus in the terminal ileum of
fistulated Göttingen minipigs. Appl Environ Microbiol.
67:4137–4143. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Cotter PD and Hill C: Surviving the acid
test: Responses of gram-positive bacteria to low pH. Microbiol Mol
Biol Rev. 67:429–453. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
66.
|
Fortier LC, Tourdot-Maréchal R, Diviès C,
Lee BH and Guzzo J: Induction of Oenococcus oeni
H+-ATPase activity and mRNA transcription under acidic
conditions. FEMS Microbiol Lett. 222:165–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67.
|
Rius N, Solé M, Francis A and Lorén JG:
Buffering capacity and membrane H+ conductance of lactic
acid bacteria. FEMS Microbiol Lett. 120:291–296. 1994. View Article : Google Scholar
|
|
68.
|
Charalampopoulos D, Pandiella SS and Webb
C: Evaluation of the effect of malt, wheat and barley extracts on
the viability of potentially probiotic lactic acid bacteria under
acidic conditions. Int J Food Microbiol. 82:133–141. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
69.
|
Corcoran BM, Stanton C, Fitzgerald GF and
Ross RP: Survival of probiotic lactobacilli in acidic environments
is enhanced in the presence of metabolizable sugars. Appl Environ
Microbiol. 71:3060–3067. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70.
|
Haghshenas B, Abdullah N, Nami Y, Radiah
D, Rosli R and Yari Khosroushahi A: Microencapsulation of probiotic
bacteria Lactobacillus plantarum 15HN using
alginate-psyllium-fenugreek polymeric blends. J Appl Microbiol.
118:1048–1057. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71.
|
Chen S, Zhao Q, Ferguson LR, Shu Q, Weir I
and Garg S: Development of a novel probiotic delivery system based
on microencapsulation with protectants. Appl Microbiol Biotechnol.
93:1447–1457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72.
|
Kailasapathy K: Microencapsulation of
probiotic bacteria: Technology and potential applications. Curr
Issues Intest Microbiol. 3:39–48. 2002.PubMed/NCBI
|
|
73.
|
Villena MJ, Lara-Villoslada F, Martínez MA
and Hernández ME: Development of gastro-resistant tablets for the
protection and intestinal delivery of Lactobacillus
fermentum CECT 5716. Int J Pharm. 487:314–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74.
|
Mei L, He F, Zhou RQ, Wu CD, Liang R, Xie
R, Ju XJ, Wang W and Chu LY: Novel intestinal-targeted
Ca-alginate-based carrier for pH-responsive protection and release
of lactic acid bacteria. ACS Appl Mater Interfaces. 6:5962–5970.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
75.
|
Zhao Q, Lee SJ, Mutukumira AN, Maddox I
and Shu Q: Viability and delivery of immobilised Lactobacillus
reuteri DPC16 within calcium alginate gel systems during
sequential passage through simulated gastrointestinal fluids. Benef
Microbes. 2:129–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76.
|
Adams CA: The probiotic paradox: Live and
dead cells are biological response modifiers. Nutr Res Rev.
23:37–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77.
|
Sashihara T, Sueki N and Ikegami S: An
analysis of the effectiveness of heat-killed lactic acid bacteria
in alleviating allergic diseases. J Dairy Sci. 89:2846–2855. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
78.
|
Zhang L, Nan L, Caicedo R and Neu J: Alive
and dead Lactobacillus rhamnosus GG decrease tumor necrosis
factor-alpha-induced interleukin-8 production in Caco-2 cells. J
Nutr. 135:1752–1756. 2005.PubMed/NCBI
|
|
79.
|
Taverniti V and Guglielmetti S: The
immunomodulatory properties of probiotic microorganisms beyond
their viability (ghost probiotics: Proposal of paraprobiotic
concept). Genes Nutr. 6:261–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80.
|
Sakai Y, Tsukahara T, Bukawa W, Matsubara
N and Ushida K: Cell preparation of Enterococcus faecalis
strain EC-12 prevents vancomycin-resistant enterococci colonization
in the cecum of newly hatched chicks. Poult Sci. 85:273–277. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
81.
|
Marin ML, Lee JH, Murtha J, Ustunol Z and
Pestka JJ: Differential cytokine production in clonal macrophage
and T-cell lines cultured with bifidobacteria. J Dairy Sci.
80:2713–2720. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
82.
|
Wagner RD, Pierson C, Warner T, Dohnalek
M, Hilty M and Balish E: Probiotic effects of feeding heat-killed
Lactobacillus acidophilus and Lactobacillus casei to
Candida albicans-colonized immunodeficient mice. J Food
Prot. 63:638–644. 2000.PubMed/NCBI
|
|
83.
|
Rachmilewitz D, Katakura K, Karmeli F,
Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J,
Takabayashi K and Raz E: Toll-like receptor 9 signaling mediates
the anti-inflammatory effects of probiotics in murine experimental
colitis. Gastroenterology. 126:520–528. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
84.
|
Orlando A, Refolo MG, Messa C, Amati L,
Lavermicocca P, Guerra V and Russo F: Antiproliferative and
proapoptotic effects of viable or heat-killed Lactobacillus
paracasei IMPC2.1 and Lactobacillus rhamnosus GG in
HGC-27 gastric and DLD-1 colon cell lines. Nutr Cancer.
64:1103–1111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
85.
|
Sanders ME, Guarner F, Guerrant R, Holt
PR, Quigley EM, Sartor RB, Sherman PM and Mayer EA: An update on
the use and investigation of probiotics in health and disease. Gut.
62:787–796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86.
|
Marchesi JR, Adams DH, Fava F, Hermes GD,
Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM,
et al: The gut microbiota and host health: A new clinical frontier.
Gut. 65:330–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87.
|
Kumar M, Nagpal R, Kumar R, Hemalatha R,
Verma V, Kumar A, Chakraborty C, Singh B, Marotta F, Jain S and
Yadav H: Cholesterol-lowering probiotics as potential
biotherapeutics for metabolic diseases. Exp Diabetes Res.
2012:9029172012. View Article : Google Scholar : PubMed/NCBI
|
|
88.
|
Tsai TY, Chen LY and Pan TM: Effect of
probiotic-fermented, genetically modified soy milk on
hypercholesterolemia in hamsters. J Microbiol Immunol Infect.
47:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89.
|
Pereira DI and Gibson GR: Cholesterol
assimilation by lactic acid bacteria and bifidobacteria isolated
from the human gut. Appl Environ Microbiol. 68:4689–4693. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
90.
|
Kadooka Y, Sato M, Imaizumi K, Ogawa A,
Ikuyama K, Akai Y, Okano M, Kagoshima M and Tsuchida T: Regulation
of abdominal adiposity by probiotics (Lactobacillus gasseri
SBT2055) in adults with obese tendencies in a randomized controlled
trial. Eur J Clin Nutr. 64:636–643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91.
|
Yoda K, Sun X, Kawase M, Kubota A,
Miyazawa K, Harata G, Hosoda M, Hiramatsu M, He F and Zemel MB: A
combination of probiotics and whey proteins enhances anti-obesity
effects of calcium and dairy products during nutritional energy
restriction in aP2-agouti transgenic mice. Br J Nutr.
113:1689–1696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92.
|
Kim SH, Huh CS, Choi ID, Jeong JW, Ku HK,
Ra JH, Kim TY, Kim GB, Sim JH and Ahn YT: The anti-diabetic
activity of Bifidobacterium lactis HY8101 in vitro and in
vivo. J Appl Microbiol. 117:834–845. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
93.
|
Moroti C, Souza Magri LF, de Rezende Costa
M, Cavallini DC and Sivieri K: Effect of the consumption of a new
symbiotic shake on glycemia and cholesterol levels in elderly
people with type 2 diabetes mellitus. Lipids Health Dis. 11:292012.
View Article : Google Scholar : PubMed/NCBI
|
|
94.
|
Huang Y, Wang J, Quan G, Wang X, Yang L
and Zhong L: Lactobacillus acidophilus ATCC 4356 prevents
atherosclerosis via inhibition of intestinal cholesterol absorption
in apolipoprotein E-knockout mice. Appl Environ Microbiol.
80:7496–7504. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95.
|
Aihara K, Kajimoto O, Hirata H, Takahashi
R and Nakamura Y: Effect of powdered fermented milk with
Lactobacillus helveticus on subjects with high-normal blood
pressure or mild hypertension. J Am Coll Nutr. 24:257–265. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
96.
|
Rashid SK, Idris-Khodja N, Auger C,
Alhosin M, Boehm N, Oswald-Mammosser M and Schini-Kerth VB:
Probiotics (VSL#3) prevent endothelial dysfunction in rats with
portal hypertension: Role of the angiotensin system. PLoS One.
9:e974582014. View Article : Google Scholar : PubMed/NCBI
|
|
97.
|
Fotiadis CI, Stoidis CN, Spyropoulos BG
and Zografos ED: Role of probiotics, prebiotics and synbiotics in
chemoprevention for colorectal cancer. World J Gastroenterol.
14:6453–6457. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
98.
|
Sobhani I, Amiot A, Le Baleur Y, Levy M,
Auriault ML, Van Nhieu JT and Delchier JC: Microbial dysbiosis and
colon carcinogenesis: Could colon cancer be considered a
bacteria-related disease? Therap Adv Gastroenterol. 6:215–229.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
99.
|
Zhang M, Fan X, Fang B, Zhu C, Zhu J and
Ren F: Effects of Lactobacillus salivarius Ren on cancer
prevention and intestinal microbiota in
1,2-dimethylhydrazine-induced rat model. J Microbiol. 53:398–405.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
100.
|
Chen ZF, Ai LY, Wang JL, Ren LL, Yu YN, Xu
J, Chen HY, Yu J, Li M, Qin WX, et al: Probiotics Clostridium
butyricum and Bacillus subtilis ameliorate intestinal
tumorigenesis. Future Microbiol. 10:1433–1445. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101.
|
Russo F, Linsalata M and Orlando A:
Probiotics against neoplastic transformation of gastric mucosa:
Effects on cell proliferation and polyamine metabolism. World J
Gastroenterol. 20:13258–13272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102.
|
Cousin FJ, Jouan-Lanhouet S,
Dimanche-Boitrel MT, Corcos L and Jan G: Milk fermented by
Propionibacterium freudenreichii induces apoptosis of HGT-1
human gastric cancer cells. PLoS One. 7:e318922012. View Article : Google Scholar : PubMed/NCBI
|
|
103.
|
Linsalata M, Cavallini A, Messa C, Orlando
A, Refolo MG and Russo F: Lactobacillus rhamnosus GG
influences polyamine metabolism in HGC-27 gastric cancer cell line:
A strategy toward nutritional approach to chemoprevention of
gastric cancer. Curr Pharm Des. 16:847–853. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
104.
|
Haghshenas B, Abdullah N, Nami Y, Radiah
D, Rosli R and Khosroushahi AY: Different effects of two
newly-isolated probiotic Lactobacillus plantarum 15HN and
Lactococcus lactis subsp. Lactis 44Lac strains from
traditional dairy products on cancer cell lines. Anaerobe.
30:51–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105.
|
Chitapanarux I, Chitapanarux T, Traisathit
P, Kudumpee S, Tharavichitkul E and Lorvidhaya V: Randomized
controlled trial of live Lactobacillus acidophilus plus
Bifidobacterium bifidum in prophylaxis of diarrhea during
radiotherapy in cervical cancer patients. Radiat Oncol. 5:312010.
View Article : Google Scholar : PubMed/NCBI
|
|
106.
|
Osterlund P, Ruotsalainen T, Korpela R,
Saxelin M, Ollus A, Valta P, Kouri M, Elomaa I and Joensuu H:
Lactobacillus supplementation for diarrhoea related to
chemotherapy of colorectal cancer: A randomised study. Br J Cancer.
97:1028–1034. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
107.
|
Crouzet L, Rigottier-Gois L and Serror P:
Potential use of probiotic and commensal bacteria as non-antibiotic
strategies against vancomycin-resistant enterococci. FEMS Microbiol
Lett. 362:fnv0122015. View Article : Google Scholar : PubMed/NCBI
|
|
108.
|
Gill EE, Franco OL and Hancock RE:
Antibiotic adjuvants: Diverse strategies for controlling
drug-resistant pathogens. Chem Biol Drug Des. 85:56–78. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
109.
|
Cano-Garrido O, Seras-Franzoso J and
Garcia-Fruitós E: Lactic acid bacteria: Reviewing the potential of
a promising delivery live vector for biomedical purposes. Microb
Cell Fact. 14:1372015. View Article : Google Scholar : PubMed/NCBI
|
|
110.
|
Mohamadzadeh M and Owen JL: Reprogramming
intestinal immunity is the answer to induced pathogenic
inflammation. Immunotherapy. 3:1415–1417. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
111.
|
Wells JM and Mercenier A: Mucosal delivery
of therapeutic and prophylactic molecules using lactic acid
bacteria. Nat Rev Microbiol. 6:349–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
112.
|
Bermúdez-Humarán LG: Lactococcus
lactis as a live vector for mucosal delivery of therapeutic
proteins. Hum Vaccin. 5:264–267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
113.
|
Marelli B, Perez AR, Banchio C, de Mendoza
D and Magni C: Oral immunization with live Lactococcus
lactis expressing rotavirus VP8 subunit induces specific immune
response in mice. J Virol Methods. 175:28–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
114.
|
Konturek PC, Sliwowski Z, Koziel J,
Ptak-Belowska A, Burnat G, Brzozowski T and Konturek SJ: Probiotic
bacteria Escherichia coli strain Nissle 1917 attenuates
acute gastric lesions induced by stress. J Physiol Pharmacol.
60(Suppl 6): S41–S48. 2009.
|
|
115.
|
Spivak MIa, Lazarenko LM, Falalieieva TM,
Virchenko OV and Neporada KS: Prophylactic effect of probiotic
strains Bifidobacterium animalis VKL and VKB on
stress-induced lesions in the gastric mucosa of rats. Fiziol Zh.
59:23–30. 2013.(In Ukrainian). PubMed/NCBI
|
|
116.
|
Nagaoka M, Hashimoto S, Watanabe T,
Yokokura T and Mori Y: Anti-ulcer effects of lactic acid bacteria
and their cell wall polysaccharides. Biol Pharm Bull. 17:1012–1017.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
117.
|
Viggiano D, Ianiro G, Vanella G, Bibbò S,
Bruno G, Simeone G and Mele G: Gut barrier in health and disease:
Focus on childhood. Eur Rev Med Pharmacol Sci. 19:1077–1085.
2015.PubMed/NCBI
|
|
118.
|
Laine L, Takeuchi K and Tarnawski A:
Gastric mucosal defense and cytoprotection: Bench to bedside.
Gastroenterology. 135:41–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
119.
|
Takeeda M, Hayashi Y, Yamato M, Murakami M
and Takeuchi K: Roles of endogenous prostaglandins and
cyclooxygenase izoenzymes in mucosal defense of inflamed rat
stomach. J Physiol Pharmacol. 55:193–205. 2004.PubMed/NCBI
|
|
120.
|
Boirivant M and Strober W: The mechanism
of action of probiotics. Curr Opin Gastroenterol. 23:679–692. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
121.
|
Vieira AT, Teixeira MM and Martins FS: The
role of probiotics and prebiotics in inducing gut immunity. Front
Immunol. 4:4452013. View Article : Google Scholar : PubMed/NCBI
|
|
122.
|
Jones SE and Versalovic J: Probiotic
Lactobacillus reuteri biofilms produce antimicrobial and
anti-inflammatory factors. BMC Microbiol. 9:352009. View Article : Google Scholar : PubMed/NCBI
|
|
123.
|
Coman MM, Verdenelli MC, Cecchini C, Silvi
S, Orpianesi C, Boyko N and Cresci A: In vitro evaluation of
antimicrobial activity of Lactobacillus rhamnosus IMC
501®, Lactobacillus paracasei IMC 502®
and SYNBIO® against pathogens. J Appl Microbiol.
117:518–527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
124.
|
Bernet MF, Brassart D, Neeser JR and
Servin AL: Lactobacillus acidophilus LA 1 binds to cultured
human intestinal cell lines and inhibits cell attachment and cell
invasion by enterovirulent bacteria. Gut. 35:483–489. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
125.
|
Mukai T, Asasaka T, Sato E, Mori K,
Matsumoto M and Ohori H: Inhibition of binding of Helicobacter
pylori to the glycolipid receptors by probiotic
Lactobacillus reuteri. FEMS Immunol Med Microbiol.
32:105–110. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
126.
|
Lin YP, Thibodeaux CH, Peña JA, Ferry GD
and Versalovic J: Probiotic Lactobacillus reuteri suppress
proinflammatory cytokines via c-Jun. Inflamm Bowel Dis.
14:1068–1083. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
127.
|
Takagi A, Matsuzaki T, Sato M, Nomoto K,
Morotomi M and Yokokura T: Enhancement of natural killer
cytotoxicity delayed murine carcinogenesis by a probiotic
microorganism. Carcinogenesis. 22:599–605. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
128.
|
Haller D, Bode C, Hammes WP, Pfeifer AM,
Schiffrin EJ and Blum S: Non-pathogenic bacteria elicit a
differential cytokine response by intestinal epithelial
cell/leucocyte co-cultures. Gut. 47:79–87. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
129.
|
Borchers AT, Selmi C, Meyers FJ, Keen CL
and Gershwin ME: Probiotics and immunity. J Gastroenterol.
44:26–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
130.
|
Madsen K, Cornish A, Soper P, McKaigney C,
Jijon H, Yachimec C, Doyle J, Jewell L and De Simone C: Probiotic
bacteria enhance murine and human intestinal epithelial barrier
function. Gastroenterology. 121:580–591. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
131.
|
Gotteland M, Cruchet S and Verbeke S:
Effect of Lactobacillus ingestion on the gastrointestinal mucosal
barrier alterations induced by indomethacin in humans. Aliment
Pharmacol Ther. 15:11–17. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
132.
|
Rao RK and Samak G: Protection and
Restitution of Gut Barrier by Probiotics: Nutritional and clinical
implications. Curr Nutr Food Sci. 9:99–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
133.
|
Gomi A, Harima-Mizusawa N, Shibahara-Sone
H, Kano M, Miyazaki K and Ishikawa F: Effect of Bifidobacterium
bifidum BF-1 on gastric protection and mucin production in an
acute gastric injury rat model. J Dairy Sci. 96:832–837. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
134.
|
Dai C, Zhao DH and Jiang M: VSL#3
probiotics regulate the intestinal epithelial barrier in vivo and
in vitro via the p38 and ERK signaling pathways. Int J Mol Med.
29:202–208. 2012.PubMed/NCBI
|
|
135.
|
Mennigen R, Nolte K, Rijcken E, Utech M,
Loeffler B, Senninger N and Bruewer M: Probiotic mixture VSL#3
protects the epithelial barrier by maintaining tight junction
protein expression and preventing apoptosis in a murine model of
colitis. Am J Physiol Gastrointest Liver Physiol. 296:G1140–G1149.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
136.
|
Wang FY, Liu JM, Luo HH, Liu AH and Jiang
Y: Potential protective effects of Clostridium butyricum on
experimental gastric ulcers in mice. World J Gastroenterol.
21:8340–8351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
137.
|
Virchenko OV, Falalyeyeva TM, Beregova TV,
Spivak MY, Lazarenko LM and Demchenko OM: Effects of mono-, poly-
and composite probiotics on the ulceration caused by restraint
stress. Fiziol Zh. 61:35–41. 2015.(In Ukrainian). PubMed/NCBI
|
|
138.
|
Virchenko OV, Falalyeyeva TM, Beregova TV
and Maryana SY: The multistrain probiotic enhances the healing
process of stress-induced lesions of the gastric mucosa of rats.
RJPBCS. 6:2492015.
|
|
139.
|
Wang JY, Yamasaki S, Takeuchi K and Okabe
S: Delayed healing of acetic acid-induced gastric ulcers in rats by
indomethacin. Gastroenterology. 96:393–402. 1989.PubMed/NCBI
|
|
140.
|
Santucci L, Fiorucci S, Di Matteo FM and
Morelli A: Role of tumor necrosis factor alpha release and
leukocyte margination in indomethacin-induced gastric injury in
rats. Gastroenterology. 108:393–401. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
141.
|
Yoshikawa T, Takano H, Naito Y, Oyamada H,
Ueda S and Kondo M: Augmentative effects of tumor necrosis
factor-alpha (human, natural type) on polymorphonuclear
leukocyte-derived superoxide generation induced by various
stimulants. Int J Immunopharmacol. 14:1391–1398. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
142.
|
Senol A, Işler M, Karahan AG, Kiliç GB,
Kuleaşan H, Gören I, Saritaş U, Kaya S, Cırış M, Aktürk O, et al:
Effect of probiotics on aspirin-induced gastric mucosal lesions.
Turk J Gastroenterol. 22:18–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
143.
|
Senol A, Isler M, Karahan AG, Kilic GB,
Kuleasan H, Kaya S, Keskin M, Goren I, Saritas U, Aridogan BC and
Delibas N: Preventive effect of probiotics and α-tocopherol on
ethanol-induced gastric mucosal injury in rats. J Med Food.
14:173–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
144.
|
Allen A and Pearson JP: Mucus
glycoproteins of the normal gastrointestinal tract. Eur J
Gastroenterol Hepatol. 5:193–199. 1993. View Article : Google Scholar
|
|
145.
|
Wong WM, Poulsom R and Wright NA: Trefoil
peptides. Gut. 44:890–895. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
146.
|
Phillipson M, Johansson ME, Henriksnäs J,
Petersson J, Gendler SJ, Sandler S, Persson AE, Hansson GC and Holm
L: The gastric mucus layers: Constituents and regulation of
accumulation. Am J Physiol Gastrointest Liver Physiol.
295:G806–G812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
147.
|
Corfield AP, Carroll D, Myerscough N and
Probert CS: Mucins in the gastrointestinal tract in health and
disease. Front Biosci. 6:D1321–D1357. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
148.
|
Singh PK and Hollingsworth MA: Cell surf
ace-associated mucins in signal transduction. Trends Cell Biol.
16:467–476. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
149.
|
Karam SM, Tomasetto C and Rio MC: Trefoil
factor 1 is required for the commitment programme of mouse oxyntic
epithelial progenitors. Gut. 53:1408–1415. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
150.
|
Ren JL, Luo JY, Lu YP, Wang L and Shi HX:
Relationship between trefoil factor 1 expression and gastric mucosa
injuries and gastric cancer. World J Gastroenterol. 11:2674–2677.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
151.
|
Fu T, Kalbacher H and Hoffmann W: TFF1 is
differentially expressed in stationary and migratory rat gastric
epithelial cells (RGM-1) after in vitro wounding: Influence of TFF1
RNA interference on cell migration. Cell Physiol Biochem.
32:997–1010. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
152.
|
Atuma C, Strugala V, Allen A and Holm L:
The adherent gastrointestinal mucus gel layer: Thickness and
physical state in vivo. Am J Physiol Gastrointest Liver Physiol.
280:G922–G929. 2001.PubMed/NCBI
|
|
153.
|
Kaunitz JD: Barrier function of gastric
mucus. Keio J Med. 2:63–68. 1999. View Article : Google Scholar
|
|
154.
|
Mattar AF, Teitelbaum DH, Drongowski RA,
Yongyi F, Harmon CM and Coran AG: Probiotics up-regulate MUC-2
mucin gene expression in a Caco-2 cell-culture model. Pediatr Surg
Int. 18:586–590. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
155.
|
Kim Y, Kim SH, Whang KY, Kim YJ and Oh S:
Inhibition of Escherichia coli O157:H7 attachment by
interactions between lactic acid bacteria and intestinal epithelial
cells. J Microbiol Biotechnol. 18:1278–1285. 2008.PubMed/NCBI
|
|
156.
|
Otte JM and Podolsky DK: Functional
modulation of enterocytes by gram-positive and gram-negative
microorganisms. Am J Physiol Gastrointest Liver Physiol.
286:G613–G626. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
157.
|
Caballero-Franco C, Keller K, De Simone C
and Chadee K: The VSL#3 probiotic formula induces mucin gene
expression and secretion in colonic epithelial cells. Am J Physiol
Gastrointest Liver Physiol. 292:G315–G322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
158.
|
Ouwehand AC, Salminen S, Tölkkö S, Roberts
P, Ovaska J and Salminen E: Resected human colonic tissue: New
model for characterizing adhesion of lactic acid bacteria. Clin
Diagn Lab Immunol. 9:184–186. 2002.PubMed/NCBI
|
|
159.
|
Nishiyama K, Nakamata K, Ueno S, Terao A,
Aryantini NP, Sujaya IN, Fukuda K, Urashima T, Yamamoto Y and Mukai
T: Adhesion properties of Lactobacillus rhamnosus
mucus-binding factor to mucin and extracellular matrix proteins.
Biosci Biotechnol Biochem. 79:271–279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
160.
|
Karam SM and Leblond CP: Dynamics of
epithelial cells in the corpus of the mouse stomach.
I.dentification of proliferative cell types and pinpointing of the
stem cell. Anat Rec. 236:259–279. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
161.
|
Karam SM, Straiton T, Hassan WM and
Leblond CP: Defining epithelial cell progenitors in the human
oxyntic mucosa. Stem Cells. 21:322–336. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
162.
|
Karam SM: A focus on parietal cells as a
renewing cell population. World J Gastroenterol. 16:538–546. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
163.
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
164.
|
Taketani Y, Kinugasa K, Kitajima R,
Nishiumi S, Ashida H, Nakamura H, Fujita T, Kanzaki K, Masutani H
and Yodoi J: Protective effects of oral administration of yeast
thioredoxin against gastric mucosal injury. Biosci Biotechnol
Biochem. 78:1221–1230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
165.
|
Schöttker B, Adamu MA, Weck MN and Brenner
H: Helicobacter pylori infection is strongly associated with
gastric and duodenal ulcers in a large prospective study. Clin
Gastroenterol Hepatol. 10:487–493.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
166.
|
Cover TL and Blaser MJ: Helicobacter
pylori in health and disease. Gastroenterology. 136:1863–1873.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
167.
|
Vergara M, Catalán M, Gisbert JP and
Calvet X: Meta-analysis: Role of Helicobacter pylori
eradication in the prevention of peptic ulcer in NSAID users.
Aliment Pharmacol Ther. 21:1411–1418. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
168.
|
Malfertheiner P, Selgrad M and Bornschein
J: Helicobacter pylori: Clinical management. Curr Opin
Gastroenterol. 28:608–614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
169.
|
Giorgio F, Principi M, De Francesco V,
Zullo A, Losurdo G, Di Leo A and Ierardi E: Primary clarithromycin
resistance to Helicobacter pylori: Is this the main reason
for triple therapy failure? World J Gastrointest Pathophysiol.
4:43–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
170.
|
Graham DY and Fischbach L: Helicobacter
pylori treatment in the era of increasing antibiotic
resistance. Gut. 59:1143–1153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
171.
|
Papastergiou V, Georgopoulos SD and
Karatapanis S: Treatment of Helicobacter pylori infection:
Meeting the challenge of antimicrobial resistance. World J
Gastroenterol. 20:9898–9911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
172.
|
Tongtawee T, Dechsukhum C, Leeanansaksiri
W, Kaewpitoon S, Kaewpitoon N, Loyd RA, Matrakool L and Panpimanmas
S: Improved Helicobacter pylori eradication rate of tailored
triple therapy by adding Lactobacillus delbrueckii and
Streptococcus thermophilus in northeast region of Thailand:
A prospective randomized controlled clinical trial. Gastroenterol
Res Pract. 2015:5180182015. View Article : Google Scholar : PubMed/NCBI
|
|
173.
|
Johnson-Henry KC, Mitchell DJ, Avitzur Y,
Galindo-Mata E, Jones NL and Sherman PM: Probiotics reduce
bacterial colonization and gastric inflammation in H.
pylori-infected mice. Dig Dis Sci. 49:1095–1102. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
174.
|
Sheu BS, Wu JJ, Lo CY, Wu HW, Chen JH, Lin
YS and Lin MD: Impact of supplement with Lactobacillus- and
Bifidobacterium-containing yogurt on triple therapy for
Helicobacter pylori eradication. Aliment Pharmacol Ther.
16:1669–1675. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
175.
|
Ushiyama A, Tanaka K, Aiba Y, Shiba T,
Takagi A, Mine T and Koga Y: Lactobacillus gasseri OLL2716
as a probiotic in clarithromycin-resistant Helicobacter
pylori infection. J Gastroenterol Hepatol. 18:986–991. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
176.
|
Chenoll E, Casinos B, Bataller E, Astals
P, Echevarría J, Iglesias JR, Balbarie P, Ramón D and Genovés S:
Novel probiotic Bifidobacterium bifidum CECT 7366 strain
active against the pathogenic bacterium Helicobacter pylori.
Appl Environ Microbiol. 77:1335–1343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
177.
|
Gotteland M and Cruchet S: Suppressive
effect of frequent ingestion of Lactobacillus johnsonii La1
on Helicobacter pylori colonization in asymptomatic
volunteers. J Antimicrob Chemother. 51:1317–1319. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
178.
|
Kabir AM, Aiba Y, Takagi A, Kamiya S, Miwa
T and Koga Y: Prevention of Helicobacter pylori infection by
lactobacilli in a gnotobiotic murine model. Gut. 41:49–55. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
179.
|
Lesbros-Pantoflickova D, Corthésy-Theulaz
I and Blum AL: Helicobacter pylori and probiotics. J Nutr.
137(3 Suppl 2): S812–S818. 2007.
|
|
180.
|
Ruggiero P: Use of probiotics in the fight
against Helicobacter pylori. World J Gastrointest
Pathophysiol. 5:384–391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
181.
|
Yang YJ and Sheu BS: Probiotics-containing
yogurts suppress Helicobacter pylori load and modify immune
response and intestinal microbiota in the Helicobacter
pylori-infected children. Helicobacter. 17:297–304. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
182.
|
Sgouras D, Maragkoudakis P, Petraki K,
Martinez-Gonzalez B, Eriotou E, Michopoulos S, Kalantzopoulos G,
Tsakalidou E and Mentis A: In vitro and in vivo inhibition of
Helicobacter pylori by Lactobacillus casei strain
Shirota. Appl Environ Microbiol. 70:518–526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
183.
|
Aiba Y, Nakano Y, Koga Y, Takahashi K and
Komatsu Y: A highly acid-resistant novel strain of Lactobacillus
johnsonii No. 1088 has antibacterial activity, including that
against Helicobacter pylori, and inhibits gastrin-mediated
acid production in mice. Microbiologyopen. 4:465–474. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
184.
|
Isobe H, Nishiyama A, Takano T, Higuchi W,
Nakagawa S, Taneike I, Fukushima Y and Yamamoto T: Reduction of
overall Helicobacter pylori colonization levels in the
stomach of Mongolian gerbil by Lactobacillus johnsonii La1
(LC1) and its in vitro activities against H. pylori motility
and adherence. Biosci Biotechnol Biochem. 76:850–852. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
185.
|
Hsieh PS, Tsai YC, Chen YC, Teh SF, Ou CM
and King VA: Eradication of Helicobacter pylori infection by
the probiotic strains Lactobacillus johnsonii MH-68 and
L. salivarius ssp. salicinius AP-32. Helicobacter.
17:466–477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
186.
|
Chen X, Liu XM, Tian F, Zhang Q, Zhang HP,
Zhang H and Chen W: Antagonistic activities of lactobacilli against
Helicobacter pylori growth and infection in human gastric
epithelial cells. J Food Sci. 77:M9–M14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
187.
|
Mack DR, Ahrne S, Hyde L, Wei S and
Hollingsworth MA: Extracellular MUC3 mucin secretion follows
adherence of Lactobacillus strains to intestinal epithelial
cells in vitro. Gut. 52:827–833. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
188.
|
Thiraworawong T, Spinler JK, Werawatganon
D, Klaikeaw N, Venable SF, Versalovic J and Tumwasorn S:
Anti-inflammatory properties of gastric-derived Lactobacillus
plantarum XB7 in the context of Helicobacter pylori
infection. Helicobacter. 19:144–155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
189.
|
Hwang SW, Kim N, Kim JM, Huh CS, Ahn YT,
Park SH, Shin CM, Park JH, Lee MK, Nam RH, et al: Probiotic
suppression of the H. pylori-induced responses by conjugated
linoleic acids in a gastric epithelial cell line. Prostaglandins
Leukot Essent Fatty Acids. 86:225–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
190.
|
Kim JM, Kim JS, Kim YJ, Oh YK, Kim IY,
Chee YJ, Han JS and Jung HC: Conjugated linoleic acids produced by
Lactobacillus dissociates IKK-gamma and Hsp90 complex in
Helicobacter pylori-infected gastric epithelial cells. Lab
Invest. 88:541–552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
191.
|
Lee JS, Paek NS, Kwon OS and Hahm KB:
Anti-inflammatory actions of probiotics through activating
suppressor of cytokine signaling (SOCS) expression and signaling in
Helicobacter pylori infection: A novel mechanism. J
Gastroenterol Hepatol. 25:194–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
192.
|
Mehling H and Busjahn A: Non-viable
Lactobacillus reuteri DSMZ 17648 (Pylopass™) as a new
approach to Helicobacter pylori control in humans.
Nutrients. 5:3062–3073. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
193.
|
Holz C, Busjahn A, Mehling H, Arya S,
Boettner M, Habibi H and Lang C: Significant reduction in
Helicobacter pylori load in humans with non-viable
Lactobacillus reuteri DSM17648: A pilot study. Probiotics
Antimicrob Proteins. 7:91–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
194.
|
Hauser G, Salkic N, Vukelic K, JajacKnez A
and Stimac D: Probiotics for standard triple Helicobacter
pylori eradication: A randomized, double-blind,
placebo-controlled trial. Medicine (Baltimore). 94:e6852015.
View Article : Google Scholar : PubMed/NCBI
|
|
195.
|
Francavilla R, Polimeno L, Demichina A,
Maurogiovanni G, Principi B, Scaccianoce G, Ierardi E, Russo F,
Riezzo G, Di Leo A, et al: Lactobacillus reuteri strain
combination in Helicobacter pylori infection: A randomized,
double-blind, placebo-controlled study. J Clin Gastroenterol.
48:407–413. 2014.PubMed/NCBI
|
|
196.
|
Pantoflickova D, Corthésy-Theulaz I, Dorta
G, Stolte M, Isler P, Rochat F, Enslen M and Blum AL: Favourable
effect of regular intake of fermented milk containing
Lactobacillus johnsonii on Helicobacter pylori
associated gastritis. Aliment Pharmacol Ther. 18:805–813. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
197.
|
Michetti P, Dorta G, Wiesel PH, Brassart
D, Verdu E, Herranz M, Felley C, Porta N, Rouvet M, Blum AL and
Corthésy-Theulaz I: Effect of whey-based culture supernatant of
Lactobacillus acidophilus (johnsonii) La1 on
Helicobacter pylori infection in humans. Digestion.
60:203–209. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
198.
|
Szajewska H, Horvath A and Piwowarczyk A:
Meta-analysis: The effects of Saccharomyces boulardii
supplementation on Helicobacter pylori eradication rates and
side effects during treatment. Aliment Pharmacol Ther.
32:1069–1079. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
199.
|
Zou J, Dong J and Yu X: Meta-analysis:
Lactobacillus containing quadruple therapy versus standard triple
first-line therapy for Helicobacter pylori eradication.
Helicobacter. 14:97–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
200.
|
Hamilton-Miller JM: The role of probiotics
in the treatment and prevention of Helicobacter pylori
infection. Int J Antimicrob Agents. 22:360–366. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
201.
|
Patel A, Shah N and Prajapati JB: Clinical
application of probiotics in the treatment of Helicobacter
pylori infection - a brief review. J Microbiol Immunol Infect.
47:429–437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
202.
|
Shane AL, Cabana MD, Vidry S, Merenstein
D, Hummelen R, Ellis CL, Heimbach JT, Hempel S, Lynch SV, Sanders
ME and Tancredi DJ: Guide to designing, conducting, publishing and
communicating results of clinical studies involving probiotic
applications in human participants. Gut Microbes. 1:243–253. 2010.
View Article : Google Scholar : PubMed/NCBI
|