Introduction
Heat shock proteins (HSPs) are a set of stress
response proteins, expressed in all living prokaryotic and
eukaryotic cells by stimuli such as increasing temperatures,
infection by a pathogen, ischemia, hypoxia, physical or chemical
factors (1). Recent findings have
shown a protective effect of of HSPs in flap ischemia-reperfusion
injuries. It seems the high expression of HSP90 in local tissues
after transplantation with pedicles or free flaps can significantly
improve the survival rate of the skin flap (2). Ischemic preconditioning can upregulate
the expression of the HSP90 gene (3), and HSP90 inhibitors can significantly
inhibit the survival rate of flap (4). Upregulation of the HSP90 gene
can be examined as a potential approach to improve the survival
rate of transplanted flaps (5).
The majority of previous studies are based on
arterial ischemic flap models (6).
However, the necrosis of transplanted flaps occurs more commonly
due to a venous reflux disorder or other reasons in the actual
clinical setting (7). Furthermore,
whether HSP90 preconditioning of ischemia-reperfusion injuries can
improve the survival rate of flaps and the optimal time to deal
with the flaps remains to be investigated.
In the present study, a model of venous-congested
flaps in rats was established to test the heat shock protein (HSP)
90α, ‘F-5’, protein as an intervention therapy to alleviate
ischemia-reperfusion injury.
Materials and methods
Experimental animals
A total of 30 healthy adult SPF Wistar male and
female rats, aged 6–8 weeks, with an average weight of 250 g were
provided by the Central Animal Laboratory of Medical College,
Qingdao University (Shandong, China). The rats were first
acclimatized to their new environment, under normal conditions with
12 h light/dark cycles and at a constant temperature of 23°C. After
coding each rat, the animals were randomly divided into three
groups of 10 animals each: group A rats were injected with normal
saline prior to flap transplantation, group B rats were injected
with ‘F-5’ gene expression protein at 1 mg/ml prior to flap
transplantation, and group C rats with the same amount of gene
expression protein after flap transplantation. The study was
approved by the ethics committee of Medical College of Qingdao
University.
Reagents used in the present study were: ABC IHC kit
(Wuhan Boster Biological Technology, Ltd., Wuhan, China), CD31
monoclonal antibody (Millipore Corp., Billerica, MA, USA), PBS
buffer (Beijing Noble Rider Technology Co., Ltd., Beijing, China),
DAB kit (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China), TRITC fluorescence labeled secondary antibody
(Wuhan Boster Biological Technology, Ltd.), DAPI dye (Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.), and neutral
balsam (neutral balsam (mounting medium) (Shanghai Sangon
Biotechnology Co., Ltd., Shanghai, China). Instruments used were:
PeriScan PIM3 laser Doppler blood flow imaging instrument (Perimed
AB, Stockholm, Sweden), ESO60D digital camera (Canon, Inc., Tokyo,
Japan), Plus v6.0 Image-Pro image analysis software (Microsoft
Corporation, Redmond, WA, USA), laser scanning confocal microscope
and image acquisition system (Olympus, Tokyo, Japan).
Establishment of the model of
ischemia-reperfusion injury in venous blood-congested flap
Following the technique described by Petry and
Worthman (8), a 3×6 cm axial flap
was formed, with the shallow abdominal blood vessel bundle as a
pedicle, in the right lower quadrant. The flap edge tissue to the
deep fascia layer along the design marking was then cut. The distal
end of the flap was lifted, using microsurgical instruments to
separate the artery and vein carefully, the venous pedicle was
clipped with a microvessel clamp to severe venous return, and the
flap was then sutured in situ. After 6 or 8 h, depending on
the group of rats being treated, the flap was reopened again and
the vascular clamp removed to allow for recanalization of the
venous return to supply blood. The veins were observed under the
operating microscope to ensure blood flow again and the
ischemia-reperfusion model was successful. The flap was then
sutured in situ again, and the same sterile material was
used to cover the flap.
Construction of HSP90α gene
vector
To construct the pET15b-F-5 recombinant plasmid,
primers were designed to amplify a fragment from the human HSP90α
cDNA published by GenBank (‘5-GGATCCGATGCCTGAGGAAACCCAG-3’ and
‘5-ACTGTCGGATCCTTAGTCTACTTCTTCCAT-3’), which included a
BamHI restriction endonuclease site. The pET15b-F-5
recombinant plasmid was genetically engineered using the amplicon,
and the correct insert sequence was verified by DNA sequencing of
the final plasmid.
Purification of ‘F-5’ gene protein was performed by
using pET15b-F-5 recombinant plasmid to transform BL21-CodonPlus
(DE3)-RP-competent cells. Protein expression was induced with IPTG.
Expression proteins were purified by nickel-nitrilotriacetic acid
(Ni-NTA) metal affinity chromatography, concentrated using
Centricon YM-50, and then further purified with fast protein liquid
chromatography (FPLC) to a final concentration of 1 mg/ml.
Qualitative and quantitative analysis of ‘F-5’ gene
expression protein was performed by producing a protein
quantification standard curve with BSA, and the Bradford method was
used for quantification. SDS-PAGE and western blot analysis were
used to verify the identity of the purified protein,
Grouping method and observation
indices
Two reperfusion time-points were established in each
group, ischemia for 6 or 8 h, (with 5 rats in each time-point).
After recanalization, saline or purified protein was instantly
injected locally to the flap subcutaneous tissue layers. The
injection site was divided into 10 points, each injection point 1
cm apart, and 0.1 ml/point, for a total of 1 ml.
The general observation of the flaps was carried out
by adhering to the following guidelines: i) Observation time: flaps
were observed at regular intervals daily following surgery, for
1–14 days; ii) observation area: observation of the areas of the
near, middle and distal ends of the flaps were recorded; and iii)
observation items: determinations of the color, dermatoglyph
presence, texture, capillary filling test, needle prick test and
the presence of blisters were recorded for each flap.
A laser doppler flow meter was used to determine the
flap blood flow after ischemia for a given time and then
reperfusion. This was performed to determine whether the
establishment of the model of ischemia-reperfusion injury in each
flap was successful.
Subsequent to surgery, the survival rate of each
flap was determined daily for 1–14 days. After immobilizing each
animal, a digital camera was used to capture images of the flap
area. The Image-Pro Plus v6.0 image analysis software was used to
calculate the survival rate of the flap, uaing the equation: flap
survival rate = (flap survival area/flap design area) × 100%.
Histological examination was also performed. Animal
models in each group were randomly selected at 1, 3, 7, 10 and 14
days after surgery, then skin tissue from the central area of the
flap was dissected, and any pathological changes of the flap were
observed under the microscope using the H&E (hematoxylin and
eosin) staining method.
Immunohistochemical staining was used to detect
angiogenesis and re-epithelialization of the flap. Briefly, the
specimen materials were taken at 1, 3, 7, 10 and 14 days after
operation, and cut into 5 µm sections after paraffin embedding.
After dewaxing and rehydration of the sections, rabbit polyclonal
anti-CD31 antibody (dilution: 1:50) (Abcam, London, England; cat
no.: ab28364) was added for incubation overnight at 4°C. The
following day the specimens were washed three times with PBS, prior
to adding TRITC-labeled secondary antibody, and incubating at room
temperature (37°C) for 1 h. The cell nucleus was stained with DAPI,
washed three times with PBS, and mounted by neutral balsam. Using a
laser scanning confocal microscope, the blood vessel density was
contrasted by calculating the average number of blood vessels in
the five random fields on each section. Immunohistochemistry was
used to detect and compare the epithelium migration of the wound in
each group.
Statistical methods
Using the SPSS statistical software for analysis,
measurement data were presented as mean ± SD. Comparison among
groups was made using ANOVA analysis, and comparison were made
between two groups using the independent-sample t-test. Enumeration
data were expressed as cases or percentage, and comparison among
groups were made using the χ2 test. P<0.05 indicated
statistically significant results.
Results
General observation of flap
The middle and distal ends of flaps showed pale or
dark red coloring in each group at 1–2 days following surgery, with
different degrees of swelling. The distal end of the flaps
gradually blackened, the surface sank and a scab shell presented
and poor flexibility was observed at 3–7 days. Generally after 7–10
days, the necrosis area of the flap was extended, and the wound
surface under the black scab shell began to shrink with time. In
addition, with time, the capillary filling time was extended, the
dermatoglyphs disappeared, and the surface became shiny, with
blisters or bleeding.
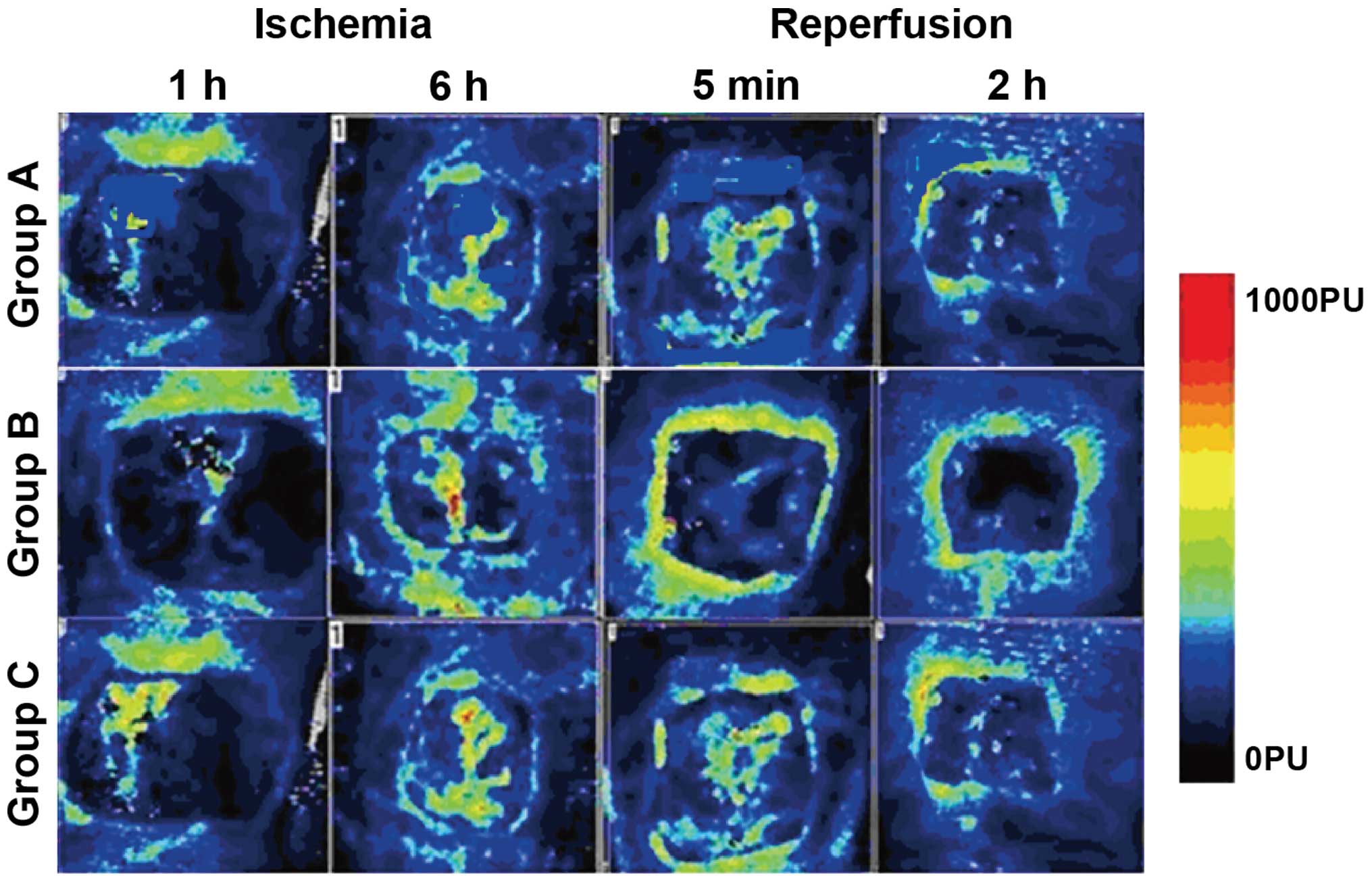
Flap blood flow
The flap blood flow in group B was significantly
higher than that in groups A and C at all ischemia and reperfusion
time-points. Group A had the lowest blood flow, and the difference
was statistically significant (P<0.05). The blood flow to the
flap was decreased in each group with the prolongation of ischemia,
and it was increased with the prolongation of reperfusion time
(Table I and Fig. 1).
 | Table I.Comparisons of tissue perfusion
parameters of blood flow, blood volume, mean transit time and
permeability surface. |
Table I.
Comparisons of tissue perfusion
parameters of blood flow, blood volume, mean transit time and
permeability surface.
| Parameters |
| Lung cancer group
(n=350) | Non-cancer group
(n=62) | t | P-value |
|---|
| Blood flow
(ml/sec) | First time | 13.4±2.3 |
3.6±0.4 |
5.627 |
0.029 |
|
| Second time | 14.2±2.5 |
3.4±0.3 |
5.839 |
0.026 |
| Blood volume
(ml) | First time |
5.6±1.3 |
1.3±0.4 |
4.748 |
0.037 |
|
| Second time |
5.8±1.2 |
1.1±0.2 |
4.923 |
0.034 |
| Mean transit time
(msec) | First time | 23.4±3.6 |
4.7±1.2 |
6.626 |
0.025 |
|
| Second time | 25.6±3.2 |
4.3±1.1 |
6.349 |
0.027 |
| Permeability
surface | First time | 35.4±6.6 | 10.2±3.1 |
6.137 |
0.029 |
|
| Second time | 36.6±5.8 | 10.4±3.3 |
6.602 |
0.031 |
Survival rate of flap
Following surgery, the survival rate of ischemia at
6 and 8 h in groups B and C was increased progressively with each
passing time-point, and the survival rate in the group with
ischemia for 6 h was higher than that in ischemia for 8 h. The
survival rate of group B was significantly higher than that of
groups A and C at each time point, with group A having the lowest
survival rates. The difference was statistically significant
(P<0.05) (Table II).
 | Table II.Survival rate of flap (%). |
Table II.
Survival rate of flap (%).
|
|
Post
operation |
|---|
|
|
|
|---|
|
| Day 1 Ischemia | Day 3 Ischemia | Day 7 Ischemia | Day 10 Ischemia | Day 14 Ischemia |
|---|
|
|
|
|
|
|
|
|---|
| Group | 6 h | 8 h | 6 h | 8 h | 6 h | 8 h | 6 h | 8 h | 6 h | 8 h |
|---|
| A | 12.3±3.6 | 10.4±3.2 | 15.6±3.3 | 12.7±3.4 | 18.2±3.7 | 15.4±3.5 | 19.3±3.8 | 16.2±3.6 | 21.3±3.5 | 18.7±3.3 |
| B | 36.6±13.2 | 30.3±14.6 | 48.5±13.6 | 36.9±15.4 | 67.9±15.2 | 59.5±15.5 | 85.6±17.6 | 76.9±18.2 | 92.8±16.9 | 84.8±17.4 |
| C | 32.1±10.3 | 26.7±11.2 | 42.7±12.6 | 35.2±12.5 | 63.5±21.7 | 52.7±18.9 | 78.9±20.5 | 73.2±23.4 | 86.5±24.7 | 79.6±24.6 |
| F-value | 4.521 | 4.863 | 5.052 | 5.274 | 5.632 | 5.754 | 5.935 | 5.867 | 6.214 | 6.539 |
| P-value | 0.041 | 0.039 | 0.037 | 0.035 | 0.031 | 0.028 | 0.026 | 0.023 | 0.017 | 0.014 |
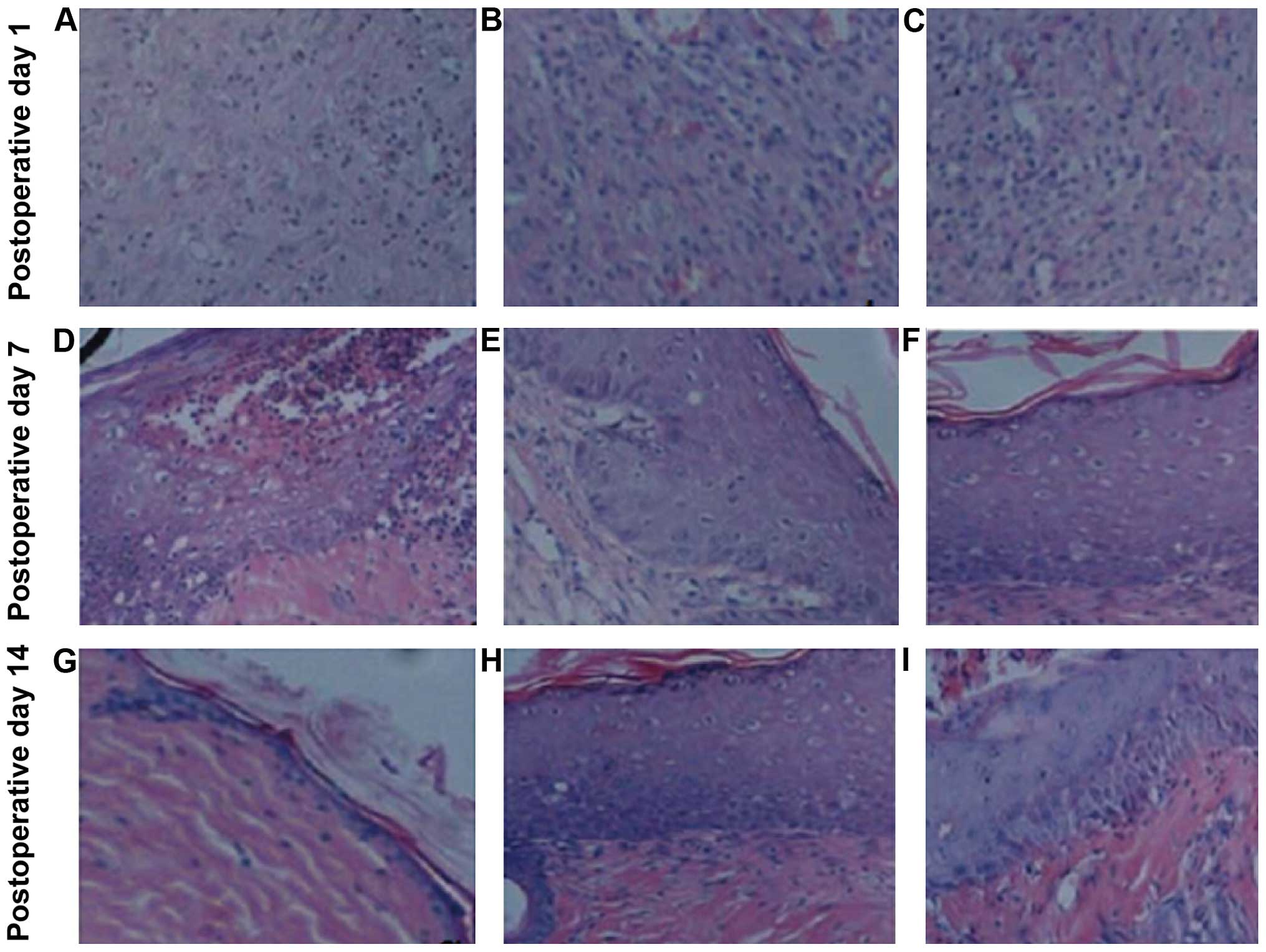
Histological examination
A large number of infiltrating inflammatory cells
were visible at wound tissues at postoperative day 1 in all the
groups, and the angiotelectasis was obviously apparent. By day 7,
groups B and C showed inflammatory cell infiltration reduction,
with the number of fibroblasts being increased, deeply stained, an
increased capillary component, and the visible part of new
epithelial tissues. Group A, however, had abundant inflammatory
cell infiltration, and less endothelial cell and fibroblast
proliferation. At post-operative day 14, groups B and C, showed new
capillaries and inflammatory cell numbers were decreased further,
while mature fibroblasts were increased, the cells were arranged in
tidier bundles, the matrix components (red staining) were uniform,
new epithelial tissues were increased, and there was visible basal
cell hyperplasia (Fig. 2).
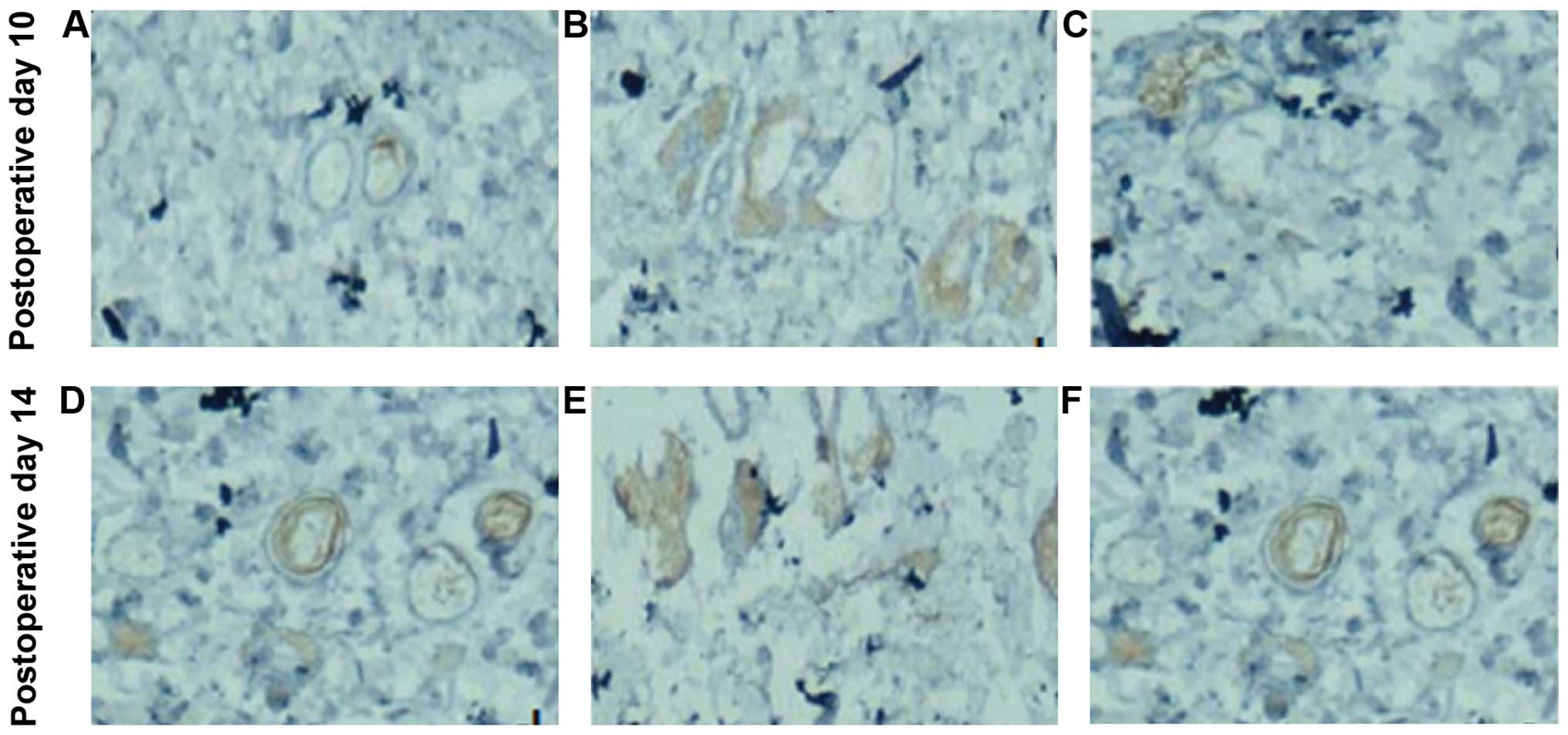
Angiogenesis and
re-epithelialization
Following surgery, the neovascular densities in
groups B and C with ischemia for 6 and 8 h were increased with each
time-point, and the densities in rats subjected to ischemia for 6 h
were higher than that in those with ischemia for 8 h. The
neovascular density of group B was significantly higher than that
of group C at each time, group A had the lowest, and the difference
was statistically significant (P<0.05) (Table III). The new skin was more abundant
on the wound tissues in groups B and C, and even some new blood
vessels were visible. At postoperative day 14, groups B and C
showed a relatively complete new skin structure, and the spikes
were visible at attachment of the epidermis and dermis, and the new
blood vessels were increased (Fig.
3).
 | Table III.Neovascular density (no./horizon). |
Table III.
Neovascular density (no./horizon).
|
|
Post
operation |
|---|
|
|
|
|---|
|
| Day 1 Ischemia | Day 3 Ischemia | Day 7 Ischemia | Day 10 Ischemia | Day 14 Ischemia |
|---|
|
|
|
|
|
|
|
|---|
| Group | 6 h | 8 h | 6 h | 8 h | 6 h | 8 h | 6 h | 8 h | 6 h | 8 h |
|---|
| A | 0.2±0.1 | 0.1±0.1 | 0.3±0.1 | 0.1±0.1 | 0.5±0.2 | 0.2±0.2 | 0.6±0.2 | 0.3±0.2 | 0.8±0.3 | 0.3±0.3 |
| B | 0.7±0.3 | 0.5±0.3 | 1.2±0.4 | 0.8±0.4 | 2.9±0.6 | 1.7±0.5 | 4.2±0.8 | 3.5±0.9 | 4.8±1.3 | 4.3±1.2 |
| C | 0.5±0.2 | 0.4±0.2 | 0.9±0.4 | 0.6±0.4 | 2.5±0.9 | 1.4±0.6 | 3.9±1.3 | 3.1±1.2 | 4.4±1.5 | 3.9±1.6 |
| F-value | 5.624 | 5.937 | 6.233 | 6.428 | 6.635 | 6.768 | 6.938 | 7.421 | 7.632 | 7.765 |
| P-value | 0.037 | 0.035 | 0.032 | 0.026 | 0.024 | 0.017 | 0.014 | 0.013 | 0.012 | 0.011 |
Discussion
In a previous study by Wang et al, HSP72
expression was induced in flaps by systemic and local preheating,
thus achieving an increased flap survival area. Their results
showed that HSP expression upregulation in flap tissues can protect
cells and alleviate ischemia-reperfusion injury, and clearly
improve tissue survival rates after transplantation (9). HSP90 is an important member of the HSPs
family, with a molecular weight of 83–90 kDa, depending on the
presence of α and β subunits containing aspartic acid amide
fragments, and the homology of the two subunits is 84%. HSP90
exists in the form of αα and ββ, the amount of both is roughly
equal, and both are cytoplasmic proteins. HSP90 is able to assist
in protein folding and maintaining the stability of intracellular
varieties of signaling proteins, thereby promoting cell survival
and growth. In addition to the intracellular form, HSP90α can also
be secreted into the extracellular environment, and is therefore
known as secretory HSP90. A previous study showed that the
hypoxia-induced factor (HIF) increases under hypoxia, which may
induce partial secretory HSP90α to increase and therefore induce
skin basal cells, while fibroblasts and vascular endothelial cells
migrate to the wound, thereby promoting wound healing (10). Additionally, another study found that
the secretion of HSP90α is effective in the promotion of wound
healing. In the same study, a highly conserved amino acid sequence
(aa236 - aa350) located in HSP90α was found, and expression of the
polypeptide containing it was more effective than the full-length
secretory HSP90α in promoting wound healing. This functional gene
sequence is known as ‘F-5’ gene (11). Therefore, for this study the F-5
sequence was induced in vitro to obtain the highly active
F-5 protein.
For the present study, two blocking points of
ischemia for 6 and 8 h were set to compare the effect of different
intervention methods under different blocking times. These
time-points were chosen based on the findings of a published study
that established that in venous occlusions of <6 h, the flaps
can survive after recanalization, whereas in venous occlusions for
8 h, the majority of the flaps cannot survive, while in blocks for
10 h, none of the flaps survive (12).
In this study, it was shown that the
ischemia-reperfusion injury model in a venous blood-congested flap
is stable and reliable. The benefit of the HSP90 preconditioning
treatment on flap survival was clearly demonstrated by showing an
increase in blood flow following recanalization, an increase in the
flap survival rate, and improved immunohistochemical parameters of
healing (lower cell infiltration, more neovascularization, and
better epidermal re-structuring) when compared to the same observed
variables in the flaps of rats treated with saline prior to the
surgical procedure or with HSP90 following the surgical procedure.
It was also clear that the flaps in all the groups were improved
after an ischemia time of 6 h than after ischemia for 8 h, and that
all the parameters of healing were improved with time after the
recanalization.
As endogenous HSPs are released in the body at
determined times, the organism or tissue needs to spend an absolute
recovery period after stimulation, during which cells synthesize
HSPs, prior to sufficient HSPs being available to act on a given
tissue (13). Future studies are
needed to establish the timings and amounts of stimulation needed
to generate protecting endogenous levels of HSP90α in tissues to be
subjected into surgical flap procedures.
In conclusion, the HSP90α intervention on
venous-congested ischemia-reperfusion injured flaps can improve the
survival rate, as the longer the ischemic time, the worse the
effect.
Acknowledgements
The present study was funded by the Natural Science
Foundation of Shandong Province.
References
|
1
|
Park CJ and Seo YS: Heat shock proteins: a
review of the molecular chaperones for plant immunity. Plant Pathol
J. 31:323–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Woodley DT, Wysong A, DeClerck B, Chen M
and Li W: Keratinocyte migration and a hypothetical new role for
extracellular heat shock protein 90 alpha in orchestrating skin
wound healing. Adv Wound Care (New Rochelle). 4:203–212. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jayaprakash P, Dong H, Zou M, Bhatia A,
O'Brien K, Chen M, Woodley DT and Li W: Hsp90α and Hsp90β together
operate a hypoxia and nutrient paucity stress-response mechanism
during wound healing. J Cell Sci. 128:1475–1480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wright L, Barril X, Dymock B, Sheridan L,
Surgenor A, Beswick M, Drysdale M, Collier A, Massey A, Davies N,
et al: Structure-activity relationships in purine-based inhibitor
binding to HSP90 isoforms. Chem Biol. 11:775–785. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Imai Y, Sakurai M, Horinouchi T, Lee YS
and Yamada A: Epithelial cells and adipose cells both have their
own temporal profile in 72-kd heat-shock protein expression
determining their tolerance for ischaemia. J Plast Reconstr Aesthet
Surg. 59:230–238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Babovic S, Angel MF, Im MJ, Ress AM and
Manson PN: Effects of tissue expansion on secondary ischemic
tolerance in experimental free flaps. Ann Plast Surg. 34:593–598.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hauge EM, Balling E, Hartmund T and
Hjortdal VE: Secondary ischemia caused by venous or arterial
occlusion shows differential effects on myocutaneous island flap
survival and muscle ATP levels. Plast Reconstr Surg. 99:825–833.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Petry JJ and Wortham KA: The anatomy of
the epigastric flap in the experimental rat. Plast Reconstr Surg.
74:410–413. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang BH, Ye C, Stagg CA, Lin M, Fawcett T,
VanderKolk CA and Udelsman R: Improved free musculocutaneous flap
survival with induction of heat shock protein. Plast Reconstr Surg.
101:776–784. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Li Y, Guan S, Fan J, Cheng CF,
Bright AM, Chinn C, Chen M and Woodley DT: Extracellular heat shock
protein-90alpha: Linking hypoxia to skin cell motility and wound
healing. EMBO J. 26:1221–1233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng CF, Sahu D, Tsen F, Zhao Z, Fan J,
Kim R, Wang X, O'Brien K, Li Y, Kuang Y, et al: A fragment of
secreted Hsp90α carries properties that enable it to accelerate
effectively both acute and diabetic wound healing in mice. J Clin
Invest. 121:4348–4361. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harashina T, Sawada Y and Watanabe S: The
relationship between venous occlusion time in island flaps and flap
survivals. Plast Reconstr Surg. 60:92–95. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Bai X, Wang Y, Li N, Li X, Han F,
Su L and Hu D: Role for heat shock protein 90α in the proliferation
and migration of HaCaT cells and in the deep second-degree burn
wound healing in mice. PLoS One. 9:e1037232014. View Article : Google Scholar : PubMed/NCBI
|