Introduction
Cholesterol embolism, which is also known as
cholesterol emboli syndrome, cholesterol crystal embolism or
atheroembolism, is a relatively rate but potentially serious
complication of atherosclerosis (1).
Cholesterol emboli occur spontaneously or iatrogenically following
vascular surgery, catheterization or anticoagulation treatment when
cholesterol crystals located in an atherosclerotic plaque in a
large caliber artery embolize to small or medium caliber arteries,
resulting in obstructive end-organ damage and an inflammatory
response (2). Previous studies have
reported that the incidence of cholesterol embolism is 0.31–2.4%
(3). In recent years, the incidence
of cholesterol embolism has increased due to a greater frequency of
triggering factors, typically catheterization or vascular surgery.
Elderly individuals with diabetes, hypertension, and dyslipidaemia
are particularly susceptible to cholesterol embolism (3). As there is no definitive treatment for
cholesterol embolism, therapeutic strategies are predominantly
preventive and supportive (3).
Prognosis is poor; 33% of patients who require renal replacement
therapy succumb to their symptoms during the first year (4).
Cholesterol embolism is ubiquitous; therefore, the
clinical manifestation are protean, non-specific, insidious and
multi-systemic (3). Diagnosis is
challenging, and can only be achieved via a biopsy. Vasculitis is
an important differential diagnosis (5). Cholesterol embolism and anti-neutrophil
cytoplasmic antibodies (ANCA)-associated vasculitis (AAV) are
distinct entities; however, their clinical manifestations,
including renal failure, skin lesions and elevation of inflammatory
markers, are markedly similar (6).
ANCA are useful indices for the diagnosis of AAV (7). Therefore, a ANCA-positive result in
patients with cholesterol embolism may complicate its differential
diagnosis and treatment.
In order to deepen the understanding of differential
diagnosis and treatment between cholesterol embolism and AAV, the
present study reports a case of cholesterol embolism in an Asian
male, who presented with subacute progressive nonoliguric renal
failure, refractory hypertension, late-developing skin lesions and
proteinase 3 targeting ANCA (PR3-ANCA).
Case report
Prior to admission to The First Affiliated Hospital
of Xiamen University (Xiamen, China), the 69-year-old male
described in the present case report had been admitted to a
regional hospital in May 2010 complaining of chest tightness,
shortness of breath and fatigue. The patient had been treated with
statins, calcium channel blockers, an angiotensin receptor
antagonist, a diuretic, antibiotics and acetylsalicylic acid. Upon
demonstrating a normal renal function, with serum creatinine (Scr)
levels of 1.13 mg/dl [normal range (NR), 0.49–1.50 mg/dl] and
normal urinalysis results, the patient was discharged. However,
during a 2-week follow-up period, a gradual rise in Scr levels
(2.45 mg/dl) was detected, as well as leukocytosis and
eosinophilia. Leukocytosis and eosinophilia peaked in July 2010,
and the white blood cell (WBC) count was 16.6×109/l (NR,
4–10.0×109/l), with 15.8% eosinophils
(2.62×109/l; NR, 0.02–0.5×109/l). Urine
volume and urinalysis results remained normal. Atherosclerotic
plaques were detected in the femoral and popliteal arteries of the
bilateral lower limbs by a color Doppler ultrasound; however, the
reason for transient eosinophilia and renal failure was not
elucidated. Scr levels fluctuated around 1.83 mg/dl (NR, 0.49–1.50
mg/dl) for several months.
The patient was admitted to the Department of
Cardiology at The First Affiliated Hospital of Xiamen University in
January 2011. The patient had a 30-year history of smoking and
suffered from chronic pulmonary disease, gout, hypertension,
hypercholesterolemia, coronary artery disease and diabetes
mellitus. A physical examination and subsequent blood tests
demonstrated the following: Blood pressure, 183/98 mmHg (NR,
<140/90 mmHg); WBC, 10.1×109/l without eosinophils;
hemoglobin, 8.8 g/dl (NR, 11–16 g/dl); erythrocyte sedimentation
rate (ESR), 82 mm/h (NR, 0–15 mm/h); blood urea nitrogen, 50.1
mg/dl (NR, 8.12–22.96 mg/dl); creatinine, 3.4 mg/dl (NR, 0.49–1.50
mg/dl); total cholesterol, 272.2 mg/dl (NR, 108.3–216.6 mg/dl);
C-reactive protein (CRP), 5.4 mg/dl (NR, 0–3 mg/dl); 24-h
proteinuria, 7.64 g (NR, <0.15 g); urine sediments containing
mild microhematuria; a normal platelet count; and no cutaneous
lesions. Tests for anti-double stranded DNA antibodies,
anti-phospholipid antibody, rheumatoid factor, hepatitis B virus,
hepatitis C virus and human immunodeficiency virus were negative,
and serum complement levels were normal. Tests for myeloperoxidase
ANCA (MPO-ANCA) and anti-glomerular basement membrane antibodies
were negative, whereas the results of a PR3-ANCA test were positive
(50.6 RU/ml; NR, <20 RU/ml). Bilateral bronchial pneumonia and
left pulmonary lingular hamartoma were detected and nasal endoscopy
demonstrated rhinitis, under armor hypertrophy and erosion in the
left plow zone.
In order to control the patient's blood pressure,
combined antihypertensive drugs (5 mg amlodipine besylate b.i.d.,
80 mg valsartan q.d., 25 mg hydrochlorothiazid q.d., 20 mg
furosemide q.d., 1 mg prazosin t.i.d., and intravenous sodium
nitroprusside or nitroglycerin) were administered; however, the
patient's blood pressure fluctuated (180–200/93–107 mmHg) and his
renal function deteriorated (Scr, 5.42 mg/dl). As a result of a
further decline in renal function and refractory hypertension, the
patient was transferred to the Department of Nephrology at The
First Affiliated Hospital of Xiamen University, where he was
treated with statins (20 mg atorvastatin calcium tablets p.o. q.n.;
Pfizer Inc., New York, NY, USA), an antibiotic (4.5 g
piperacillin/tazobactam i.v. g.t.t. q12 h; Pfizer Inc.) and an
anti-aggregant (75 mg clopidogrel hydrogen sulfate p.o. q.d.;
Sanofi S.A., Paris, France), and his renal function subsequently
improved (Scr decreased to 3.5 mg/dl).
In March 2011, the patient underwent a renal biopsy.
Renal biopsy specimens were examined by light and
immunofluorescence microscopy. For light microscopy, specimens were
fixed in 10% buffered-formaldehyde, embedded in paraffin, and 2 µm
sections were stained with hematoxylin and eosin, periodic
acid-Schiff and periodic acid-silver methenamine. For
immunofluorescence examination, the specimens were embedded in an
optimal cutting temperature compound (Miles Laboratories, Elkhart,
IN, USA) and frozen in an acetone-dry ice mixture. Frozen sections
were cut into 5-µm on a cryostat, rinsed in 0.01 mol/l
phosphate-buffered saline (PBS; pH 7.4), fixed in absolute acetone
for 10 min, and incubated for 30 min at room temperature with
fluorescein isothiocyanate-conjugated rabbit antihuman IgG, IgA,
IgM, C1q, C3, C4 or fibrinogen antisera (Dakopatts, Copenhagen,
Denmark). Stained sections were rinsed in PBS and examined under a
fluorescence photomicroscope (Zeiss Axiophot, Oberkochen, Germany).
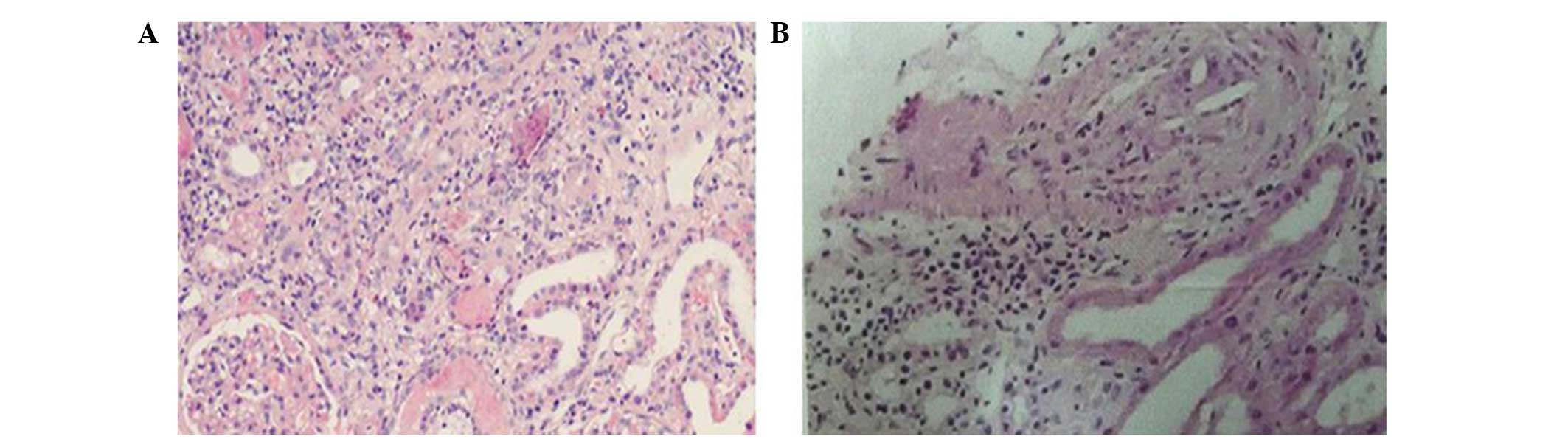
Analysis of the specimen demonstrated 40 glomeruli in the renal
cortex, of which 16 exhibited global sclerosis and two exhibited
segmental sclerosis. The remaining glomeruli were diminished to
varying extents. Extracapillary proliferation, crescents or
segmental necrotizing lesions were not detected in the glomeruli.
Tubular atrophy was severe and the renal interstitium exhibited
mild edema and fibroplastic proliferation. In addition, the renal
interstitium was saturated with inflammatory cells, including
eosinophils, neutrophils, monocytes, lymphocytes and plasmocytes
(Fig. 1A). As shown in Fig. 1B, the vascular wall of the
interlobular arteries was thickened, and the lumen of the
interlobular arteries was narrowed. Furthermore, individual
interlobular arteries exhibited hyalinosis, and needle-shaped
cholesterol emboli were observed in the respective lumens of two
interlobular arteries (Fig. 1B).
However, neither immunoglobulin nor complement deposition were
observed, according to a routine immunofluorescence analysis.
Taking all the results together, the patient was
diagnosed with cholesterol embolism and subacute interstitial
nephritis, associated with PR3-ANCA. Immunosuppressive therapy with
a steroid, prednisone, and cyclophosphamide (CTX; both SINE
Shanghai Pharma Co., Ltd., Shanghai, China) was initiated. A total
of 40 mg prednisone was initially administered per day; however,
the dosage was reduced to 30 mg/day after 4 weeks, and was
subsequently tapered gradually at 2- or 4-week intervals until
suspension at 6 months. CTX (0.8 g) was administered intravenously
six times at 4-week intervals. During the course of
immunosuppressive therapy, Scr levels fluctuated (4.43–5.96 mg/dl),
and urinalysis (Aution Max AX-4030, Arkray, Tokyo, Japan) revealed
nephritic proteinuria and trace hematuria. PR3-ANCA titer decreased
to 35.3 RU/ml; however, it remained positive at the end of
immunosuppressive therapy.
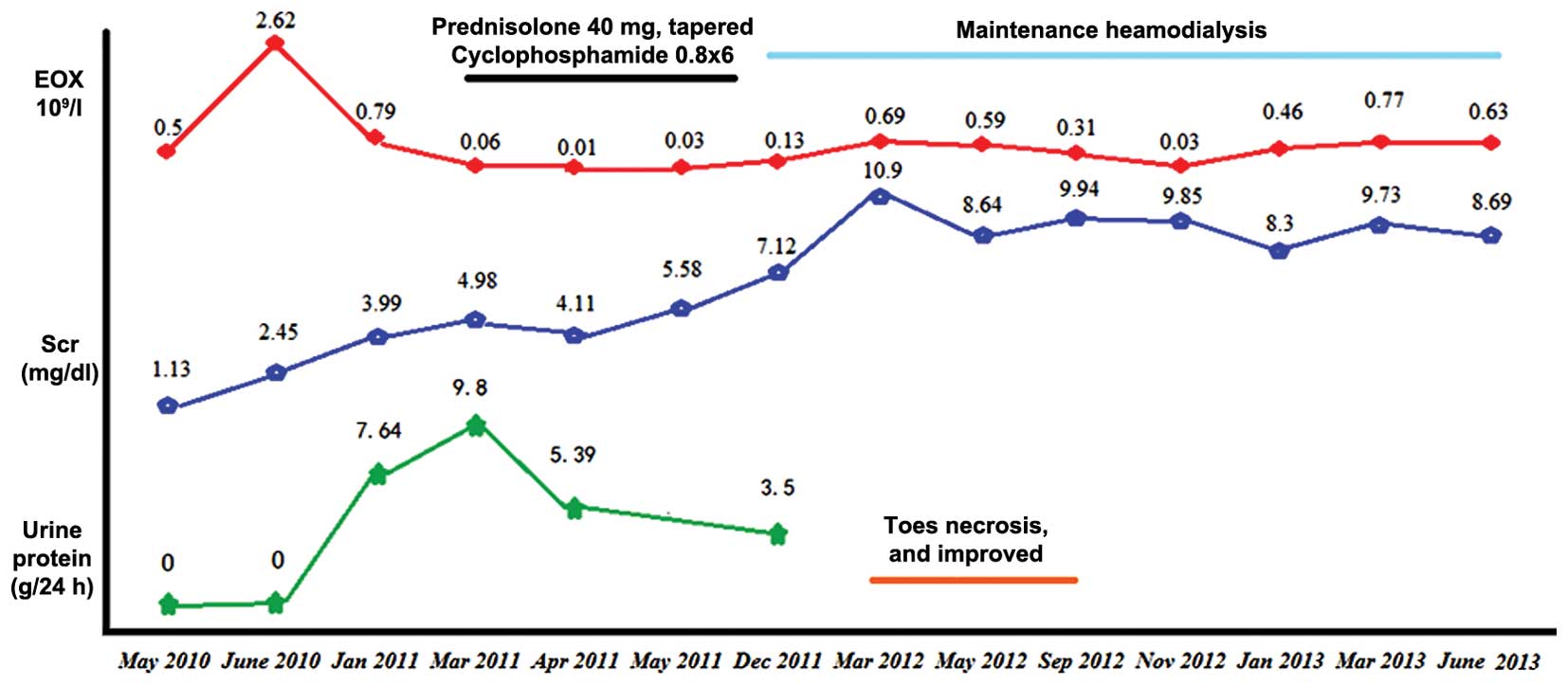
In December 2011, due to a further decline in renal
function (Scr level, 7.52 mg/dl), hemodialysis was performed three
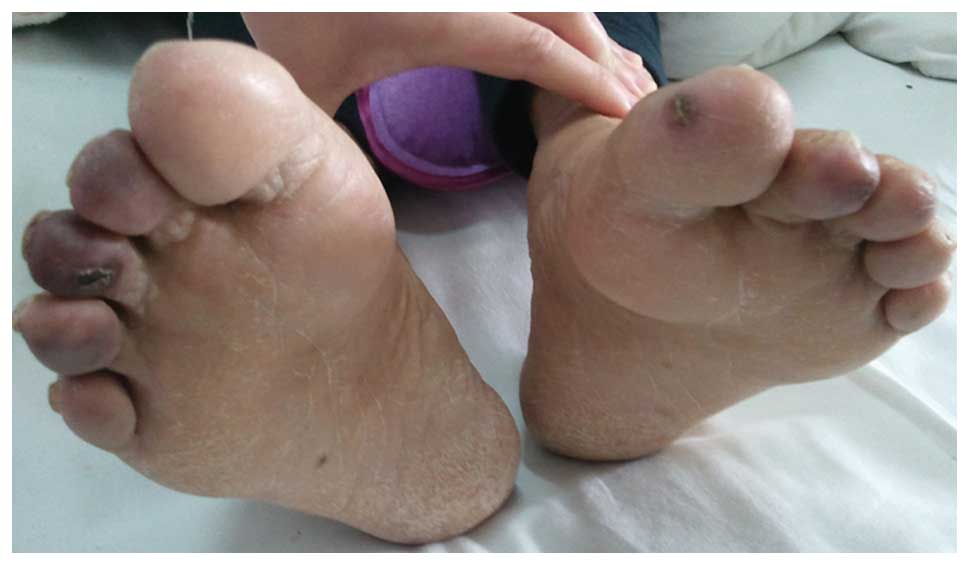
times per week. A month later, cyanosis was observed on the
patient's toes and he complained of severe pain. In addition, the
right pedal pulse was weakened. Color Doppler ultrasound analysis
demonstrated atherosclerosis and plaques in the femoral and
popliteal arteries of the bilateral lower limbs, and hypoperfusion
in the right anteriortibial artery and right dorsalis pedis.
Cyanosis of the patient's toes worsened and four toes exhibited
signs of necrosis or gangrene (Fig.
2). The patient refused to undergo a skin biopsy of the
affected toes and an angiography of the lower limb arteries.
Following 5 months of treatment with atorvastatin (20 mg q.d.;
Pfizer, Inc.) and anti-aggregants, including aspirin (100 mg q.d.;
Bayer, Shanghai, China), sarpogrelate (100 mg b.i.d.; Mitsubishi
Gas Chemical Company, Inc., Tokyo, Japan), clopidogrel (50 mg q.d.;
CardinalHealth China, Shanghai, China) and cilostazol (50 mg
b.i.d.; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan), the
necrosis gradually improved. The patient was maintained on
hemodialysis for ~2 years (Fig. 3).
At the most recent follow-up, the patient exhibited general good
health and has been undergoing maintenance hemodialysis in our
hemodialysis center for >4 years.
Discussion
Diagnosis of cholesterol embolism remains
challenging due to its diverse manifestations. Cholesterol embolism
is a well-documented cause of renal disease (8). Renal failure and cutaneous lesions are
two of the most common clinical manifestations of cholesterol
embolism (3); however, they are also
frequently observed in patients with systemic vasculitis (6). Furthermore, the presence of ANCA in
cholesterol embolism typically complicates its differential
diagnosis and treatment, since ANCA are a defining feature of
ANCA-associated vasculitis (AAV) (7). Cholesterol embolism remains a rare
disease, therefore its incidence is unclear (3). Cholesterol embolism associated with
ANCA has rarely been reported. To date, only 11 cases reported in
English could be retrieved from the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed) using the
term ‘cholesterol embolism plus ANCA’ (9–18).
Clinical characteristics and laboratory findings of these 12
patients (including the present case) are listed in Table I.
 | Table I.Clinical and laboratory features of
patients with cholesterol embolism and ANCA in the literature. |
Table I.
Clinical and laboratory features of
patients with cholesterol embolism and ANCA in the literature.
|
|
Case |
|---|
|
|
|
|---|
| Feature | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|
| (Refs.) | Present | (13) | (8) | (8) | (12) | (10) | (6) | (5) | (11) | (7) | (14) | (9) |
| Age/gender/race | 69/M/A | 73/M/C | 63/M/C | 69/F/C | M/C | 50/M/A | 47/M/C | 67/M/C | 70/M/C | 65/F/C | 75/M/A | 76/M/A |
| Prior medical
problems |
|
|
|
|
|
|
|
|
|
|
|
|
|
Hypertension | Y | Y | Y | Y | NM | NM | Y | N | Y | Y | N | N |
|
Hypercholesterolemia | Y | NM | NM | Y | NM | NM | Y | N | Y | NM | N | N |
|
Atherosclerotic vascular
disease | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| Inciting events | Sp | Sp | Sp | Thrombolytic
therapy | NM | Angiography | Coronary artery
angioplasty | Cardiac
catheterization | Anticoagulant
therapy | Angiography | Coronary artery
bypass graft | Sp |
| Clinical
manifestation |
|
|
|
|
|
|
|
|
|
|
|
|
|
Kidney | SARF; severe
uncontrolled hypertension | ARF | SARF; severe
uncontrolled hypertension | SARF; Bp 180/90
mmHg | SARF | ARF; Bp 178/98
mmHg | Creatinine 0.94
mg/dl | ARF; Bp 180/100
mmHg | SARF | SARF; Bp 220/110
mmHg | ARF | Normal renal
function |
|
Skin | BTS; ulceration;
gangrene | Ulceration;
gangrene | Ulceration;
gangrene | Blue toe
syndrome | No | Ulceration;
gangrene | Livedo reticularis;
necrosis | Livedo
reticularis | Necrotic livedo
reticularis | Acrocyanosis;
livedo reticularis | Systemic purpura
rashes; BTS | Micro livedo;
ulcers |
| Laboratory
findings |
|
|
|
|
|
|
|
|
|
|
|
|
|
Leukocytosis | Y | Y | N | N | NM | Y | N | N | Y | Y | N | N |
|
Anemia | Y | Y | N | N | NM | Y | N | N | N | Y | Y | N |
|
Eosinophilia | Y | N | N | Y | NM | N | N | N | N | N | Y | N |
|
Elevated | Y | NM | Y | Y | NM | Y | Y | N | NM | Y | Y | Y |
| ESR or
CRP |
|
|
|
|
|
|
|
|
|
|
|
|
|
Urinalysis | Nephrotic-range
proteinuria; mild hematuria | NM | Proteinuria 1.08
g/l; microscopic haematuria | Mild proteinuria
without erythrocyturia | NM | NM | NM | Proteinuria 3.4
g/24 h; intense micro-hematuria | Proteinuria 1,000
mg/d | Proteinuria 300
mg/24 h without erythrocyturia | Protein++ (0.6
g/d), occult blood 3+ | Normal |
|
Hypocomplementaemia | N | N | N | N | N | N | N | N | N | N | N | N |
|
ANCA | PR3-ANCA (50.6
RU/ml) | ANCA+ | MPO-ANCA (34
RU/ml) | MPO-ANCA (43
RU/ml) | ANCA+ | PR3-ANCA+
MPO-ANCA | PR3-ANCA (62
IU/ml) | MPO-ANCA (48I
U/ml) | C-ANCA [1:80
(IIF)] | MPO-ANCA (39
AU) | MPO-ANCA (24
EU) | MPO-ANCA (236
EU) |
| Tissue biopsy |
|
|
|
|
|
|
|
|
|
|
|
|
| Renal
biopsy | CE; subacute
interstitial nephritis | CE; global or
partial sclerosis; focal interstitial fibrosis; tubular
atrophy | CE; hypertensive
nephro-angiosclerosis | CE; hypertensive
nephro-angiosclerosis | CE | NM | NM | CE; crescents with
segmental necrotizing lesions; nephro-angiosclerotic damage | CE | CE | NM | NM |
| Skin or
muscle biopsy | NM | CE | CE, vasculitis | CE | NM | CE | NM | NM | No
abnormalities | No
abnormalities | CE, vasculitis | CE, vasculitis |
| Treatment | Prednisone + CTX,
statin | Prednisolone + CTX,
antibiotics | Steroid | NM | Steroid + CTX | Steroid + CTX | Prednisolone + CTX,
azathioprine | Steroid + CTX | Supportive
therapy | Prednisolone,
hydro-xychloroquine | Prednisolone,
statin, apheresis | Prednisolone |
| Outcome | Maintenance HD | HD; died due to
cardiac failure on day 39 | HD; toes were
amputated | NM | Survived 1.5 years
post-onset | HD | Survived | Stable Scr (2.5
mg/dl) | HD; died after 12
weeks due to multi-organ failure | Stable Scr (1.58
mg/dl) | HD discont.; Scr
decreased to 2.5 mg/dl | Survived for 2
months |
The average age of the patients was 69 years (age
range, 47–76 years) and 82% of the patients were aged >63 years.
Of the 12 patients reported in the literature, 10 were male, two
were female, four were Asian and eight were Caucasian. A number of
these patients suffered from one or more medical problems prior to
admission, including hypertension (n=7), hypercholesterolemia
(n=4), chronic obstructive pulmonary disease (n=3) and
atherosclerotic vascular disease (n=11). In addition, 91% of the
patients suffered from one or more atherosclerotic vascular
diseases, including coronary heart disease, abdominal aortic
aneurysm, peripheral vascular disease or ischemic nephropathy, and
a third of the patients were smokers.
According to the preliminary clinical data, 33% of
the patients (4/12) developed cholesterol embolism spontaneously;
whereas the remaining eight patients developed the disease
following a vascular procedure, including anticoagulation or
thrombolysis therapy (19). Four
patients presented with acute renal failure, and pronounced renal
impairment typically occurred within 2 weeks. Six patients
exhibited subacute renal failure several weeks after an inciting
event and two patients demonstrated normal renal function. Notably,
50% of the patients exhibited severe uncontrolled hypertension. In
the present case, the blood pressure of the patient remained
difficult to control, despite treatment with antihypertensive
drugs. Skin lesions are the most common extrarenal manifestation of
cholesterol embolism and the majority of patients suffer from blue
toe syndrome, livedo reticularis, ulceration or gangrene (3). Typically, the skin lesions developed at
the onset of the disease; however, in the present case, skin
lesions were observed at 1.5 years after disease presentation.
Since the skin lesions presented within the period of maintained
hemodialysis, the episode may have occurred due to the use of
anticoagulants for hemodialysis (20).
It has previously been reported that eosinophilia
occurs in 80% of cases of atheroembolic renal disease, therefore it
may help to establish the diagnosis (3). However, eosinophilia was only detected
in 25% of the patients (3/12) with cholesterol embolism in the
literature. A possible explanation for this discrepancy is that
eosinophilia frequently occurs during the acute phase of the
disease and is typically transient (3). In the present case, eosinophil counts
peaked in the early stage and subsequently fluctuated during the
course of the disease. In the majority of patients, elevated levels
of inflammatory markers, including ESR and CRP, were also observed.
Among the 12 patients, urinalysis results were typically benign
with mild hematuria, and only minor proteinuria was detected in the
present case. However, there was no record of urinalysis having
taken place for four patients. One patient exhibited normal
urinalysis results, five patients showed moderate proteinuria with
or without hematuria and proteinuria was demonstrated in one
patient (3.4 g/24 h) with intense microhematuria and hyaline
granular casts. Nephrotic-range proteinuria, which was observed in
the present case, has previously been described in a number of
cholesterol embolism patients with focal glomerulosclerosis or
diabetic nephropathy (3,5).
Of the 12 patients reported in the literature, three
were positive for cytoplasmic ANCA (C-ANCA), six were positive for
perinuclear ANCA (P-ANCA), one patient was positive for both C-ANCA
and P-ANCA and two patients were ANCA-positive, as determined by
early indirect immunofluorescence. ANCA positivity in patients with
cholesterol embolism typically confounds the differential diagnosis
and treatment of the disease, and the role of ANCA in cholesterol
embolism remains unclear. Whether the occurrence of ANCA in
cholesterol embolism is merely a coincidence or bears cause and
effect is yet to be elucidated. In addition, it is unclear whether
ANCA underlies the thromboembolic pathogenic process. It has
previously been suggested that ANCA may have a pathogenetic role in
AAV (21); however, other studies
have argued against the involvement of ANCA due to the presence of
naturally occurring ANCA in healthy individuals and the lack of an
association between ANCA titres and disease activity (7). Furthermore, it has been hypothesized
that not all PR3- or MPO-ANCAs are pathogenic (7). A previous study reported that ANCA did
not have a major role in premature atherosclerosis, since no
increased level of the autoantibody was observed (22). In some cases, including the present
case, the presence of ANCA may occur synchronously with cholesterol
embolism, resulting in impairment of renal function and systemic
inflammation (11,12,14,15,17). In
two cases, persistent ANCA and vasculitis developed following
cholesterol embolism (10,16). Conversely, in other cases, vasculitis
and cholesterol embolism were deemed coexistent, based on the
identification of prior medical problems, intense hematuria,
variable ANCA titres or a high ANCA titre (9,13,18).
Notably, the clinical features of cholesterol embolism with ANCA
typically resemble those of patients with cholesterol embolism
alone (3,6). These data indicate that cholesterol
embolism may enhance the induction of ANCA during neutrophil
activation as a result of damage to the vascular system, which is
consistent with the induction of ANCA following in silica
exposure to or the administration of propylthiouracil (23). However, further large
population-based studies are required in order to clarify the role
of ANCA in cholesterol embolism.
Histological confirmation is regarded as the
definitive method for the diagnosis of cholesterol embolism, and a
renal biopsy was performed for eight of the 12 patients with
cholesterol embolism in the literature (9,11,12,15–17).
Consistent with previous reports (9,12,17),
cholesterol emboli were observed in the lumen of the interlobular
arteries in the present case, and histological changes to the
glomeruli and interstitium were observed. Skin or muscle biopsies
were performed for eight of the 12 patients (11–15,17,18). Two
cases showed no evidence of vasculitis or cholesterol crystal
clefts (11,15), whereas six cases demonstrated
cholesterol embolism, of which three also exhibited inflammatory
infiltration of neutrophils into the walls of the small arteries,
thus indicating that vasculitis and cholesterol embolism may have
been coexistent (12,13,18).
The British Society for Rheumatology/British Health
Professionals in Rheumatology guideline recommends that newly
diagnosed AAV should be assessed for treatment with glucocorticoids
and CTX (24). However, at present,
no definitive treatment has been established and no clinical trials
have been conducted in patients with atheroembolic renal disease;
therefore, the majority of therapeutic measures are preventive
(3). Although anticoagulants may
trigger atheroembolization, the results of a previous study did not
support the hypothesis that peritoneal dialysis is superior to
hemodialysis (20), and the use of
steroids remains controversial (3).
Of the cases of cholesterol embolism with positive ANCA, ten (83%)
were treated with steroids, of which six (60%) were also
administered CTX. Combination therapy with steroids and CTX has
been shown to be effective in five cases (9,10,14,16,17).
One patient improved following treatment with prednisolone and CTX,
but relapsed after the prednisolone dosage was reduce and CTX was
discontinued (17). Treatment with a
steroid alone was effective in three cases, including one case of
pleuritis and two cases of cholesterol embolism with vasculitis and
cutaneous lesions; the renal functions of these three patients were
improved following treatment (11,13,18). One
patient was able to discontinue hemodialysis after steroid therapy
(18). One patient was treated with
hemodialysis and supportive therapy; however, the skin lesions
deteriorated and the patient succumbed to multiorgan failure after
12 weeks (15). In the present case,
steroids combined with CTX therapy were administered at 8 months
post-disease onset, upon a renal biopsy and detection of subacute
interstitial nephritis and gradual deterioration of renal
function.
In conclusion, the present study reported the case
of a 69-year-old Asian male who successively presented with
eosinophilia, subacute progressive renal failure, refractory
hypertension, PR3-ANCA positivity, cholesterol embolism with
interstitial nephritis and late-developing skin lesions. The
present study provides an overall perspective on the
ANCA-associated cholesterol embolism through a comparative
discussion of the clinical data of 11 cases of ANCA-associated
cholesterol embolism in the literature and the present case. The
roles of ANCA in cholesterol embolism, and efficient treatment
strategies for patients with ANCA-associated cholesterol embolism,
remain to be elucidated and require further investigation.
Acknowledgements
The authors would like to thank the staff of the
Hemodialysis Unit at The First Affiliated Hospital of Xiamen
University.
References
|
1
|
Quinones A and Saric M: The cholesterol
emboli syndrome in atherosclerosis. Curr Atheroscler Rep.
15:315–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saric M and Kronzon I: Aortic
atherosclerosis and embolic events. Curr Cardiol Rep. 14:342–349.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scolari F and Ravani P: Atheroembolic
renal disease. Lancet. 375:1650–1660. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thériault J, Agharazzi M, Dumont M,
Pichette V, Ouimet D and Leblanc M: Atheroembolic renal failure
requiring dialysis: Potential for renal recovery? A review of 43
cases. Nephron Clin Pract. 94:c11–c18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meyrier A: Cholesterol crystal embolism:
Diagnosis and treatment. Kidney Int. 69:1308–1312. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minota S: ANCA in atheroembolism; just a
coincidence or bearing cause and effect? Intern Med. 45:495–496.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schönermarck U, Csernok E and Gross WL:
Pathogenesis of anti-neutrophil cytoplasmic antibody-associated
vasculitis: Challenges and solutions 2014. Nephrol Dial Transplant.
30(Suppl 1): i46–i52. 2015.PubMed/NCBI
|
|
8
|
Scolari F, Tardanico R, Zani R, Pola A,
Viola BF, Movilli E and Maiorca R: Cholesterol crystal embolism: A
recognizable cause of renal disease. Am J Kidney Dis. 36:1089–1109.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aviles B, Ubeda I, Blanco J and Barrientos
A: Pauci-immune extracapillary glomerulonephritis and atheromatous
embolization. Am J Kidney Dis. 40:847–851. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De RS, Serratrice J, Granel B, Disdier P,
Bartoli JM, Pache X, Astoul P, Garbe L, Branchereau A and Weiller
PJ: Periaortitis heralding Wegener's granulomatosis. J Rheumatol.
29:392–394. 2002.PubMed/NCBI
|
|
11
|
Delen S, Boonen A, Landewé R, Kroon AA,
van der Linden S and Tervaert JW: An unusual case of ANCA positive
disease. Ann Rheum Dis. 62:780–781. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaplan-Pavlovcic S, Vizjak A, Vene N and
Ferluga D: Antineutrophil cytoplasmic autoantibodies in
atheroembolic disease. Nephrol Dial Transplant. 13:985–987. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maejima H, Noguchi T and Tanei R:
Cholesterol embolism associated with MPO-ANCA. Eur J Dermatol.
20:539–540. 2010.PubMed/NCBI
|
|
14
|
Maeshima E, Yamada Y, Mune M and Yukawa S:
A case of cholesterol embolism with ANCA treated with
corticosteroid and cyclophosphamide. Ann Rheum Dis. 60:7262001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miguélez A, Barrientos N, López-Rios F,
Vanaclocha F and Iglesias L: Necrotic livedo reticularis, multiple
cholesterol emboli and ANCA. J Eur Acad Dermatol Venereol.
17:351–352. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Palmgren E, Hartford M and Herlitz H:
Cholesterol embolism-a serious systemic disease. Lakartidningen.
97:1263–1266. 2000.(In Swedish). PubMed/NCBI
|
|
17
|
Peat DS and Mathieson PW: Cholesterol
emboli may mimic systemic vasculitis. BMJ. 313:546–547. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sugimoto T, Morita Y, Yokomaku Y, Isshiki
K, Kanasaki K, Eguchi Y, Koya D and Kashiwagi A: Systemic
cholesterol embolization syndrome associated with
myeloperoxidase-anti-neutrophil cytoplasmic antibody. Intern Med.
45:557–561. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scolari F, Ravani P, Gaggi R, Santostefano
M, Rollino C, Stabellini N, Colla L, Viola BF, Maiorca P,
Venturelli C, et al: The challenge of diagnosing atheroembolic
renal disease: Clinical features and prognostic factors.
Circulation. 116:298–304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ravani P, Gaggi R, Rollino C, Santostefano
M, Stabellini N, Colla L, Dallera N, Ravera S, Bove S, Faggiano P
and Scolari F: Lack of association between dialysis modality and
outcomes in atheroembolic renal disease. Clin J Am Soc Nephrol.
5:454–459. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kallenberg CG: Pathogenesis and treatment
of ANCA-associated vasculitides. Clin Exp Rheumatol. 33(4 Suppl
92): S11–S14. 2015.PubMed/NCBI
|
|
22
|
van Haelst PL, Asselbergs FW, van Doormaal
JJ, Veeger NJ, May JF, Holvoet P, Gans RO and Tervaert JW:
Antineutrophil cytoplasmatic antibodies in patients with premature
atherosclerosis: Prevalence and association with risk factors. J
Intern Med. 251:29–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Lind van Wijngaarden RA, van Rijn L,
Hagen EC, Watts RA, Gregorini G, Tervaert JW, Mahr AD, Niles JL, de
Heer E, Bruijn JA and Bajema IM: Hypotheses on the etiology of
antineutrophil cytoplasmic autoantibody associated vasculitis: The
cause is hidden, but the result is known. Clin J Am Soc Nephrol.
3:237–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ntatsaki E, Carruthers D, Chakravarty K,
D'Cruz D, Harper L, Jayne D, Luqmani R, Mills J, Mooney J, Venning
M, et al: BSR and BHPR guideline for the management of adults with
ANCA-associated vasculitis. Rheumatology (Oxford). 53:2306–2309.
2014. View Article : Google Scholar : PubMed/NCBI
|