Introduction
Ischemic cerebral apoplexy is a worldwide. It is
reported that the prevalence of ischemic stroke is 20–40% with a
high disability rate (60–85%) and high mortality rate (30–45%) for
individuals over the age of 40 years (1) It is believed that multiple factors
result in the temporary or permanent ischemia of the artery.
Furthermore, there are a series of secondary mechanisms of brain
tissue and cells e.g., ischemic/hypoxic injury, energy metabolic
disorder, oxidative stress and calcium overload (2). Approximately 50–65% patients have
transient ischemic attack (TIA), which may be a different
development process for another disease compared with cerebral
apoplexy, but they have the same pathological basis i.e., stenosis
of feeding artery (3). Early
diagnosis of the target vessel lesion was significant in terms of
improving the clinical remedy rate and life quality. Conventional
magnetic resonance imaging (MRI) has a resolution ratio on
components of brain tissue, but also has poor results on showing
blood flow perfusion (4). Dynamic
magnetic sensitivity contrast agent perfusion-weighted imaging
(PWI) increases complications and counterfeit image interference
from exogenous contrast agents (5).
Magnetic resonance angiography (MRA) has unsatisfactory development
on small artery lesions, small and moderate artery with mild or no
obvious stenosis (6). It cannot
provide accurate imaging information in time. Cerebral blood flow
(CBF) imaging, which is obtained through using free water proton in
arterial blood as an endogenous tracer and reverse pulse technology
for labeling and then post-processing, is able to sensitively and
specifically indicate abnormal changes of cerebral perfusion
(7). It has been approved to
different degrees for ischemic and hemorrhagic cerebrovascular
diseases, and has better developing effects compared with
conventional diffusion-weighted imaging (DWI), PWI and functional
MRI, and also better signal-to-noise ratio (SNR), high
repeatability and consensus stability (8).
The present study examined the application value of
arterial spin labeling (ASL) in TIA and mild and moderate
intracranial atherosclerotic stenosis, which provide references for
the early recognition of target lesions and immediate treatment
clinically.
Patients and methods
Patients
A total of 58 cases of TIA and 60 cases of ischemic
cerebral apoplexy were diagnosed at the Yantaishan Hospital
(Shandong, China) and were selected between January 2014 and
January 2016. These cases were 32 men and 26 women in the TIA
group, with an average age of 45.6±10.3 years. The average onset
time of disease was 1.5±0.4 min, and the average number of attack
times was 1.5±0.6. There were 12 hypertensive, 8 smoking, 5
diabetic and 7 hyperlipidaemic cases. In the cerebral apoplexy
group, there were 36 male and 34 female cases, with an average age
of 47.7±11.5 years, an average onset time of disease of 26.4±7.2
min, and an average number of attack times of 1.3±0.5. There were
32 cases with lesion location at basal ganglia region, 14 cases at
cerebrum, 7 cases at cerebellum and 7 cases at brainstem.. There
were 15 hypertensive, 10 smoking, 3 diabetic and 5 hyperlipidaemic
cases. There was no statistical differences between the two groups
regarding gender, age, attack times and combined disease type
(P>0.05).
Infored consent was obtained from the patients and
their family members. Approval for the study was obtained by the
ethics committee of Yantaishan Hospital.
Study method
GE Healthcare Signa HDx 3.0T (GE Healthcare,
Piscataway, NJ, USA) superconducting whole-body magnetic resonance
scan was performed on all the patients within 24 h of attack. The
maximum uniaxial gradient strength was ≥45 mT/m. Eight-channel head
phased array coils and conventional sequence were used to obtain
T1-weighted images (T1WI), T2WI, DWI, MRA and ASL imaging. The
scanning parameters were OAxT1WI: TR=1,750 msec, TE=24 msec, number
of layers was 20, thickness of layer was 5.0 mm, spacing between
layers was 1.0 mm, vision FOV: 22×22 cm, NEX was 1 and matrix was
320×224. OAxT2WI: TR=5,100 msec, TE=118.9 msec, number of layers
was 20, thickness of layer was 5.0 mm, spacing between layers was
1.0 mm, FOV: 22×22 cm, NEX was 1.5 and matrix was 512×512. DWI:
TR=5,400 msec, TE=minimum, number of layers were 20, thickness of
layer was 5.0 mm, spacing between layers was 1.0 mm, vision FOV:
22×22 cm and NEX was 1. MRA selected three-dimensional
time-of-flight method and TR=40 msec, TE=8 msec, number of layers
was 20, thickness of layer was 5.0 mm, spacing between layers was
1.0 mm, vision FOV: 22×22 cm and NEX was 1. Intracranial vascular
examination included intracranial artery (intracalvarium, siphon
bend and terminal segment), middle cerebral artery M1–3 segments
and anterior cerebral artery A1–3 segments.
For ASL technology, FAIR was used and TR=1,000 msec,
TE=13.8 msec, TI=2,000 msec, number of layers was 20, thickness of
layer was 1.3 mm, spacing between layers was 1.0 mm, vision FOV:
22×22 cm, NEX was 80, matrix was 256×128 and scanning time was 2
min and 48 sec. Scanning was performed at the plane of 1 cm above
the corpus callosum, because this area was supplied with blood from
the anterior cerebral and middle cerebral arteries, and therefore
was less influenced by magnetic sensitivity or vein pollution.
Reconstruction of ASL imaging was shown in pseudo color map, where
the red area was high perfusion, green was moderate perfusion and
blue was low perfusion. A qualitative and semiquantitative analysis
on ASL imaging was performed and the corresponding value of
relative CBF (rCBF) was used to distinguish perfusion, where rCBF
<0.9 referred to low perfusion, 0.9–1.1 was moderate perfusion
and >1.1 high perfusion. Two experienced MRI doctors jointly
observed the distribution of grey and white matter, and
subsequently confirmed the abnormal area of perfusion and
determined the lesion location based on clinical manifestations of
TIA. The doctors manually drew the region of interest (ROI) for
grey and white matter of abnormal perfusion. RESTplus 1.1 software
(RESTplus, Hangzhou, China; http://restfmri.net/forum/index.php?q=rest) was used
to manually test ROI was used to manually test ROI signal strength,
and the image method was used to place ROI at the other side to
measure signal strength of the corresponding side and perform
semiquantitative analysis. The average signal strength at the
abnormal perfusion side was the healthy side, and was considered
abnormal if the value was >20%.
Observation index
The correlation and differences between ASL and
conventional imaging were compared and analyzed in terms of lesion
location, size, blood perfusion and the signal range of rCBF.
Statistical analysis
SPSS 19.0 (SPSS, Inc., Chicago, IL, USA) was used to
analyze and process data. Quantitative data were presented as mean
value ± standard deviation, where comparisons between groups were
made using a t-test. Qualitative data were presented as number of
cases, or percentage, and comparisons between groups were made
using the χ2 test. Correlation analysis was performed
using Pearson's or Spearman's tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
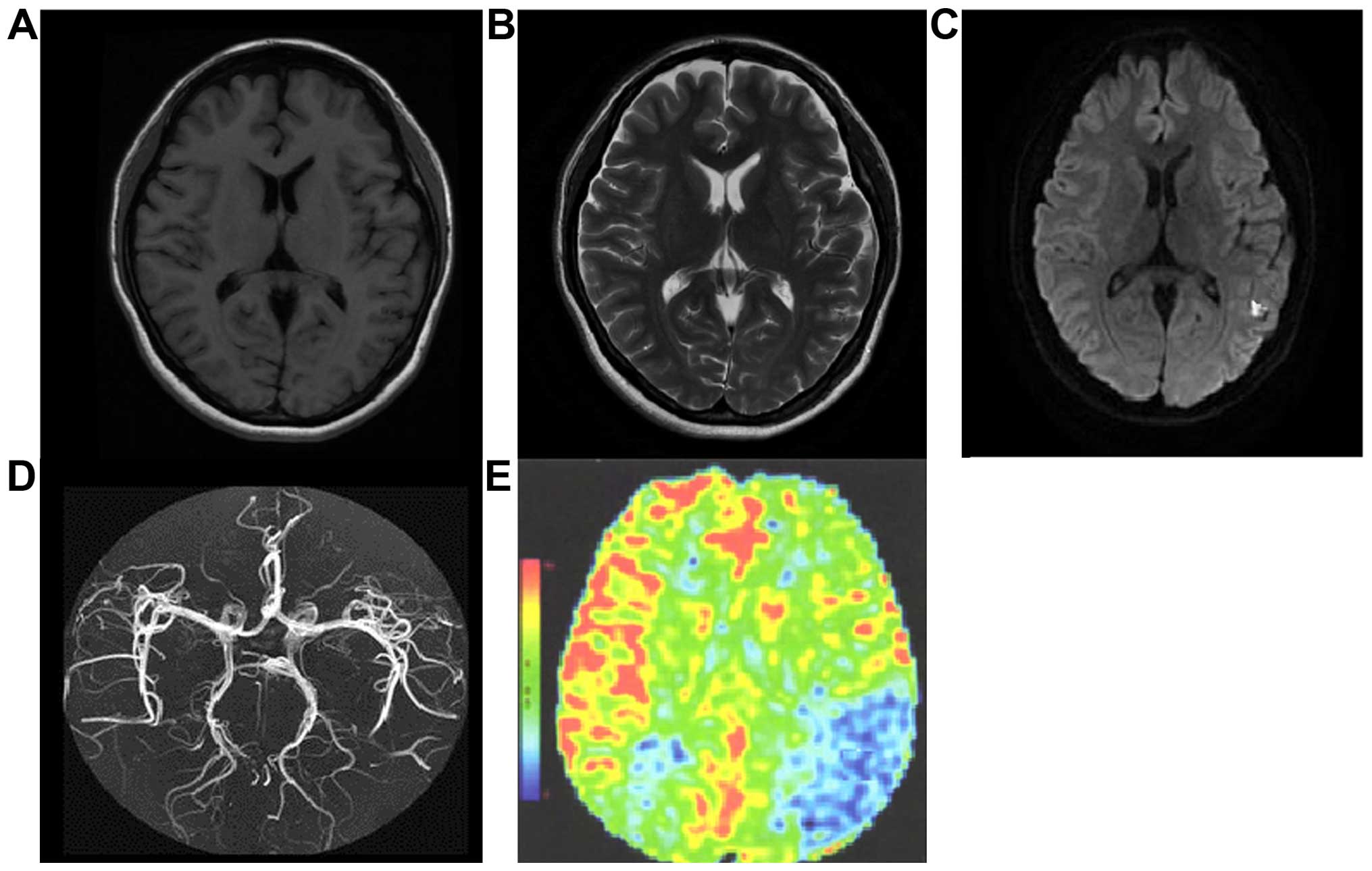
Analysis on the development in TIA
group
There were 13 cases of an abnormal signal tested in
conventional MRI (manifesting as T1 and T2 low signal) and the
positive rate was 22.4%. The hypoperfusion area of DWI was 0.5–1.8
mm2 and the average was 1.1±0.6 mm2. The
diameter stenosis of intracranial arterial lumen tested in MRA was
10–50% and average was 32.5±10.4%. In ASL, 37 cases with
hypoperfusion area were tested (63.8%), the rCBF was 0.6–1.2 and
the average was 0.8±0.3. The semiquantitative analysis revealed
that the rate of abnormal perfusion signals was 72.4% (42/58), the
hypoperfusion area was 1.2–5.4 mm2 and the average was
3.3±1.4 mm2. For the location of the hypoperfusion area,
18 cases were at basal ganglia, 10 cases were at cerebrum, 6 cases
were at cerebellum and 3 cases were at brainstem. A total of 13
cases of abnormal signal in conventional MRI were identified
through ASL technology. Diameter stenosis beyond 30% in MRA were
tested in ASL. A positive rate in ASL was significantly higher than
that of conventional MRI (χ2=29.078, P<0.001) and the
hypoperfusion area was greatly increased (t=32.526, P<0.001).
The rCBF value was positively correlated with the degree of
diameter stenosis shown in MRA (r=0.524, P=0.012). Additionally,
the positive rate of ASL was positively correlated with the attack
times of TIA (r=0.352, P=0.027) (Fig.
1).
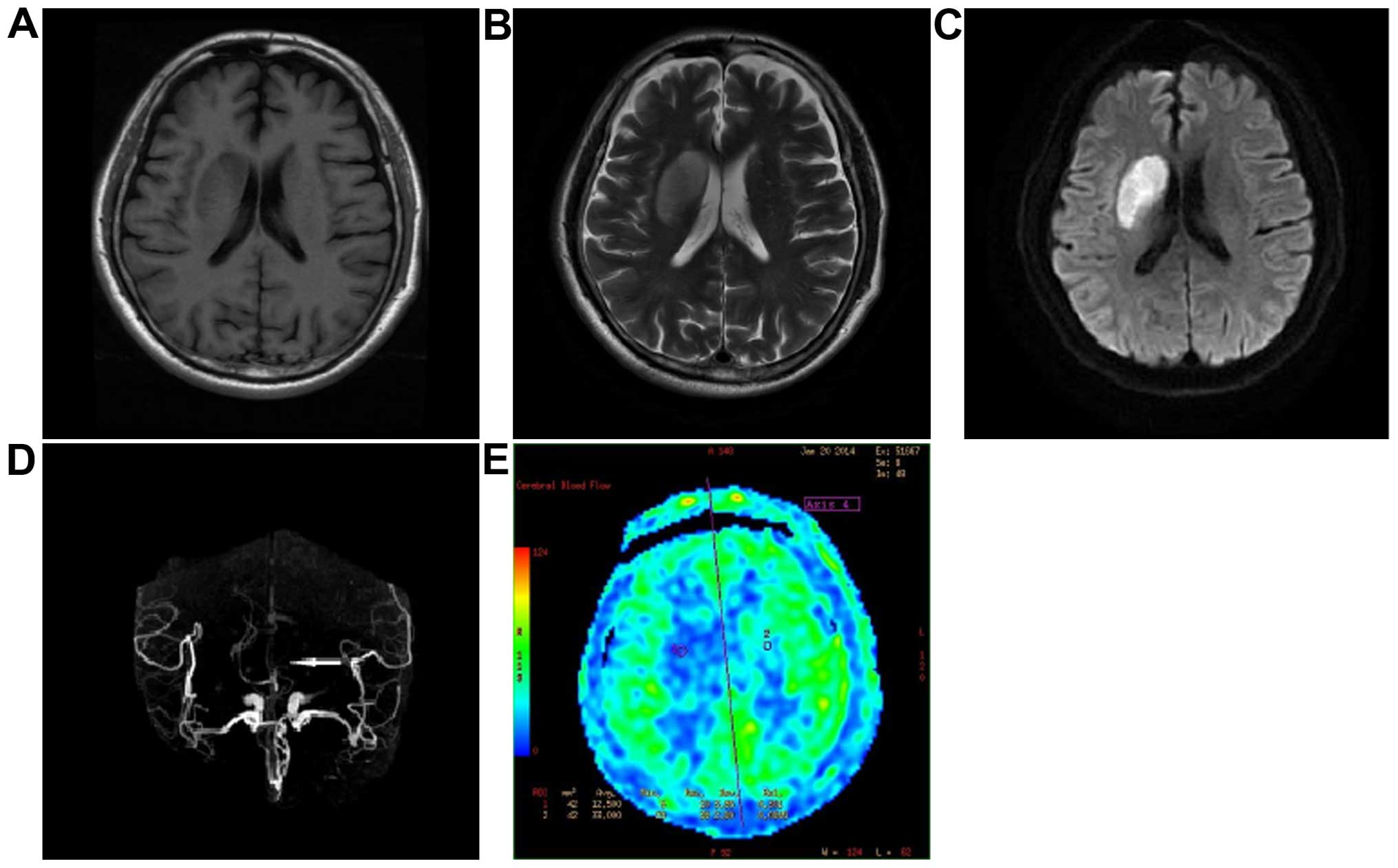
Analysis on the development in
cerebral apoplexy group
There were 39 cases of abnormal signal tested in
conventional MRI (manifesting as T1 and T2 low signal) and the
positive rate was 65.0%, with a hypoperfusion area of DWI was
1.6–4.5 mm2 and an average was 3.2±1.3 mm2.
The diameter stenosis of intracranial arterial lumen tested in MRA
was 30–75% and the average was 56.5±14.8%. In ASL, 52 cases had a
hypoperfusion area (86.7%), rCBF 0.4–1.0 and an average of 0.6±0.2.
The rate of abnormal perfusion signals was 91.7% (55/60), the
hypoperfusion area was 1.8–8.5 mm2 and the average was
5.7±1.6 mm2. For the location of the hypoperfusion area,
26 cases were at basal ganglia, 14 cases at cerebrum, 7 cases at
cerebellum and 5 cases at brainstem. A total of 39 cases of
abnormal signal in the conventional MRI were identified through ASL
technology. A positive rate in ASL was significantly higher than
that of the conventional MRI (χ2=7.685, P=0.006) and the
hypoperfusion area was greatly increased (t=9.425, P<0.001). The
rCBF value was positively correlated with the degree of diameter
stenosis (r=0.635, P=0.009) (Fig.
2).
Discussion
ASL is a non-invasive, safe and simple technology
that tests blood perfusion of brain tissue. Decrease or abnormality
of brain blood perfusion was the pathological basis of ischemic
cerebrovascular disease and the main diagnostic basis. There were
corresponding clinical symptoms when whole-brain CBF was <50%,
in other words 23 ml/min·100 g (9).
ASL technology was used to test the decrease or absence of
perfusion in the early period and calculate rCBF, which provided a
significant reference value for the quantitative reaction of
hemodynamic changes of cerebrovascular diseases as well as clinical
diagnosis and treatment (10). Based
on different inversion labeling methods for arterial blood, ASL
technology was divided into continuous ASL (CASL) and pulsing ASL
(PASL). CASL has a higher SNR and narrow labeling plane, its
sensitive area of radiofrequency coil need not be long and
consequently, it is considered more suitable for a lower anatomical
layer (5). However, CASL has a
higher radiofrequency energy deposition and its tissue was greatly
influenced by the magnetization transfer effect (11). Nevertheless, PASL was able to
eliminate the magnetization vector from static structure at the
notch pulse and control pulse, which may minimize defective
artifacts of profiled outline. In addtion, the inversion labeling
rate of PASL is greatly improving (12). With the development of MR software
and hardware and increasing investigations of ASL technology, the
ASL sequence is also improving continuously, such as FAIREST
technology performing the third imaging on imaging layer after
saturation based on core sequence (FAIR sequence), which is able to
effectively separate blood oxygen level-dependent (BOLD) weight and
perform quantitative CBF measurement (13). BOLD and T1-weighted imaging can
subsequently be obtained to reflect blood perfusion and metabolic
status of brain tissue more accurately (13).
Calamante et al applied CASL technology to
examine transient middle cerebral artery occlusion in a rat model
(14). The results of that study
showed that, ASL can be used to reveal low blood perfusion of
cerebral ischemia for animals and monitor the time course of
ischemia reperfusion and treatment effects of embolism (14). Chalela et al (15) have previously reported a CASL study
with 15 cases of acute ischemic apoplexy patients. The findings of
that study showed that CASL is able to identify insufficient
perfusion of acute cerebral ischemia and is correlated with
clinical symptoms (15). In
addition, Bokkers et al (16)
used ASL technology to scan 23 cases of carotid artery stenosis
(including cases with and without ischemic symptoms) and 20 healthy
cases and observed the perfusion of brain tissue prior to and after
acetazolamide. The results of those authors showed that the
perfusion area of brain tissue for carotid artery stenosis with
symptoms was smaller than those with no symptoms (16). After intravenous injection of
acetazolamide, cerebral blood perfusion increased, but the increase
in scope for carotid artery stenosis with symptoms were lower than
that of cases with symptoms, but both of them were lower than those
of healthy individuals. The results of that study indicated that
ASL was able to test blood perfusion of brain tissue, providing CBF
information for cerebral ischemia patients, which was useful in
conventional MRI testing.
The 13 cases of abnormal signal in conventional MRI
(positive rate 22.4%) were also identified through ASL technology
(positive rate 63.8%). Diameter stenosis beyond 30% in MRA was also
tested in ASL. The positive rate in ASL was significantly higher
than that of conventional MRI and hypoperfusion area was greatly
increased. The rCBF value was positively correlated with the degree
of diameter stenosis shown in MRA and the positive rate of ASL was
positively correlated with the attack times of TIA. A total of 39
abnormal signal cases of cerebral apoplexy in conventional MRI
(positive rate 65.0%) were identified through ASL technology
(positive rate 86.7%). A positive rate in ASL was significantly
higher than that of the conventional MRI and hypoperfusion area was
greatly increased. The rCBF value was positively correlated with
the degree of diameter stenosis. In conclusion, 3.0T ASL was
equally important in the early diagnosis of TIA and mild, moderate
intracranial arterial stenosis of cerebral apoplexy.
References
|
1
|
Bu X, Li C, Zhang Y, Xu T, Wang D, Sun Y,
Peng H, Xu T, Chen CS, Bazzano LA, Chen J and He J: CATIS
Investigators. Early Blood Pressure Reduction in Acute Ischemic
Stroke with Various Severities: A subgroup analysis of the CATIS
trial. Cerebrovasc Dis. 42:186–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Psychogios K, Stathopoulos P, Takis K,
Vemmou A, Manios E, Spegos K and Vemmos K: The Pathophysiological
Mechanism Is an independent predictor of long-term outcome in
stroke patients with large vessel atherosclerosis. J Stroke
Cerebrovasc Dis. 24:2580–2587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kivikko M, Kuoppamäki M, Soinne L,
Sundberg S, Pohjanjousi P, Ellmen J and Roine RO: Oral levosimendan
increases cerebral blood flow velocities in patients with a history
of stroke or transient ischemic attack: A pilot safety study. Curr
Ther Res Clin Exp. 77:46–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Snow NJ, Peters S, Borich MR, Shirzad N,
Auriat AM, Hayward KS and Boyd LA: A reliability assessment of
constrained spherical deconvolution-based diffusion-weighted
magnetic resonance imaging in individuals with chronic stroke. J
Neurosci Methods. 257:109–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang SX, Yao YH, Zhang S, Zhu WJ, Tang
XY, Qin YY, Zhao LY, Liu CX and Zhu WZ: Comparative study of
DSC-PWI and 3D-ASL in ischemic stroke patients. J Huazhong Univ Sci
Technolog Med Sci. 35:923–927. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eugène F, Gauvrit JY, Ferré JC, Gentric
JC, Besseghir A, Ronzière T and Raoult H: One-year MR angiographic
and clinical follow-up after intracranial mechanical thrombectomy
using a stent retriever device. AJNR Am J Neuroradiol. 36:126–132.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Faraco CC, Strother MK, Dethrage LM,
Jordan L, Singer R, Clemmons PF and Donahue MJ: Dual echo
vessel-encoded ASL for simultaneous BOLD and CBF reactivity
assessment in patients with ischemic cerebrovascular disease. Magn
Reson Med. 73:1579–1592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nael K, Meshksar A, Liebeskind DS, Wang
DJ, Ellingson BM, Salamon N and Villablanca JP: UCLA Stroke
investigators: Periprocedural arterial spin labeling and dynamic
susceptibility contrast perfusion in detection of cerebral blood
flow in patients with acute ischemic syndrome. Stroke. 44:664–670.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang DJ, Alger JR, Qiao JX, Gunther M,
Pope WB, Saver JL, Salamon N and Liebeskind DS: UCLA Stroke
Investigators: Multi-delay multi-parametric arterial spin-labeled
perfusion MRI in acute ischemic stroke - Comparison with dynamic
susceptibility contrast enhanced perfusion imaging. Neuroimage
Clin. 3:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaichi Y, Okada G, Takamura M, Toki S,
Akiyama Y, Higaki T, Matsubara Y, Okamoto Y, Yamawaki S and Awai K:
Changes in the regional cerebral blood flow detected by arterial
spin labelingafter 6-week escitalopram treatment for major
depressive disorder. J Affect Disord. 194:135–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garraux G, Hallett M and Talagala SL: CASL
fMRI of subcortico-cortical perfusion changes during memory-guided
finger sequences. Neuroimage. 25:122–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bibic A, Knutsson L, Schmidt A,
Henningsson E, Månsson S, Abul-Kasim K, Åkeson J, Gunther M,
Ståhlberg F and Wirestam R: Measurement of vascular water transport
in human subjects using time-resolved pulsed arterial spin
labelling. NMR Biomed. 28:1059–1068. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo L, Zhang Q, Ding L, Liu K, Ding K,
Jiang C, Liu C, Li K and Cui L: Pseudo-continuous arterial spin
labeling quantifies cerebral blood flow in patients with acute
ischemic stroke and chronic lacunar stroke. Clin Neurol Neurosurg.
125:229–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calamante F, Thomas DL, Pell GS, Wiersma J
and Turner R: Measuring cerebral blood flow using magnetic
resonance imaging techniques. J Cereb Blood Flow Metab. 19:701–735.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chalela JA, Alsop DC, Gonzalez-Atavales
JB, Maldjian JA, Kasner SE and Detre JA: Magnetic resonance
perfusion imaging in acute ischemic stroke using continuous
arterial spin labeling. Stroke. 31:680–687. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bokkers RPH, van Osch MJP, van der Worp
HB, de Borst GJ, Mali WP and Hendrikse J: Symptomatic carotid
artery stenosis: Impairment of cerebral autoregulation measured at
the brain tissue level with arterial spin-labeling MR imaging.
Radiology. 256:201–208. 2010. View Article : Google Scholar : PubMed/NCBI
|