Introduction
Postoperative cognitive dysfunction (POCD) is
generally considered a subtle decline in the cognitive function
that occurs in patients following surgery, for which the elderly
population is at high risk. While it is believed that general
anesthetics produce reversible restraining effects on the brain, it
has been shown that use of anesthetics results in prolonged
anesthesia-related neuropathophysiological effects after their
elimination, such as neuroinflammation (1), blood-brain barrier disruption (2), neurotoxicity (3,4) or
persistent depression of synaptic function (5), and are likely involved in the
pathogenesis of POCD.
N-methyl-D-aspartic acid receptors (NMDARs) are a
major type of excitatory neurotransmitter-gated ionotropic channels
with a particularly important role in synaptic plasticity, learning
and memory (6,7). NMDARs are one of the important targets
of general anesthetics. Previous evidence suggested that isoflurane
exposure induced a prolonged NMDAR subunit, and GluN2B upregulation
after anesthetic elimination, which was accompanied by changes in
cognitive function, albeit the behavioral outcomes were
inconsistent (8–12). Functional NMDARs are composed of two
obligatory GluN1 subunits, together with two GluN2 subunits or a
combination of GluN2 and GluN3 subunits, and most central NMDARs
are GluN1/GluN2 assemblies. GluN2A and GluN2B are the primary GluN2
subunits in the forebrain including the hippocampus. After
maturation, most GluN2A-containing NMDA receptors are incorporated
into synapses. GluN2B-containing NMDA receptors are also present in
synapses, but are mainly found extrasynaptically. NMDARs with
different subunits and localization trigger different signaling
pathways (13), which play different
roles in synaptic plasticity, learning and memory, cell survival,
excitotoxicity and cell death (6,14,15).
It is not clear whether synaptic and extrasynaptic
GluN2B subunits have different roles in anesthetic-induced
cognitive dysfunction. Therefore, the aim of the study was to
determine the effects of isoflurane on the expression of the NMDAR
subunits, GluN2A and GluN2B, as well as the associated alteration
of cognitive function in aged rats. The specific GluN2B antagonist,
Ro25–6981, was administered to test the role of GluN2B in
anesthetic-induced changes of spatial learning and memory.
Materials and methods
Animals
Sprague-Dawley rats (male, 20 months of age and
weighing 500–600 g) obtained from the Dongchuang Laboratory Animal
Center, Changsha (Hunan, China) were used for the experiments. The
animals were housed in a temperature- and humidity-controlled room
(21±20°C, 60%) using a 12-h light/dark cycle (light on at 06:00)
with food and water available ad libitum. The animals were
given an interval of at least 14 days to adapt to their new
environment prior to experiments. The animal protocol was approved
by the Peking University Biomedical Ethics Committee, Experimental
Animal Ethics Branch (approval no. LA 2012-38).
One week prior to anesthesia exposure, the animals
were trained to swim in the Morris water maze (MWM) without a
platform, four times per day for at least 3 days, and there was a 1
min interval between each 2 min of swimming. Animals with
continuous thigmotactic behavior or those floating without swimming
for the last 2 days were excluded from experiments.
Isoflurane exposure
The animals were randomly exposed to isoflurane
(Baxter Healthcare of Puerto Rico, Guayama, Puerto Rico).
Isoflurane (1.5%) with 2 l/min 100% oxygen as carrying gas (n=30)
or vehicle gas (Beijing Millennium City Gas Sales Center (Beijing,
China) was employed at 2 l/min 100% oxygen (n=30) for 4 h. To
examine the role of GluN2B in anesthetic-induced changes of spatial
learning and memory, an additional two groups (n=30, respectively)
were administered the specific GluN2B antagonist, Ro25-6981 (3
mg/kg, 2 ml/kg, i.p.; Sigma-Aldrich, St. Louis, MO, USA) daily for
6 days, 24 h after exposure to isoflurane or vehicle gas.
The animals were maintained in an anesthesia chamber
during isoflurane or vehicle gas exposure. At the outlet of the
chamber, the concentrations of isoflurane, oxygen, and carbon
dioxide in the chamber were continuously analyzed with a gas
monitor (Datex-Ohmeda, Inc., Louisville, CO, USA). In a previous
study, it was shown that this anesthesia protocol did not cause
significant changes in blood gas or glucose (1,2).
MWM
After 24 h of treatment, 10 animals from each group
were trained for MWM tests for 6 days. Another 10 animals from each
group were used for the same tests after 30 days of treatment. The
MWM tests were conducted by investigators blind to the group
conditions as previously described (1,2). The
swimming route was observed by video (Beijing Sunny Instruments Co.
Ltd., Beijing, China). The time spent to locate the submerged
platform by the animals (defined by the latency cut-off time point
of 120 sec) and the swimming velocity were recorded. On day 6, a
probe trial was performed without the platform. The percentage of
time spent in the previous platform quadrant in a 120-sec period
was determined.
Hippocampal tissue harvest and
separation of synaptic and extrasynaptic membranes
Following anesthesia exposure, 3 animals from the
isoflurane and vehicle gas exposure groups were sacrificed by
decapitation at 1, 7 and 30 days post-treatment for harvesting of
the hippocampus without MWM tests. The separation of synaptic and
extrasynaptic fractions was performed as described by Zhang et
al and Goebel-Goody et al (16,17),
which was based on the principle that the postsynaptic density
(PSD) protein-associated or synaptic fraction was insoluble in
Triton X-100, whereas the non-PSD protein-associated or
extrasynaptic fraction was soluble in Triton X-100.
Antibodies and immunoblotting
The protein extracted from the hippocampal tissue
homogenate, including the total protein, PSD protein-associated (or
synaptic) fraction and the non-PSD protein-associated (or
extrasynaptic) fraction were used for western blot analysis to test
the expression of GluN2A and GluN2B. Total protein (60 µg), as well
as 40 µg protein from synaptic fraction and extrasynaptic fraction
per lane were separated electrophoretically in 8% SDS-PAGE gels,
and transferred to nitrocellulose membranes (Millipore, Newyork,
USA). The membranes were incubated in rat polyclonal antibodies
against GluN2B (1:1,000, cat no.: ab65783) and polyclonal
antibodies against GluN2A (1:1,000, cat no.: ab14596) (both from
Abcam, Cambridge, MA, USA). The binding of the primary antibodies
was detected by fluorescently-labeled secondary antibody
(1:10,000), and was visualized by scan-ning membranes in an Odyssey
infrared imaging system (both from LI-COR Biosciences, Lincoln, NE,
USA). For densito-metric analysis, the signal intensity was
quantified as a ratio of GluN2A or 2B/actin and normalized to the
values of the corresponding control animals.
Statistical analysis
Statistical analyses were performed using SPSS 20.0
for Windows (SPSS, Inc., Chicago, IL, USA). The values of latency
and swimming speed in the MWM test were analyzed using two-way
repeated-measures analysis of variance (ANOVA), with Bonferroni
post-hoc analysis. The percentage of time spent in the previous
platform quadrant and data from the western blot analysis were
compared between isoflurane and control groups by one-way ANOVA
using Bonferroni post-hoc analysis. Data were presented as mean ±
SEM and statistical significance was set at P<0.05.
Results
Isoflurane exposure induces reversible
spatial learning and memory impairment
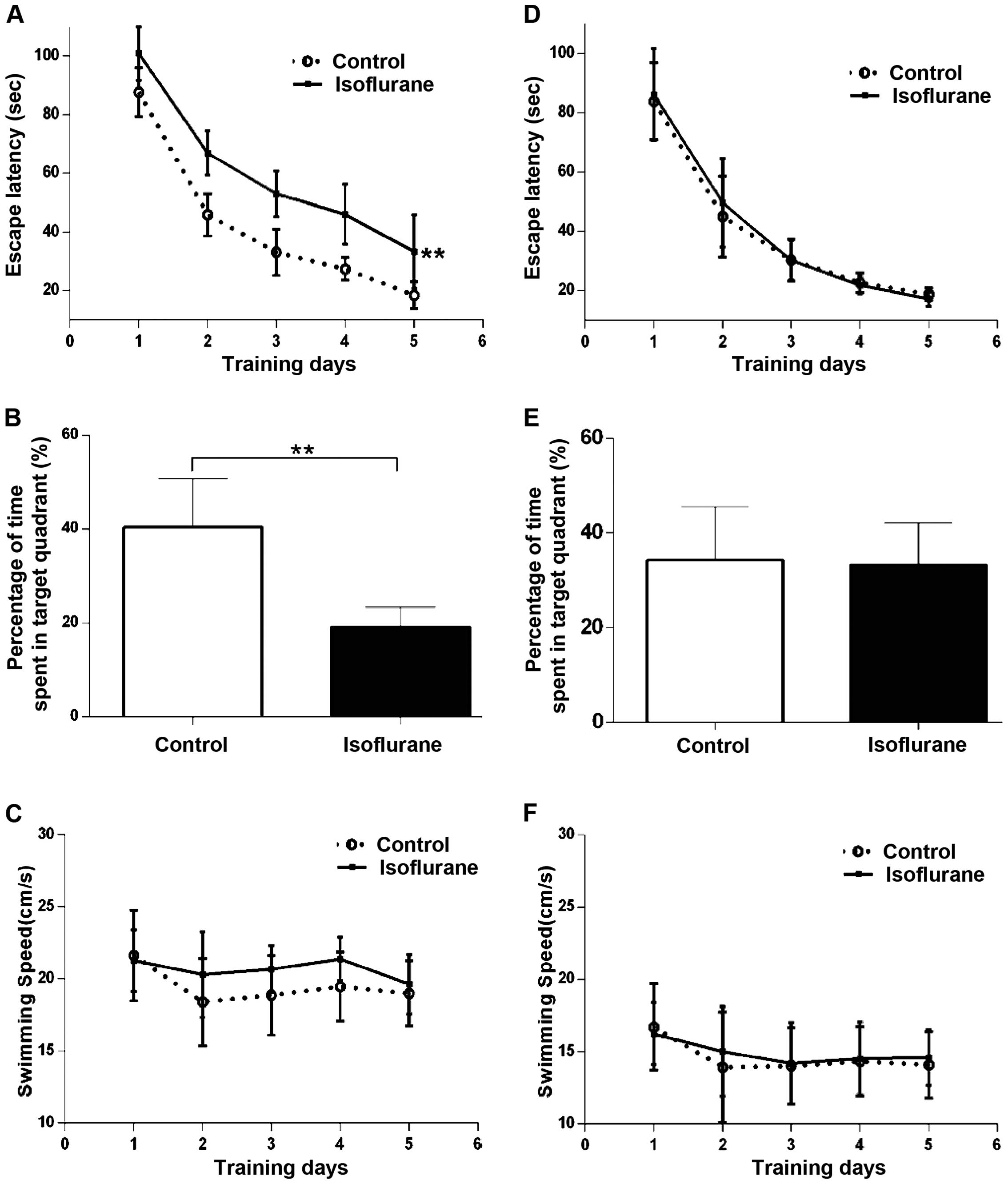
We used the MWM test to investigate whether
isoflurane affects spatial learning and memory. Fig. 1 shows the results of these
experiments in the first week (Fig.
1A–C) and 30 days (Fig. 1D–F)
after anesthesia. During the first week after treatment, the time
required to locate the platform (latency) in the spatial
acquisition training was significantly affected by isoflurane
treatment compared to the control group (Fig. 1A; P<0.01). A probe trial was
conducted to evaluate reference memory at the end of learning. The
time spent in the platform area by the rats in the isoflurane group
was shorter than that in the control group (Fig. 1B; P<0.01). Isoflurane treatment
had no effect on swimming speed compared to the control group
(Fig. 1C; P>0.05). Rats from each
group not receiving MWM test during the first week post-anesthesia
underwent the same procedures of MWM test 30 days after the
treatment. We found that there were no differences in escape
latency and percentage of time spent in the target quadrant between
the isoflurane and control groups (Fig.
1D and E; P>0.05). The swimming speed between the two groups
showed no difference during the first week and 30 days after
treatment (Fig. 1F; P>0.05).
These results indicated that the spatial learning and memory in MWM
test was impaired at least 1 week following isoflurane exposure,
but recovered to the control level 30 days after the treatment.
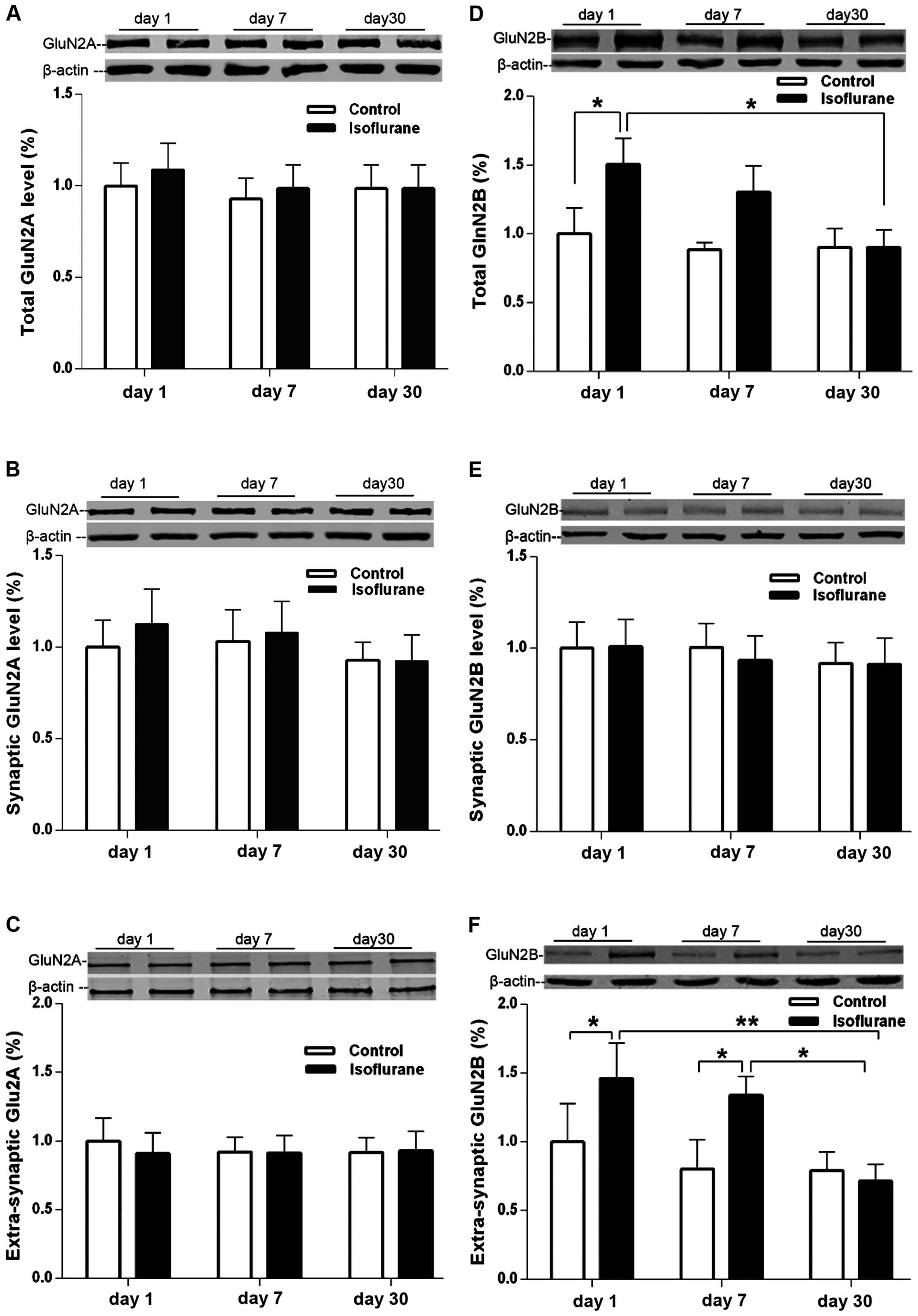
GluN2 subunit expression in the
hippocampus increases following isoflurane exposure
Rats in the isoflurane and control groups were
decapitated at 1, 7 and 30 days post-treatment to collect the
hippocampus without MWM tests. The hippocampal NMDARs GluN2A and
GluN2B subunit protein expression is shown in Fig. 2. The synaptic GluN2A levels showed no
difference between isoflurane and control groups at 1, 7 and 30
days following treatment (Fig. 2A–C;
P>0.05, between isoflurane and control groups). The levels of
total GluN2B protein in the hippocampus increased 24 h after the
isoflurane exposure compared with the control group (Fig. 2D; P<0.05, between the isoflurane
and control groups). At 7 days after treatment, the GluN2B levels
in the isoflurane group were higher than those in the control group
although there was no statistical significance (Fig. 2D; P>0.05), and they returned to
the control levels after 30 days following the isoflurane exposure
(Fig. 2D; P>0.05 between the
isoflurane and control groups). The levels of extrasynaptic GluN2B
in the hippocampus also increased 24 h following isoflurane
exposure compared with the control group (Fig. 2F; P<0.05), and remained higher
than that in the control group at 7 days after treatment (Fig. 2F, p<0.05). However, the levels
also returned to the control levels 30 days after the treatment
(Fig. 2F, P>0.05 between the
isoflurane and control groups). The synaptic levels of GluN2B
showed no difference between the isoflurane and control groups at
1, 7 or 30 days after treatment (Fig.
2E, P>0.05).
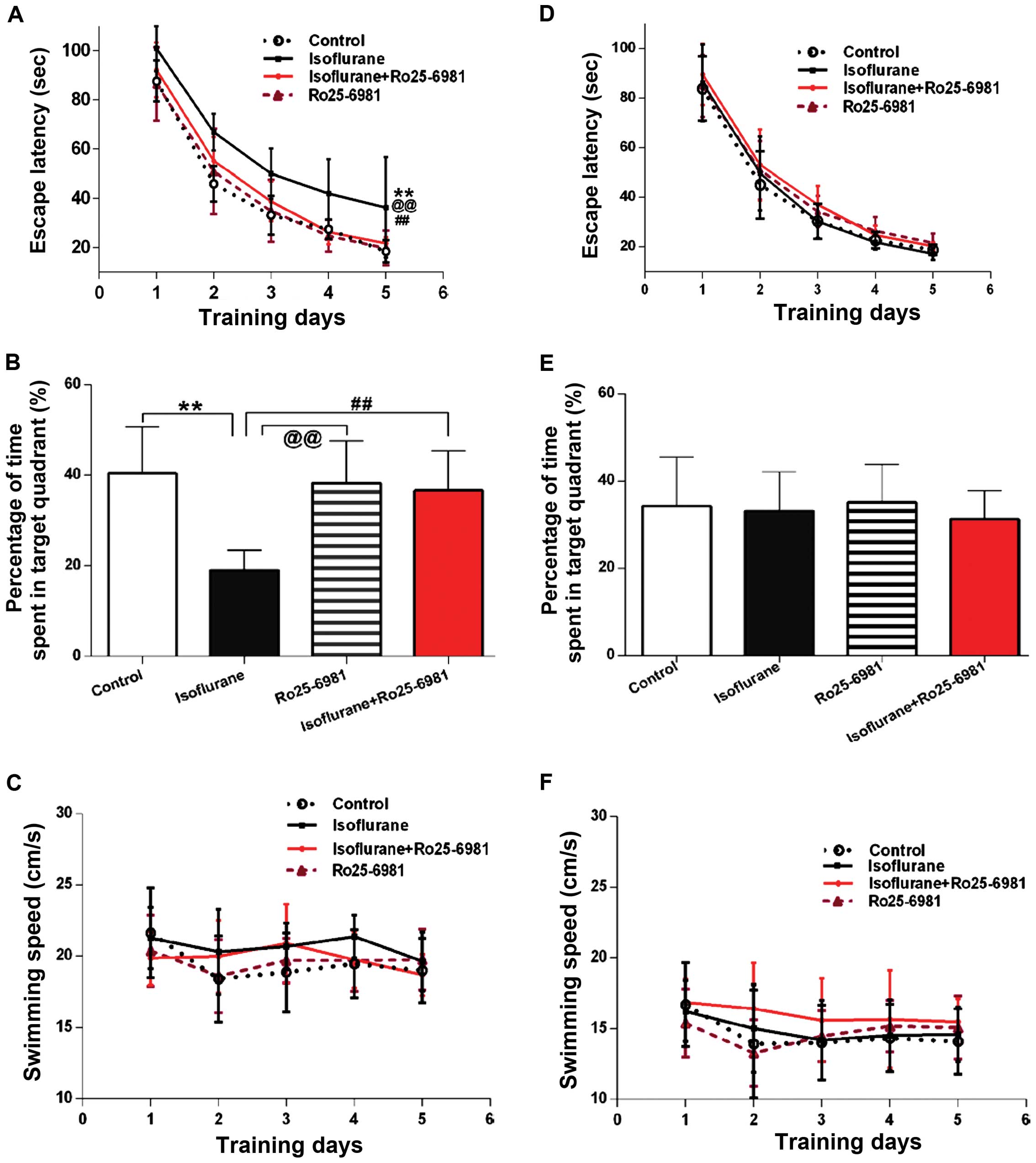
GluN2B-specific antagonist Ro25-6981
alleviates spatial learning and memory impairment induced by
isoflurane exposure
During the first week after treatment, Ro25-6981
significantly reduced the latency of rats exposed to isoflurane in
spatial acquisition training (Fig.
3A; P<0.05 between the isoflurane and isoflurane + Ro25-6981
groups), and the time spent in the platform area in the probe trial
was prolonged by Ro25–6981, compared to the rats treated only with
isoflurane (Fig. 3B, P<0.01
between the isoflurane and isoflurane + Ro25-6981 groups). However,
there were no differences in the two aforementioned outcomes in the
four groups 30 days after treatment (Fig. 3D and E; P>0.05). No differences in
swimming speed in the four groups were identified (P>0.05). No
differences in swimming speed in the four groups were identified
(Fig. 3C and F; P>0.05).
The latency during the acquisition training was not
affected by Ro25-6981 alone, when compared with the control levels
during the first week and 30 days thereafter (Fig. 3A and D; P>0.05). The time spent in
the platform area in the probe trial was not different between the
control and Ro25-6981 groups during the 7 or 30 days after
treatment (Fig. 3B and E;
P>0.05).
Discussion
POCD has become a more common phenomenon in the
aging population, albeit its mechanism remains unclear. Anesthetics
may partially consitute the underlying problem. The majority of
investigations pertaining to anesthetic-induced cognitive
dysfunction were focused on neurotoxicity and cell death, and the
majority of supporting evidence stemmed from cultured cells and the
developing brain (18,19). Although there are clinical studies
that support that age is a risk factor for cognitive dysfunction,
there is little evidence to show that the aforementioned
neurotoxicity and cell death are involved in cognitive dysfunction
in animal models on aging (3,20). On
the other hand, epidemiological evidence has demonstrated that the
prevalence rate of POCD has decreased over time, suggesting that
POCD in most patients may be temporary and self-limiting (21–23).
Stratmann et al identified that aged rats exposed to
isoflurane showed no evidence of ongoing cell death, impaired
hippocampal neurogenesis, or long-term cognitive impairment
(24). Cognitive impairment by
anesthetics in the aging brain manifest certain temporary
characteristics of Alzheimer's disease, such as elevated Aβ
production and τ hyperphosphorylation (25), which caused synaptic dysfunction
without obvious neural loss at the early phase of this disease
(26–30). Evidence suggests that inhibitory
ionotropic receptors, α-5 subunit containing γ aminobutyric acid-A
receptors (α-5GABAARs) can be upregulated and maintain a persistent
inhibitory current for 1 week even after a single dosage of
etomidate (5). Given the
aforementioned evidence, it is reasonable to hypothesize that
delayed inhibitory effects in neural activity secondary to
anesthetic exposure, even after their elimination, may partially
account for cognitive dysfunction, in addition to cell death.
NMDARs are a target of general anesthetics, and play
an important role in synaptic plasticity, learning and memory
(31). Previous findings have shown
that the effects on NMDARs by isoflurane can be sustained beyond
the elimination of anesthetics and are involved in changes in
cognitive function, albeit the behavioral outcomes were
inconsistent (8–12). Our results demonstrate spatial
learning and memory in aged rats tested in the MWM. These rats
showed impairment of these parameters for at least 1 week following
isoflurane exposure, and returned to the control levels 30 days
later, suggesting that isoflurane did not induce permanent damage
to the central nervous system. We also found that isoflurane
exposure for 4 h may induce prolonged changes in GluN2B expression
levels for at least 1 week following isoflurane treatment. It is a
common pharmacological phenomenon that chronic treatment with
receptor antagonists can lead to an increased density of receptors.
Evidence suggests that acute (8,9,12) and chronic (32) treatment with anesthetics targeting
NMDARs results in the upregulation of NMDA receptors as assessed by
the levels of GluN2B subunit protein and mRNA. Since
GluN2B-containing receptors are highly sensitive to isoflurane
compared to GluN2A-containing receptors (33), the finding that only GluN2B was
upregulated in the hippocampus, whereas GluN2A was not altered, can
be attributed to the high affinity of isoflurane for
GluN2B-containing receptors, which allows for the specific
upregulation of GluN2B expression in the hippocampus following
acute isoflurane exposure.
Furthermore, we found that extrasynaptic GluN2B
protein expression, but not synaptic GluN2B, increased
significantly after 4 h of isoflurane exposure compared to
non-isoflurane exposure, and treatment with the GluN2B antagonist
Ro25-6981 was able to alleviate this impairment. Since the majority
of GluN2A is incorporated into synapses, and GluN2B is mainly found
extrasynaptically, these outcomes indicate that extrasynaptic
GluN2B subunit upregulation may be involved in isoflurane-induced
cognitive dysfunction.
GluN2B subunits are particularly important for
plasticity, learning and memory, and their location in synaptic and
extrasynaptic compartments play different roles (6,14,16,17,34–36).
Elevations in the synaptic GluN2B subunit levels and
GluN2B-containing NMDARs are involved in spatial learning tasks and
hippocampal long-term potentiation (LTP) enhancement (36,37),
whereas intrasynaptic GluN2B reduction is accompanied by cognitive
impairment (16,38). On the other hand, activating
extrasynaptic GluN2B can facilitate hippocampal long-term
depression (LTD) and inhibit LTP (34,35).
Therefore, increasing extrasynaptic GluN2B expression by isoflurane
exposure may produce inhibitory effects on synaptic transmission
and cause loss of cognitive function.
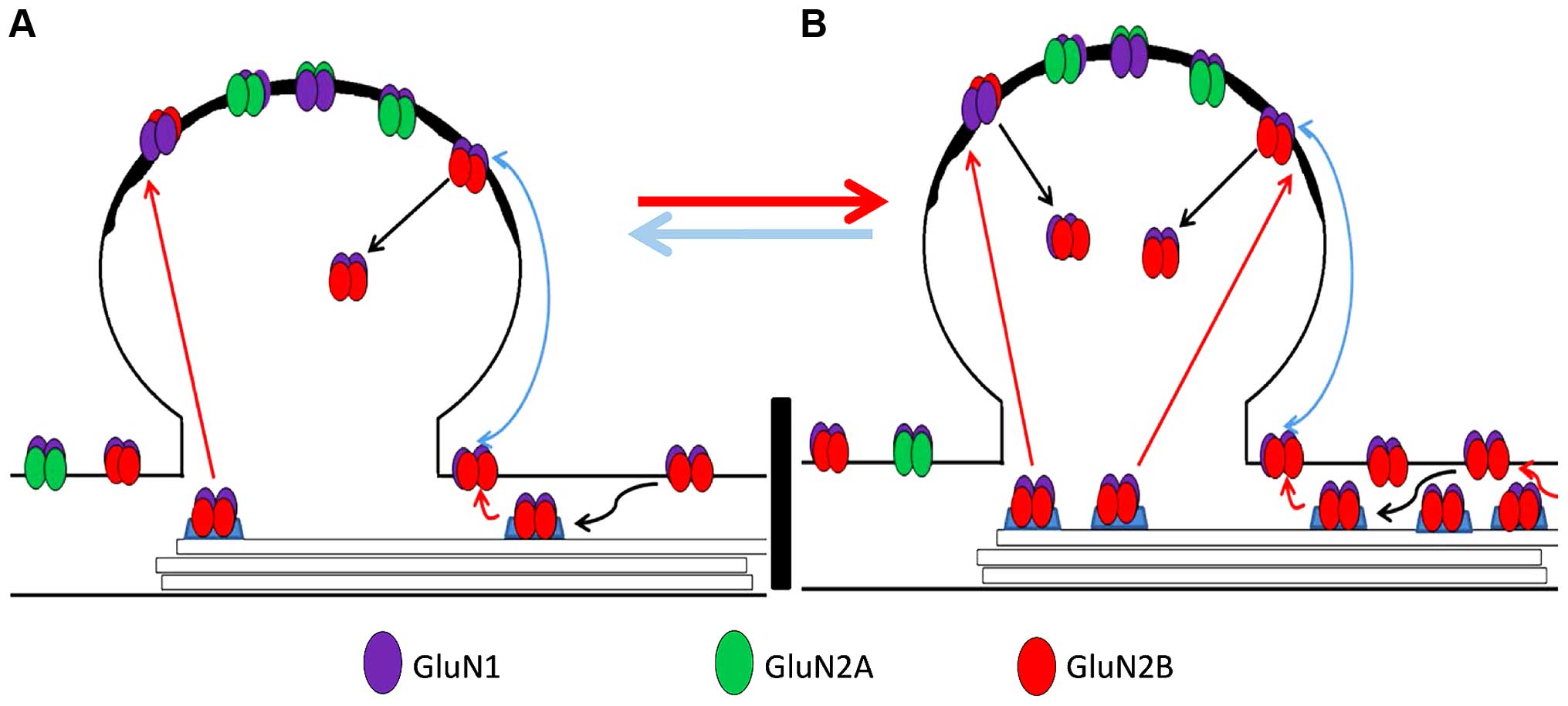
The molecular machinery regulating the sub-cellular
localization of GluN2B subunits remains to be elucidated. Evidence
suggests that anesthetics and aging can disturb these mechanisms.
For example, it has been recognized that phosphorylation of GluN2B
is important for the regulation of such processes (39), which can be influenced by aging and
exposure to anesthetics (40–42).
Increasing levels of CDK5 with age are capable of regulating the
binding of Src to PSD-95, leading to dephosphorylation of
tyrosine-1472 of GluN2B at synapses and its endocytosis, and
reducing levels of synaptic GluN2B, but does not affect
extrasynaptic GluN2B levels (43,44).
Therefore, isoflurane-induced upregulation of GluN2B, combined with
increased CDK5 in aging rats, which curtails the synaptic location
of GluN2B by dephosphorylation of tyrosine-1472, or by directly
phosphorylating GluN2B at Serine-1116, decreasing its synaptic
expression (45) may explain our
result that intrasynaptic GluN2B expression showed no changes,
while there was upregulation of total and extrasynaptic GluN2B. The
schematic in Fig. 4 outlines the
process. The animal model used by Rammes et al (8) was an adult model and may have had a
relatively lower activity of CDK5 and lower GluN2B endocytosis
compared to aged animals, and consequently, the upregulation of
total GluN2B may lead to increased synaptic GluN2B and improved
cognitive function.
Although previous findings have shown that systemic
or intracerebral administration of NMDAR antagonists affects
learning and memory performance on various tasks, including spatial
learning and memory test in MWM (46–48), our
findings showed that rats treated with Ro25-6981 alone have no
changes in spatial learning and memory compared with the control
animals. However, rats exposed to isoflurane and Ro25-6981 present
improved cognitive function compared to the isoflurane group.
Possible explanations include that intrasynaptic GluN2B-containing
NMDA receptors are not sensitive to the small dose of Ro25-6981
used in our investigation under ‘normal’ physiological conditions,
or the latter cannot pass through blood-brain barrier without
isoflurane (2). A higher dose of
Ro25-6981 may interfere with NMDARs function severely and lead to
cognitive impairment (8,48).
In conclusion, the present data suggest that
isoflurane may induce reversible cognitive impairment, and
isoflurane-induced sustained upregulation of extrasynaptic GluN2B,
not synaptic GluN2B, after anesthesia, may be involved in this
cognitive impairment. The precise mechanisms for general
anesthetic-mediated modulation of the subcellular localization of
GluN2B subunits and their role in cognitive function remain to be
elucidated.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81371205,
81571036 and 81571044), and the National Basic Research Program of
China (973 Program, no. 2012CB911004).
References
|
1.
|
Li ZQ, Rong XY, Liu YJ, Ni C, Tian XS, Mo
N, Chui DH and Guo XY: Activation of the canonical nuclear
factor-κB pathway is involved in isoflurane-induced hippocampal
interleukin-1β elevation and the resultant cognitive deficits in
aged rats. Biochem Biophys Res Commun. 438:628–634. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Cao Y, Ni C, Li Z, Li L, Liu Y, Wang C,
Zhong Y, Cui D and Guo X: Isoflurane anesthesia results in
reversible ultrastructure and occludin tight junction protein
expression changes in hippocampal blood-brain barrier in aged rats.
Neurosci Lett. 587:51–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Jevtovic-Todorovic V, Absalom AR, Blomgren
K, Brambrink A, Crosby G, Culley DJ, Fiskum G, Giffard RG, Herold
KF, Loepke AW, et al: Anaesthetic neurotoxicity and
neuroplasticity: an expert group report and statement based on the
BJA Salzburg Seminar. Br J Anaesth. 111:143–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Rappaport BA, Suresh S, Hertz S, Evers AS
and Orser BA: Anesthetic neurotoxicity-clinical implications of
animal models. N Engl J Med. 372:796–797. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Zurek AA, Yu J, Wang DS, Haffey SC,
Bridgwater EM, Penna A, Lecker I, Lei G, Chang T, Salter EW, et al:
Sustained increase in α5GABAA receptor function impairs memory
after anesthesia. J Clin Invest. 124:5437–5441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Shipton OA and Paulsen O: GluN2A and
GluN2B subunit-containing NMDA receptors in hippocampal plasticity.
Philos Trans R Soc Lond B Biol Sci. 369:201301632013. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lee YS and Silva AJ: The molecular and
cellular biology of enhanced cognition. Nat Rev Neurosci.
10:126–40. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Rammes G, Starker LK, Haseneder R,
Berkmann J, Plack A, Zieglgänsberger W, Ohl F, Kochs EF and Blobner
M: Isoflurane anaesthesia reversibly improves cognitive function
and long-term potentiation (LTP) via an up-regulation in NMDA
receptor 2B subunit expression. Neuropharmacology. 56:626–636.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Mawhinney LJ, de Rivero Vaccari JP, Alonso
OF, Jimenez CA, Furones C, Moreno WJ, Lewis MC, Dietrich WD and
Bramlett HM: Isoflurane/nitrous oxide anesthesia induces increases
in NMDA receptor subunit NR2B protein expression in the aged rat
brain. Brain Res. 1431:23–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Chi H, Kawano T, Tamura T, Iwata H,
Takahashi Y, Eguchi S, Yamazaki F, Kumagai N and Yokoyama M:
Postoperative pain impairs subsequent performance on a spatial
memory task via effects on N-methyl-D-aspartate receptor in aged
rats. Life Sci. 93:986–993. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wang WY, Jia LJ, Luo Y, Zhang HH, Cai F,
Mao H, Xu WC, Fang JB, Peng ZY, Ma ZW, et al: Location- and
subunit-specific NMDA receptors determine the developmental
sevoflurane neurotoxicity through ERK1/2 signaling. Mol Neurobiol.
53:216–230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Liu J, Zhang X, Zhang W, Gu G and Wang P:
Effects of sevoflurane on young male adult C57BL/6 mice spatial
cognition. PLoS One. 10:e01342172015. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ivanov A, Pellegrino C, Rama S, Dumalska
I, Salyha Y, Ben-Ari Y and Medina I: Opposing role of synaptic and
extrasynaptic NMDA receptors in regulation of the extracellular
signal-regulated kinases (ERK) activity in cultured rat hippocampal
neurons. J Physiol. 572:789–798. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hardingham GE and Bading H: Synaptic
versus extrasynaptic NMDA receptor signalling: Implications for
neurodegenerative disorders. Nat Rev Neurosci. 11:682–696. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Soria FN, Pérez-Samartín A, Martin A, Gona
KB, Llop J, Szczupak B, Chara JC, Matute C and Domercq M:
Extrasynaptic glutamate release through cystine/glutamate
antiporter contributes to ischemic damage. JClin Invest.
124:3645–3655. 2014. View
Article : Google Scholar
|
|
16.
|
Zhang X, Xin X, Dong Y, Zhang Y, Yu B, Mao
J and Xie Z: Surgical incision-induced nociception causes cognitive
impairment and reduction in synaptic NMDA receptor 2B in mice. J
Neurosci. 33:17737–17748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Goebel-Goody SM, Davies KD, Alvestad
Linger RM, Freund RK and Browning MD: Phospho-regulation of
synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult
hippocampal slices. Neuroscience. 158:1446–1459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hudson AE and Hemmings HC Jr: Are
anaesthetics toxic to the brain? Br J Anaesth. 107:30–7. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Rappaport BA, Suresh S , Hertz S, Evers AS
and Orser BA: Anesthetic neurotoxicity-clinical implications of
animal models. N Engl J Med. 372:796–7. 2015.
|
|
20.
|
Brambrink AM, Orfanakis A and Kirsch JR:
Anesthetic neurotoxicity. Anesthesiol Clin. 30:207–228. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Moller JT, Cluitmans P, Rasmussen LS, Houx
P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD,
et al: Long-term postoperative cognitive dysfunction in the elderly
ISPOCD1 study. ISPOCD investigators. International study of
post-operative cognitive dysfunction. Lancet. 351:857–861. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Newman S, Stygall J, Hirani S, Shaefi S
and Maze M: Postoperative cognitive dysfunction after noncardiac
surgery: A systematic review. Anesthesiology. 106:572–590. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Nadelson MR, Sanders RD and Avidan MS:
Perioperative cognitive trajectory in adults. Br J Anaesth.
112:440–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Stratmann G, Sall JW, Bell JS, Alvi RS,
May L, Ku B, Dowlatshahi M, Dai R, Bickler PE, Russell I, et al:
Isoflurane does not affect brain cell death, hippocampal
neurogenesis, or long-term neurocognitive outcome in aged rats.
Anesthesiology. 112:305–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Liu H and Weng H: Up-regulation of
Alzheimer's disease-associated proteins may cause enflurane
anesthesia induced cognitive decline in aged rats. Neurol Sci.
35:185–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Selkoe DJ: Alzheimer's disease is a
synaptic failure. Science. 298:789–791. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Arendt T: Synaptic degeneration in
Alzheimer's disease. Acta Neuropathol. 118:167–179. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Querfurth HW and LaFerla FM: Alzheimer's
disease. N Engl J Med. 362:329–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Shipton OA, Leitz JR, Dworzak J, Acton CE,
Tunbridge EM, Denk F, Dawson HN, Vitek MP, Wade-Martins R, Paulsen
O, et al: Tau protein is required for amyloid {beta}-induced
impairment of hippocampal long-term potentiation. J Neurosci.
31:1688–1692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Pooler AM, Noble W and Hanger DP: A role
for tau at the synapse in Alzheimer's disease pathogenesis.
Neuropharmacology. 76(Pt A): 1–8. 2014.doi:
10.1016/j.neuropharm.2013.09.018. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Parsons MP and Raymond LA: Extrasynaptic
NMDA receptor involvement in central nervous system disorders.
Neuron. 82:279–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Nagy J: The NR2B subtype of NMDA receptor:
a potential target for the treatment of alcohol dependence. Curr
Drug Targets CNS Neurol Disord. 3:169–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Ming Z, Griffith BL, Breese GR, Mueller RA
and Criswell HE: Changes in the effect of isoflurane on
N-methyl-D-aspartic acid-gated currents in cultured cerebral
cortical neurons with time in culture: evidence for subunit
specificity. Anesthesiology. 97:856–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Liu DD, Yang Q and Li ST: Activation of
extrasynaptic NMDA receptors induces LTD in rat hippocampal CA1
neurons. Brain Res Bull. 93:10–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Li S, Jin M, Koeglsperger T, Shepardson
NE, Shankar GM and Selkoe DJ: Soluble Aβ oligomers inhibit
long-term potentiation through a mechanism involving excessive
activation of extrasynaptic NR2B-containing NMDA receptors. J
Neurosci. 31:6627–6638. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Tang YP, Shimizu E, Dube GR, Rampon C,
Kerchner GA, Zhuo M, Liu G and Tsien JZ: Genetic enhancement of
learning and memory in mice. Nature. 401:63691999.
|
|
37.
|
Hawasli AH, Benavides DR, Nguyen C, Kansy
JW, Hayashi K, Chambon P, Greengard P, Powell CM, Cooper DC and
Bibb JA: Cyclin-dependent kinase 5 governs learning and synaptic
plasticity via control of NMDAR degradation. Nat Neurosci.
10:880–886. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Zhao X, Rosenke R, Kronemann D, Brim B,
Das SR, Dunah AW and Magnusson KR: The effects of aging on
N-methyl-D-aspartate receptor subunits in the synaptic membrane and
relationships to long-term spatial memory. Neuroscience.
162:933–945. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Chen BS and Roche KW: Regulation of NMDA
receptors by phosphorylation. Neuropharmacology. 53:3622007.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Pehar M, Ko MH, Li M, Scrable H and
Puglielli L: P44, the ‘longevity-assurance’ isoform of P53,
regulates tau phosphorylation and is activated in an age-dependent
fashion. Aging Cell. 13:449–456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Song WJ, Son MY, Lee HW, Seo H, Kim JH and
Chung SH: Enhancement of BACE1 activity by p25/Cdk5-mediated
phosphorylation in Alzheimer's disease. PLoS One. 10:e01369502015.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Wang WY, Luo Y, Jia LJ, Hu SF, Lou XK,
Shen SL, Lu H, Zhang HH, Yang R, Wang H, et al: Inhibition of
aberrant cyclin-dependent kinase 5 activity attenuates isoflurane
neurotoxicity in the developing brain. Neuropharmacology. 77:90–99.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Zhang S, Edelmann L, Liu J, Crandall JE
and Morabito MA: Cdk5 regulates the phosphorylation of tyrosine
1472 NR2B and the surface expression of NMDA receptors. J Neurosci.
28:415–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Sato S, Xu J, Okuyama S, Martinez LB,
Walsh SM, Jacobsen MT, Swan RJ, Schlautman JD, Ciborowski P and
Ikezu T: Spatial learning impairment, enhanced CDK5/p35 activity,
and downregulation of NMDA receptor expression in transgenic mice
expressing tau-tubulin kinase 1. J Neurosci. 28:14511–14521. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Plattner F, Hernández A, Kistler TM, Pozo
K, Zhong P, Yuen EY, Tan C, Hawasli AH, Cooke SF, Nishi A, et al:
Memory enhancement by targeting Cdk5 regulation of NR2B. Neuron.
81:1070–1083. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Howland JG and Cazakoff BN: Effects of
acute stress and GluN2B-containing NMDA receptor antagonism on
object and object-place recognition memory. Neurobiol Learn Mem.
93:261–267. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Ge Y, Dong Z, Bagot RC, Howland JG,
Phillips AG, Wong TP and Wang YT: Hippocampal long-term depression
is required for the consolidation of spatial memory. Proc Natl Acad
Sci USA. 107:16697–16702. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Mathur P, Graybeal C, Feyder M, Davis MI
and Holmes A: Fear memory impairing effects of systemic treatment
with the NMDA NR2B subunit antagonist, Ro 25–6981, in mice:
attenuation with ageing. Pharmacol Biochem Behav. 91:453–460. 2009.
View Article : Google Scholar : PubMed/NCBI
|