Introduction
Painful bladder syndrome/interstitial cystitis
(PBS/IC) occurs far more frequently in women than in men. It has
been reported that among 1.3 million Americans having the symptoms
of PBS/IC, >1.2 million were women in the year 2007 (1). The typical age predilection is ~40
years (2). The common symptoms of
PBS/IC include urinary urgency, frequency, nocturia and suprapubic
or pelvic pain without any known etiological factor. Diagnostic
criteria have been established by the National Institute of
Diabetes and Digestive and Kidney Diseases (NIDDK) in order to
standardize the diagnosis of PBS/IC; these include objective
findings of glomerulations or Hunner's ulcer at cystoscopy and
subjective symptoms of bladder pain or urinary urgency, in addition
to multiple exclusion criteria such as age <18 years, duration
of symptoms <9 months and absence of nocturia (3). Even though numerous treatments have
been reported, including intravesical botulinum toxin A injections,
pentosan polysulfate sodium (PPS), dietary modification, lifestyle
interventions/behavioral therapies and bladder training, no
specific effective therapy has yet been identified.
As an immunosuppressive agent, cyclosporine A (CyA)
suppresses the activation of T cells by inhibiting the enzymatic
activity of calcineurin (4). It has
been successfully used in various conditions, such as in transplant
recipients, hepatitis C (5), and
autoimmune diseases such as psoriasis (6), Crohn's disease (7) and rheumatoid arthritis (8). Furthermore, CyA is emerging as a
potential therapeutic strategy for PBS/IC, which is an intractable
condition to treat. Several studies have reported promising results
for the treatment effects of CyA in PBS/IC. However, the strength
of the evidence of efficacy in each independent study is limited by
various factors, such as limitation of included subjects, or
absence of a control group. Therefore, the present systematic
review was performed in order to assess the global evidence
concerning the treatment effects of CyA in PBS/IC.
Materials and methods
Search strategy
The search strategy included the following key words
variably combined: ‘cyclosporine A,’ ‘bladder pain syndrome’ and
‘interstitial cystitis’. Databases including China National
Knowledge Infrastructure (CNKI), PubMed, Highwire, EMBASE and
Science Direct were searched for relevant references. In addition,
other retrieval strategies were performed to screen for relevant
articles, including manual retrieval and scanning of the reference
sections of eligible articles.
Inclusion/exclusion criteria
Studies were regarded as qualified for inclusion if
they met all the following inclusion criteria: i) the disease type
was PBS/IC with or without meeting the NIDDK criteria; ii) the
study was a clinical trial, including randomized controlled trials
(RCTs), and prospective or retrospective cohort studies; iii) the
treatment effects of CyA in PBS/IC were measured. Studies were
excluded on the basis of any of the following criteria: i) the
article was a review, letter or case report; ii) the article did
not relate to the treatment effects of CyA in PBS/IC.
Data selection
Two researchers independently screened the initially
included articles on the basis of the inclusion/exclusion criteria.
Any disagreement was resolved by consensus. Baseline data and
result information were extracted and assessed by the same
researchers, including first author and publication year, country,
inclusion/exclusion criteria of each study, subjects, gender
(female/male), mean age, regimen protocol, outcome parameters,
follow-up and complications. In addition, all included articles
were assessed for methodological quality, according to the
Newcastle-Ottawa scale (9) for
nonrandomized studies and Jadad score for RCTs (10).
Results
Eligible articles
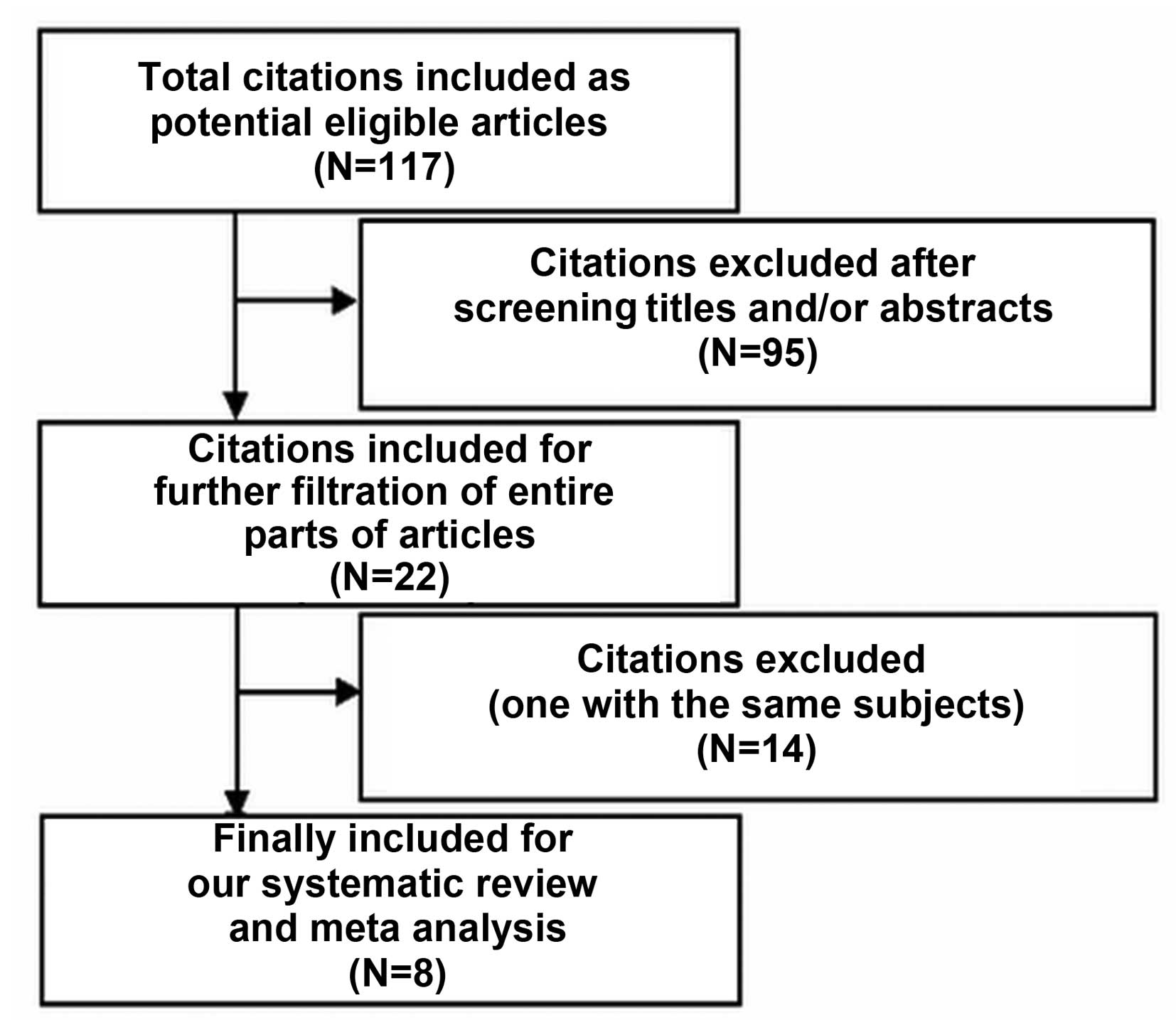
The various steps of the filtration procedure used
in this review are presented in Fig.
1. Initially, 117 articles were included (26 from PubMed, 11
from CNKI, 36 from Highwire, 17 from EMBASE and 31 from
ScienceDirect). No further relevant references were identified by
manual search and filtration of the reference sections of included
articles. Following careful screening of the titles and abstracts
of each article, 95 articles were excluded, leaving 22 articles for
further filtration. After screening the entire contents of these
articles, 14 articles were further excluded. Eight articles
(11–18) were finally included as eligible
articles, which including a total of 298 subjects with a median
number of 37.25 subjects per study. These 8 studies included 3 RCTs
(16–18), 4 prospective cohort studies (11–13,15) and
1 retrospective cohort study (14).
In the 7 studies that reported the ages of the subjects, the age
predilection was 40–70 years. In one of the articles (12), the patient age could not be
identified because only the abstract was available. The included
subjects were mostly females; the percentage of males was
relatively low. The sample sizes of the included studies were
uniformly small, ranging from 10 to 64. Other characteristics of
the included articles are listed in Table I.
 | Table I.Baseline characteristics of the
included studies. |
Table I.
Baseline characteristics of the
included studies.
| First author,
publication year (Refs.) | Country | Study type | Inclusion/exclusion
criteria | No. of subjects | Gender
(female/male) | Mean age (years) | Regimen protocol | Outcome
parameters | Follow up | Complications |
|---|
| Ehrén, 2013 (11) | Sweden | Cohort,
prospective | Diagnosed with IC for
>2 years (some for up to 10 years). Exclusion: Use of
angiotensin-converting enzyme inhibitors, angiotensin II
antagonists, potassium-containing drugs, any medicine that inhibits
or induces CYP3A4, pregnancy, lactation, or regular intake of
hypericin or grapefruit | 10 | 6/4 | 58±13 | CyA dosage: 3
mg/kg/day for 12 weeks; followed by 4 weeks of deescalation in two
steps (1.5 mg/kg/day for 2 weeks and 0.75 mg/kg/day for 2 weeks,
respectively) and no medication for the last 2 weeks | NO formation; ICSI
and ICPI scores | 18 weeks | Mild adverse events;
no serum creatinine elevation or increase in blood pressure |
| Chade, 2014 (12) | Brazil | Cohort,
prospective | Fulfilled the NIDDKD
criteria | 45 | 43/2 | N/A | CyA dosage: 1.5 mg/kg
twice a day, for 5 years | ICSI and ICPI scores;
mean voided vol. (ml) | 5 years | None |
| Forrest, 2012
(13) | USA | Cohort,
prospective | Patients with no
contraindications to CyA and in whom the usual IC/PBS treatments
had failed | 44 | 30/14 | 55.5 | CyA dosage: 2–3 mg/kg
divided into 2 daily dosages with a maximum of 300 mg daily; if
adverse events occurred the dose was reduced or the drug was
stopped | Symptom response
(based on ICSI or GRA); response with vs. without HL; CyA
levels | mean, 20.8 months
(range, 3–81) | Serum creatinine was
increased in 9 patients, causing 4 patients to stop CyA treatment;
Hypertension in 3 patients |
| Sairanen, 2004
(14) | Finland | Cohort,
retrospective | Fulfilled the NIDDKD
criteria, and multiple first-line therapies had been tried without
clinical success | 23 | 20/3 | 65.7±7.6 | CyA dosage: 3 mg/kg
divided into 2 daily dosages. When symptoms were alleviated the
dose was gradually decreased to as low as 1 mg/kg as a single daily
dose | Mean voided vol.
(ml); maximum awake bladder capacity (ml); 24-h voiding
frequency | 60.8±35.7 months | Hypertension and
gingival hyperplasia occurred in 3 patients, respectively; induced
hair growth occurred in 2 patients |
| Forsell, 1996
(15) | Finland | Cohort,
prospective | Fulfilled the
criteria for IC according to an international accrual form | 11 | 10/1 | 27–75 | CyA dosage: 3–6
months at an initial dose of 2.5–5 mg/kg daily and a maintenance
dose of 1.5–3 mg/kg daily | No. of voids/24 h;
daily urinary output; mean voided vol.; maximum voided vol. | N/A | Noted in 6 patients,
including mild hypertension (n=2), mild hyperplasia (n=2),
transient tremor (n=1) and growth of facial hair (n=1) |
| Sairanen, 2005
(16) | Finland | RCT | Fulfilled the NIDDK
criteria for IC. Exclusion criteria: History of cancer in the last
10 years, untreated hypertension or renal insufficiency [serum
creatinine higher than normal limits (>90 mg/dl in females or
>100 mg/dl in males)] | 64 | 53/11, | Case, 56.2±14.7;
control, 59.7±13.0 | Case group: 1.5 mg/kg
CyA twice daily (27 women, 5 men); control group: 100 mg PPS 3
times daily (26 women, 6 men) | Frequency in 24 h;
max awake bladder capacity; mean voided vol.; VAS; nocturia
episodes; ICSI and ICPI symptom scores, GRA; PST | 6 months | Mild adverse
events |
| Sairanen, 2008
(17) | Finland | RCT | Fulfilled the NIDDKD
criteria | 37 | 32/5, | Case, 55.7±14.8;
control, 56.4±13.2 | Case group: 1.5 mg/kg
CyA twice daily; control group: 100 mg PPS 3 times daily | GRA; EGF, IL-6; pain
score (VAS); urinary frequency 24 h; mean voided volume; ICSI
score | 6 months | N/A |
| Sairanen, 2009
(18) | Finland | RCT | Fulfilled the NIDDKD
criteria | 64 | 51/13 | Case, 53.2±11.9;
control, 57.3±10.0 | Case group: 1.5 mg/kg
CyA twice daily; control group: 100 mg PPS 4 times daily | General health
perceptions; pain; emotional wellbeing; vitality; social
functioning; physical capacity; sexual interest; sexual
functioning | 6 months | N/A |
Six studies used the NIDDK criteria for diagnosing
IC (12,14,16–18); the
other two studies used clinical symptoms to diagnose PBS/IC
(11,13). The subjects of two studies (13,14) were
patients in whom multiple first line therapies had failed. Regimen
protocols were presented in all studies; the initial dosage of CyA
was typically 2–3 mg/kg/day, sometimes divided into 2 daily
dosages. If the symptoms were alleviated, the dosage of CyA was
gradually decreased to a tolerance dose; if adverse events occurred
the dose was reduced or the drug was stopped. In the three RCTs
(16–18), the treatment therapy administered to
the control group was 100 mg PPS 4 times daily.
Methodological quality
All studies have detailed descriptions of the
ascertainment of diagnosis, assessment of outcome and the
instruments used to assess outcomes. All of the articles, with the
exception of one (15), reported the
follow-up time; the longest duration of follow-up was 5 years
(12) and, the proportion of
patients lost to follow-up was <20% in all studies. On the basis
of the Jadad scale, the scores for the included RCTs ranged from 1
to 3 (regard as low methodological quality).
Outcome assessment
The outcome assessment of the included studies is
presented in Table II. The
O'Leary-Sant Symptom and Problem Indices of Interstitial Cystitis
(ICSI and ICPI, respectively) were used as outcome measures in 5
studies (11–13,16,17).
Other outcome parameters were reported, including mean voided
volume (ml) (12,14–17),
maximum awake bladder capacity (ml) (14–16),
urinary frequency (24 h) (14–17),
pain score (visual analog scale, VAS) (16,17),
cytokine levels (11,17), daily urinary output (ml) (15), nocturia episodes (16) and improvement of quality of life
(QoL) (18). All outcome parameters,
with the exception of the biomarker IL-6, were reported to exhibit
improvement following treatment of CyA, even though the degree of
improvement varied. However, Sairanen et al (17) reported that IL-6 was significantly
(P=0.04) decreased in patients ≥53 years old following treatment
with CyA, but not following treatment with PPS (P=0.73). All
reported parameters in the three RCTs implied that the treatment
effects of CyA were far superior to those of PPS treatment. In
addition, long-term treatment with a low dosage of CyA could
provide a persistent improvement of symptoms. The symptoms were
recurrence if the treatment of CyA was discontinued.
 | Table II.Outcome assessment of included
studies. |
Table II.
Outcome assessment of included
studies.
| Outcome | First author, year
(Refs.) | Results |
|---|
| ICSI and ICPI | Ehren, 2013 (11) | ICSI: reduced
from16±1 to 8±1; ICPI: reduced from 14±1 to 6±1, after 12 weeks;
ICSI and ICPI remained at a low level when treatment was continued;
without treatment ICSI and ICPI increased again, to 12±2 and 9±2,
respectively |
|
| Chade, 2014 (12) | ICSI: reduced from 36
initially to 21.6 at 6 months, and 8.4 at 5 years (P<0.001);
ratio of patients with ICPI scores >8 reduced from 100 to
22% |
|
| Forrest, 2012
(13) | 26/44 (59%) patients
with symptom response, 10/44 (23%) patients without symptom
response; patients with HL had a higher response (23/34, 68%) than
patients without HL; low dosage of treatment for symptom
maintance |
|
| Sairanen, 2005
(16) | Sum of ICSI + ICPI:
−15.0±9.4 in CyA group vs. −3.1±4.3 in PPS group (P<0.001);
ICSI: −7.9±4.5 in CyA group vs. −2.0±2.6 in PPS group (P<0.001);
ICPI: −7.1±4.4 in CyA group vs. −1.5±1.8 in PPS group
(P<0.001) |
|
| Sairanen, 2008
(17) | Sum of ICSI + ICPI:
Reduced from 28.8 to 15.1 in CyA group vs. 30.5 to 28.5 in PPS
group; responder (GRA, 5 or 6): 13/18 (72%) in CyA group vs. 3/19
(16%) in PPS group |
| Mean voided volume
(ml) | Chade, 2014 (12) | Upregulated from 103
pretreatment to 170 after CyA treatment |
|
| Sairanen, 2004
(14) | Upregulated from
101.47 (SD, 42.7) pretreatment to 246.4 (SD, 97.9) after CyA
treatment (P<0.001) |
|
| Forsell, 1996
(15) | Upregulated from
100.4 (SD, 58.4) pretreatment to 173.7 (SD, 84.0) after CyA
treatment |
|
| Sairanen, 2005
(16) | Improvement: 59±57 in
CyA group vs. 1±31 in PPS group (P<0.001) |
|
| Sairanen, 2008
(17) | Improvement: From
28.8 (SD, 4.8) to 15.1 (SD, 8.6) in CyA group vs. 30.5 (SD, 3.8) to
28.5 (SD, 5.6) in PPS group (P=0.003) |
| Maximum awake | Sairanen, 2004
(14) | Upregulated from
168.8 (SD, 74.6) pretreatment to 360.7 (SD, 99.3) after CyA
treatment (P<0.001) |
| bladder capacity
(ml) | Forsell, 1996
(15) | Upregulated from
179.1 (SD, 96.3) pretreatment to 361.4 (SD, 137.6) after CyA
treatment |
|
| Sairanen, 2005
(16) | Improvement: 81±94 in
CyA group vs. 2.8±60 in PPS group (P=0.003) |
| Urinary frequency (24
h) | Sairanen, 2004
(14) | Reduced from 20.8
(SD, 6.3) pretreatment to 10.2 (SD, 3.8) after CyA treatment
(P<0.001) |
|
| Forsell, 1996
(15) | Reduced from 20.55
(SD, 6.2) pretreatment to 11.9 (SD, 4.0) after CyA treatment |
|
| Sairanen, 2005
(16) | Improvement:
−6.7±4.7 in CyA group vs. −2.0±5.1 in PPS group (P<0.001) |
|
| Sairanen, 2008
(17) | Reduced from 16.4
(SD, 3.5) to 11.0 (SD, 4.2) in CyA group vs. 19.5 (SD, 5.9) to 18.4
(SD, 6.3) in PPS group (P=0.005) |
| Pain score
(VAS) | Sairanen, 2005
(16) | Improvement:
−4.7±3.5 in CyA group vs. −1.6±3.3 in PPS group (P<0.001). |
|
| Sairanen, 2008
(17) | Reduced from 7.0
(SD, 2.2) to 2.2 (SD, 2.5) in CyA group vs. 7.5 (SD, 2.1) to 5.8
(SD, 3.1) in PPS group (P=0.005). |
| Cytokine
levels | Ehren, 2013
(11) | NO (ppm): Reduced
after treatment with CyA, from 489±141 initially to 16±6 (2 weeks),
10±7 (4 weeks), 7±3 (6 weeks) and 3±1 (8 weeks); increased to
144±70 when the dosage of CyA was reduced to 0.75 mg/kg/day;
without treatment in last 2 weeks, the level rose to 478±187. |
|
| Sairanen, 2008
(17) | EGF (mean, ng/mg
creatinine): reduced from 35 to 28 in CyA group (P=0.034) vs. 33 to
32 in PPS group (P=0.81); ≥ 53 years old, reduced from 34 to 23 in
CyA group (P=0.001) vs. 31 to 30 in PPS group (P=1.00); IL-6 (mean,
pg/ml): reduced from 7.1 to 3.6 in CyA group (P=0.18) vs. 11 to 11
in 0.PPS group (P=38); ≥53 years old, reduced from 10.3 to 4.1 in
CyA group (P=0.04) vs. 13.7 to 10.3 in PPS group (P=0.73) |
| Daily urinary
output (ml) | Forsell, 1996
(15) | Upregulated from
1,832.7 (SD, 754) pretreatment to 1,954 (SD, 691.6) after CyA
treatment |
| Nocturia
episodes | Sairanen, 2005
(16) | Improvement:
−2.2±1.6 in CyA group vs. −0.2±2.1 in PPS group (P<0.001) |
| Improvement of
QoL | Sairanen, 2009
(18) | Improvement greater
in the CyA group than in the PPS group, including general health
perceptions, pain, emotional wellbeing, social functioning,
physical capacity, limitation days, % of patients undertaking
sexual activity (masturbation or intercourse) |
Adverse effects
Five of the 8 included studies reported
complications, which mainly comprised tiredness, abdominal pain,
nausea, diarrhea and gingival hyperplasia (1 patient), induced hair
growth (3 patients), serum creatinine elevation (12 patients) and a
rise in blood pressure (8 patients). Following symptomatic
treatment, the majority of patients were able to undergo sustained
CyA treatment. However, 4 patients were required to stop treatment
with CyA following increases in serum creatinine levels.
Discussion
The evidence from the included studies demonstrates
that oral treatment with CyA may be an appropriate therapeutic
strategy for patients with PBS/IC, and there is a trend towards
benefit with a long-term low-dosage regimen. To the best of our
knowledge, he present review summarizes all the prospective or
retrospective non-randomized cohort trials and RCTs that exist on
this topic.
CyA may be more advantageous in severe PBS/IC,
because two studies (11,13) included patients in whom the usual
IC/BPS treatments had failed, and the symptoms and parameters in
those patients improved following treatment with CyA (2–3 mg/kg
divided into 2 daily dosages with a maximum of 300 mg daily). In
addition CyA exhibited a higher curative effect than PPS. Long-term
low-dosage CyA treatment can provide and maintain good symptom
relief (19). However, symptoms may
recur if the therapy with CyA is stopped. Hence, it is suggested
that the use of CyA may be primarily as a drug for symptom
improvement rather than a fundamental solution or cure. In
addition, a series of adverse effects (in particular, upregulation
of serum creatinine levels and hypertension) are of concern when
oral CyA therapy is carried out.
There are several advantages of this systematic
review. Firstly, a critical issue has been addressed. The search
strategy was full-scale and without language restrictions.
Secondly, two reviewers independently screened the eligible
articles and extracted data with minimum errors. Thirdly, attempts
were made to contact authors of published and unpublished studies
to obtain relevant details for this systematic review.
However, the included studies contained small
numbers of patients with short-term or long-term follow-up. For one
included study (12), only the
abstract was available for analysis in this systematic review, and
it was not possible to obtain further details. The heterogeneity of
the diagnosis criteria of PBS/IC, differences in methods, varied
number of outcome measures employed and small number of
participants, create challenges in the analysis of the treatment
effect of CyA in PBS/IC. Therefore, further higher quality clinical
trials are required to further explore the effectiveness of oral
CyA in the treatment of PBS/IC.
References
|
1.
|
Clemens JQ, Link CL, Eggers PW, Kusek JW,
Nyberg LM and McKinlay JB: BACH Survey Investigators: Prevalence of
painful bladder symptoms and effect on quality of life in black,
Hispanic and white men and women. J Urol. 177:1390–1394. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Held PJ, Hanno PM, Wein AJ, Pauly MV and
Cahn MA: Epidemiology of interstitial cystitis. Interstitial
Cystitis. Hanno PM, Staskin DR, Krane RJ and Wein AJ: (London).
Springer. 29–48. 1990. View Article : Google Scholar
|
|
3.
|
Gillenwater JY and Wein AJ: Summary of the
National Institute of Arthritis, Diabetes, Digestive and Kidney
Diseases workshop on interstitial cystitis, National Institutes of
Health, Bethesda, Maryland, August 28–29, 1987. J Urol.
140:203–206. 1988.PubMed/NCBI
|
|
4.
|
Ogawa H, Kameda H, Amano K and Takeuchi T:
Efficacy and safety of cyclosporine A in patients with refractory
systemic lupus erythematosus in a daily clinical practice. Lupus.
19:162–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Forns X and Navasa M: Cyclosporine A or
tacrolimus for hepatitis C recurrence? An old debate. Am J
Transplant. 11:1559–1560. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Maza A, Montaudiè H, Sbidian E, Gallini A,
Aractingi S, Aubin F, Bachelez H, Cribier B, Joly P, Jullien D, et
al: Oral cyclosporin in psoriasis: A systematic review on treatment
modalities, risk of kidney toxicity and evidence for use in
non-plaque psoriasis. J Eur Acad Dermatol Venereol. 25(Suppl 2):
S19–S27. 2011. View Article : Google Scholar
|
|
7.
|
Sternthal MB, Murphy SJ, George J,
Kornbluth A, Lichtiger S and Present DH: Adverse events associated
with the use of cyclosporine in patients with inflammatory bowel
disease. Am J Gastroenterol. 103:937–943. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Dijkmans B and Gerards A: Cyclosporin in
rheumatoid arthritis: Monitoring for adverse effects and clinically
significant drug interactions. BioDrugs. 10:437–445. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analysis. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ehrén I, Grufman Hallén K, Vrba M,
Sundelin R and Lafolie P: Nitric oxide as a marker for evaluation
of treatment effect of cyclosporine A in patients with bladder pain
syndrome/interstitial cystitis type 3C. Scand J Urol. 47:503–508.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chade J, Chade D, Lucon AM, Bruschini H
and Srougi M: 462 5-year follow-up of patients with refractory
interstitial cystitis treated with cyclosporine A: A prospective
single-institution study. Eur Urol. (Supp 13): e4622014. View Article : Google Scholar
|
|
13.
|
Forrest JB, Payne CK and Erickson DR:
Cyclosporine A for refractory interstitial cystitis/bladder pain
syndrome: Experience of 3 tertiary centers. J Urol. 188:1186–1191.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Sairanen J, Forsell T and Ruutu M:
Long-term outcome of patients with interstitial cystitis treated
with low dose cyclosporine A. J Urol. 171:2138–2141. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Forsell T, Ruutu M, Isoniemi H, Ahonen J
and Alfthan O: Cyclosporine in severe interstitial cystitis. J
Urol. 155:1591–1593. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sairanen J, Tammela TL, Leppilahti M,
Multanen M, Paananen I, Lehtoranta K and Ruutu M: Cyclosporine A
and pentosan polysulfate sodium for the treatment of interstitial
cystitis: A randomized comparative study. J Urol. 174:2235–2238.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Sairanen J, Hotakainen K, Tammela TL,
Stenman UH and Ruutu M: Urinary epidermal growth factor and
interleukin-6 levels in patients with painful bladder
syndrome/interstitial cystitis treated with cyclosporine or
pentosan polysulfate sodium. Urology. 71:630–633. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Sairanen J, Tammela TL, Leppilahti M,
Onali M, Forsell T and Ruutu M: Potassium sensitivity test (PST) as
a measurement of treatment efficacy of painful bladder
syndrome/interstitial cystitis: A prospective study with
cyclosporine A and pentosan polysulfate sodium. Neurourol Urodyn.
26:267–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Gao X, Ma Y, Sun L, Chen D, Mei C and Xu
C: Cyclosporine A for the treatment of refractory nephritic
syndrome with renal dysfunction. Exp Ther Med. 7:447–450.
2014.PubMed/NCBI
|